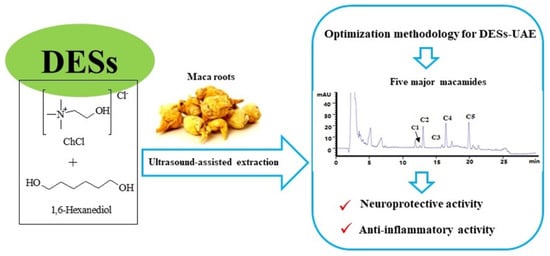
Deep Eutectic Solvent-Based Ultrasound-Assisted Strategy for Simultaneous Extraction of Five Macamides from Lepidium meyenii Walp and In Vitro Bioactivities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. DES Preparation
2.3. HPLC Quantification
2.4. Extraction Procedure
2.5. DES–UAE Process Optimization
2.5.1. Single-Factor Optimization
2.5.2. BBD Optimization
2.6. Other Extraction Methods for Comparison
2.7. Recovery of Target Macamides from DES Extracts
2.8. Evaluation of Neuroprotective Activities
2.9. Evaluation of Anti-Inflammatory Activities
2.10. Statistical Analysis
3. Results and Discussion
3.1. Single-Factor Experimentation
3.1.1. Screening of DESs
3.1.2. Screening of UAE Parameters
3.2. BBD Optimization of Extraction Process Conditions
5.94X12 − 6.47X22 − 3.86X32
− 82.95X12 − 78.10X22 − 26.69X32
5.90X12 − 7.06X22 − 3.20X32
11.07X2X3 − 106.67X12 − 79.47X22 − 24.07X32
73.02X2X3 − 126.45X12 − 129.17X22 − 61.55X32
3.3. Validation of HPLC Analysis
3.4. Model Verification
3.5. Comparison of Extraction Efficiency
3.5.1. Petroleum Ether and DESs
3.5.2. Heating, Heating + Stirring, and UAE
3.6. Recovery of Target Macamides
3.7. Pharmacological Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, Y.; McNeil, B.; Harvey, L.M. Maca: An Andean crop with multi-pharmacological functions. Food Res. Int. 2007, 40, 783–792. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Fan, L. The nutritional composition of maca in hypocotyls (Lepidium meyenii Walp.) cultivated in different regions of China. J. Food Qual. 2017, 2017, 3749627. [Google Scholar] [CrossRef]
- Chen, R.; Wei, J.; Gao, Y. A review of the study of active components and their pharmacology value in Lepidium meyenii (Maca). Phytother. Res. 2021, 35, 6706–6719. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Peng, X.R.; Zhang, R.R.; Liu, J.H.; Li, Z.R.; Zhou, L.; Qiu, M.H. Lepithiohydimerins A-D: Four pairs of neuroprotective thiohydantoin dimers bearing a disulfide bond from maca (Lepidium meyenii Walp.). Chin. J. Chem. 2021, 39, 2738–2744. [Google Scholar] [CrossRef]
- Peres, N.S.L.; Bortoluzzi, L.C.P.; Marques, L.L.M.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Cardoso, F.A.R. Medicinal effects of Peruvian maca (Lepidium meyenii) a review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef]
- Tenci, B.; Mannelli, L.D.; Maresca, M.; Micheli, L.; Pieraccini, G.; Mulinacci, N.; Ghelardini, C. Effects of a water extract of Lepidium meyenii root in different models of persistent pain in rats. Z. Nat. C J. Biosci. 2017, 72, 449–457. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, B.; Hua, H.; Liu, C.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Macamides: A review of structures, isolation, therapeutics and prospects. Food Res. Int. 2020, 138, 109819. [Google Scholar] [CrossRef]
- Chen, S.X.; Li, K.K.; Pubu, D.; Jiang, S.P.; Chen, B.; Chen, L.R.; Yang, Z.; Ma, C.; Gong, X.J. Optimization of ultrasound-assisted extraction, HPLC and UHPLC-ESI-Q-TOF-MS/MS analysis of main macamides and macaenes from Maca (cultivars of Lepidium meyenii Walp). Molecules 2017, 22, 2196. [Google Scholar] [CrossRef]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and antitumoral activities of isolated macamide and macaene fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef]
- Lin, L.; Huang, J.; Sun-Waterhouse, D.; Zhao, M.; Zhao, K.; Que, J. Maca (Lepidium meyenii) as a source of macamides and polysaccharide in combating of oxidative stress and damage in human erythrocytes. Int. J. Food Sci. Technol. 2018, 53, 304–312. [Google Scholar] [CrossRef]
- Yang, Q.; Jin, W.; Lv, X.; Dai, P.; Ao, Y.; Wu, M.; Deng, W.; Yu, L. Effects of macamides on endurance capacity and anti-fatigue property in prolonged swimming mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef]
- Stojanovska, L.; Law, C.; Lai, B.; Chung, T.; Nelson, K.; Day, S.; Haines, C. Maca reduces blood pressure and depression, in a pilot study in postmenopausal women. Climacteric. J. 2015, 18, 69–78. [Google Scholar] [CrossRef]
- Melnikovova, I.; Fait, T.; Kolarova, M.; Fernandez, E.C.; Milella, L. Effect of Lepidium meyenii Walp. on semen parameters and serum hormone levels in healthy adult men: A double-blind, randomized, placebo-controlled pilot study. J. Evid. Based Complement Altern. Med. 2015, 2015, 324369. [Google Scholar] [CrossRef]
- McCollom, M.M.; Villinski, J.R.; McPhail, K.L.; Craker, L.E.; Gafner, S. Analysis of macamides in samples of maca (Lepidium meyenii) by HPLC-UV-MS/MS. Phytochem. Anal. 2005, 16, 463–469. [Google Scholar] [CrossRef]
- Turgud, F.K.; Narinç, D. Influences of dietary supplementation with Maca (Lepidium meyenii) on performance, parameters of growth curve and carcass characteristics in Japanese quail. Animals 2022, 12, 318. [Google Scholar] [CrossRef]
- Hajdu, Z.; Nicolussi, S.; Rau, M.; Lorántfy, L.; Forgo, P.; Hohmann, J.; Csupor, D.; Gertsch, J. Identification of endocannabinoid system-modulating N-alkylamides from Heliopsis helianthoides var. scabra and Lepidium meyenii. J. Nat. Prod. 2014, 77, 1663–1669. [Google Scholar] [CrossRef]
- Ye, Y.Q.; Ma, Z.H.; Yang, Q.F.; Sun, Y.Q.; Zhang, R.Q.; Wu, R.F.; Ren, X.; Mu, L.J.; Jiang, Z.Y.; Zhou, M. Isolation and synthesis of a new benzylated alkamide from the roots of Lepidium meyenii. Nat. Prod. Res. 2019, 33, 2731–2737. [Google Scholar] [CrossRef]
- Xia, C.; Deng, J.L.; Chen, J.; Zhu, Y.Q.; Song, Y.; Zhang, Y.J.; Li, H.J.; Lin, C.B. Simultaneous determination of macaenes and macamides in maca using an HPLC method and analysis using a chemometric method (HCA) to distinguish maca origin. Rev. Bras. Farmacogn. 2019, 29, 702–709. [Google Scholar] [CrossRef]
- Xing, C.; Cui, W.Q.; Zhang, Y.; Zou, X.S.; Hao, J.Y.; Zheng, S.D.; Wang, T.T.; Wang, X.Z.; Wu, T.; Liu, Y.Y.; et al. Ultrasound-assisted deep eutectic solvents extraction of glabridin and isoliquiritigenin from Glycyrrhiza glabra: Optimization, extraction mechanism and in vitro bioactivities. Ultrason. Sonochem. 2022, 83, 105946. [Google Scholar] [CrossRef]
- Aparecida de Marco, B.; Saú Rechelo, B.; Gandolpho Tótoli, E.; Carolina Kogawa, A.; Regina Nunes Salgado, H. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S.J. Utilization of pomegranate peel waste: Natural deep eutectic solvents as a green strategy to recover valuable phenolic compounds. J. Clean Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Peng, X.; Duan, M.H.; Yao, X.H.; Zhang, Y.H.; Zhao, C.J.; Zu, Y.G.; Fu, Y.J. Green extraction of five target phenolic acids from Lonicerae Japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Alshammari, O.A.O.; Almulgabsagher, G.A.A.; Ryder, K.S.; Abbott, A.P. Effect of solute polarity on extraction efficiency using deep eutectic solvents. Green Chem. 2021, 23, 5097–5105. [Google Scholar] [CrossRef]
- Zhen, S.; Chen, S.; Geng, S.; Zhang, H.; Chen, Y.; Liu, B. Ultrasound-assisted natural deep eutectic solvent extraction and bioactivities of flavonoids in Ampelopsis grossedentata leaves. Foods 2022, 11, 668. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Alam, P.; Alrehaily, A.J.; Alqahtani, A.S.; Akhtar, A.; Alhowiriny, T.A.; Almarfadi, O.M.; Mothana, R.A. Optimization of ultrasound-assisted parthenolide extraction from Tarchonanthus camphoratus leaves using response surface methodology: HPTLC and cytotoxicity analysis. Arab. J. Chem. 2021, 14, 103194. [Google Scholar] [CrossRef]
- Tang, Z.; Lin, W.; Yang, J.; Feng, S.; Qin, Y.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T.; et al. Ultrasound-assisted extraction of Cordyceps cicadae polyphenols: Optimization, LC-MS characterization, antioxidant and DNA damage protection activity evaluation. Arab. J. Chem. 2022, 15, 103953. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Li, J.; Wang, A.; Li, G.; Ren, X.; Yin, W. Ultrasound-assisted deep eutectic solvent extraction of echinacoside and oleuropein from Syringa pubescens Turcz. Ind. Crop. Prod. 2020, 151, 112442. [Google Scholar] [CrossRef]
- Ivanović, M.; Albreht, A.; Krajnc, P.; Vovk, I.; Razboršeka, M.I. Sustainable ultrasound-assisted extraction of valuable phenolics from inflorescences of Helichrysum arenarium L. using natural deep eutectic solvents. Ind. Crop. Prod. 2021, 160, 113102. [Google Scholar] [CrossRef]
- Yin, X.; Jia, H.; Zhang, Q.; Jiang, Y.; Tu, P. (+)- and (–)-Corydecumbenines A and B, two pairs of novel quaternary protoberberine alkaloid cycloadduct enantiomers with anti-neuroinflammatory and neuroprotective activities from the rhizomes of Corydalis decumbens. Bioorg. Chem. 2020, 104, 104251. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Shi, L.; Cheng, Y.; Hu, H.; Zeng, B.; Zhao, F. Water based-deep eutectic solvent for ultrasound-assisted liquid–liquid microextraction of parabens in edible oil. Food Chem. 2022, 383, 132586. [Google Scholar] [CrossRef]
- Yin, X.; Zhong, Z.; Bian, G.; Cheng, X.; Li, D. Ultra-rapid, enhanced and eco-friendly extraction of four main flavonoids from the seeds of Oroxylum indicum by deep eutectic solvents combined with tissue-smashing extraction. Food Chem. 2020, 319, 126555. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Zhong, J.L.; Yan, H.; Xu, H.D.; Muhammad, N.; Yan, W.D. Preparation from Lepidium meyenii Walpers using high-speed counter current chromatography and thermal stability of macamidesin air at various temperatures. J. Pharm. Biomed. Anal. 2019, 164, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Mako´s, P.; Przyjazny, A.; Boczkaj, G. Hydrophobic deep eutectic solvents as “green” extraction media for polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2018, 1570, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, K.S.; Vu, N.; Rondón-Ortiz, A.N.; Böhlke, M.; Maher, T.J.; Pino-Figueroa, A.J. Neuroprotective activity of macamides on manganese-induced mitochondrial disruption in U-87 MG glioblastoma cells. Toxicol. Appl. Pharmacol. 2018, 340, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ponzetti, M.; Rucci, N. Updates on osteoimmunology: What’s new on the cross-talk between bone and immune system. Front. Endocrinol. 2019, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, W.; Fu, C.; Dai, P.; Yu, Y.; Huo, Q.; Yu, L. Discovering anti-osteoporosis constituents of maca (Lepidium meyenii) by combined virtual screening and activity verification. Food Res. Int. 2015, 77, 215–220. [Google Scholar] [CrossRef]
- Wang, T.; Sun, C.H.; Zhong, H.B.; Gong, Y.; Cui, Z.K.; Xie, J.; Wang, Y.P.; Liang, C.; Cao, H.H.; Chen, X.R.; et al. N-(3- methoxybenzyl)-(9Z,12Z,15Z)-octadecatrienamide promotes bone formation via the canonical Wnt/β-catenin signaling pathway. Phytother. Res. 2019, 33, 1074–1083. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, W.; Cui, Y.; Ao, M.; Liu, H.; Xu, H.; Yu, L. Protective effects of macamides from Lepidium meyenii Walp. against corticosterone-induced neurotoxicity in PC12 cells. RSC Adv. 2019, 9, 23096–23108. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. The macamide relieves fatigue by acting as inhibitor of inflammatory response in exercising mice: From central to peripheral. Eur. J. Pharmacol. 2022, 917, 174758. [Google Scholar] [CrossRef]



| Abbreviation | HBA | HBD | Molar Ratio |
|---|---|---|---|
| DES-1 | Choline chloride | Levulinic acid | 1:2 |
| DES-2 | Propanedioic acid | 1:2 | |
| DES-3 | 1,4-Butanediol | 1:2 | |
| DES-4 | Glycerol | 1:2 | |
| DES-5 | Urea | 1:2 | |
| DES-6 | Triethylene glycol | 1:2 | |
| DES-7 | 1,6-Hexanediol | 1:2 | |
| DES-8 | Xylitol | 1:2 | |
| DES-9 | DL-Malic acid | 1:2 | |
| DES-10 | Ethyl glycol | 1:2 |
| Run | X1 (mL/g) | X2 (°C) | X3 (min) | Extraction Yield (μg/g) | ||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | ||||
| 1 | 0 (10:1) | 0 (40) | 0 (30) | 77.50 | 602.14 | 57.08 | 719.40 | 1201.25 |
| 2 | 0 (10:1) | 0 (40) | 0 (30) | 78.60 | 622.04 | 58.13 | 710.43 | 1197.92 |
| 3 | −1 (8:1) | −1 (30) | 0 (30) | 71.14 | 504.41 | 49.35 | 568.32 | 1063.07 |
| 4 | 0 (10:1) | −1 (30) | 1 (40) | 67.69 | 486.67 | 43.71 | 607.95 | 962.07 |
| 5 | 0 (10:1) | 1 (50) | 1 (40) | 68.32 | 491.35 | 46.65 | 584.90 | 1047.88 |
| 6 | −1 (8:1) | 0 (40) | −1 (20) | 73.31 | 578.83 | 53.12 | 644.78 | 1123.30 |
| 7 | 0 (10:1) | 0 (40) | 0 (30) | 80.40 | 606.05 | 56.97 | 705.52 | 1223.63 |
| 8 | 0 (10:1) | 1 (50) | −1 (20) | 66.69 | 486.54 | 44.66 | 580.04 | 936.22 |
| 9 | 0 (10:1) | 0 (40) | 0 (30) | 79.02 | 598.54 | 54.50 | 707.97 | 1236.01 |
| 10 | 0 (10:1) | −1 (30) | −1 (20) | 73.09 | 550.41 | 50.62 | 647.35 | 1142.50 |
| 11 | 0 (10:1) | 0 (40) | 0 (30) | 80.82 | 613.87 | 56.69 | 699.71 | 1205.63 |
| 12 | −1 (8:1) | 1 (50) | 0 (30) | 65.71 | 463.57 | 42.64 | 528.33 | 953.95 |
| 13 | −1 (8:1) | 0 (40) | 1 (40) | 70.13 | 504.98 | 48.51 | 585.49 | 1066.53 |
| 14 | 1 (12:1) | 0 (40) | 1 (40) | 68.98 | 490.85 | 45.46 | 543.04 | 964.98 |
| 15 | 1 (12:1) | 0 (40) | −1 (20) | 65.49 | 420.89 | 43.22 | 538.13 | 944.76 |
| 16 | 1 (12:1) | 1 (50) | 0 (30) | 65.91 | 409.79 | 41.04 | 490.77 | 895.90 |
| 17 | 1 (12:1) | −1 (30) | 0 (30) | 64.71 | 412.15 | 41.83 | 502.41 | 916.16 |
| Variables | C1 | C2 | C3 | C4 | C5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Square | F-Value | p-Value a | Mean Square | F-Value | p-Value a | Mean Square | F-Value | p-Value a | Mean Square | F-Value | p-Value a | Mean Square | F-Value | p-Value a | |
| Model | 56.34 | 37.07 | <0.0001 | 9358.95 | 76.79 | <0.0001 | 62.52 | 35.71 | <0.0001 | 10,744.64 | 106.55 | <0.0001 | 25,878.04 | 94.97 | <0.0001 |
| X1 | 28.88 | 19.00 | 0.0033 | 12,649.25 | 103.79 | <0.0001 | 60.89 | 34.78 | 0.0006 | 7973.95 | 79.08 | <0.0001 | 29,409.19 | 107.93 | <0.0001 |
| X2 | 12.50 | 8.22 | 0.0241 | 1310.46 | 10.75 | 0.0135 | 13.83 | 7.90 | 0.0261 | 2520.15 | 24.99 | 0.0016 | 7803.13 | 28.64 | 0.0011 |
| X3 | 1.50 | 0.98 | 0.3541 | 493.29 | 4.05 | 0.0841 | 6.64 | 3.79 | 0.0924 | 988.35 | 9.80 | 0.0166 | 1386.54 | 5.09 | 0.0387 |
| X1X2 | 10.99 | 7.23 | 0.0311 | 370.18 | 3.04 | 0.1249 | 8.76 | 5.00 | 0.0603 | 200.93 | 1.99 | 0.2009 | 1974.02 | 7.24 | 0.0310 |
| X1X3 | 11.12 | 7.32 | 0.0304 | 5170.33 | 42.42 | 0.0003 | 11.73 | 6.70 | 0.0360 | 1030.41 | 10.22 | 0.0151 | 1481.87 | 5.44 | 0.0325 |
| X2X3 | 12.36 | 8.13 | 0.0246 | 1174.78 | 9.64 | 0.0172 | 19.80 | 11.31 | 0.0120 | 489.74 | 4.86 | 0.0634 | 21,329.14 | 78.27 | <0.0001 |
| X12 | 148.33 | 97.59 | <0.0001 | 28,972.43 | 237.72 | <0.0001 | 146.36 | 83.60 | <0.0001 | 47,913.24 | 475.15 | <0.0001 | 67,320.92 | 247.06 | <0.0001 |
| X22 | 176.00 | 115.79 | <0.0001 | 25,680.27 | 210.70 | <0.0001 | 210.06 | 119.99 | <0.0001 | 26,594.34 | 263.73 | <0.0001 | 70,253.80 | 257.82 | <0.0001 |
| X32 | 62.58 | 41.17 | 0.0004 | 2999.17 | 24.61 | 0.0016 | 43.14 | 24.64 | 0.0016 | 2439.79 | 24.19 | 0.0017 | 15,950.65 | 58.54 | 0.0001 |
| Lack of fit | 1.1100 | 0.6000 | 0.6464 | 165.1100 | 1.8500 | 0.2793 | 1.7200 | 0.9700 | 0.4900 | 165.6600 | 3.1700 | 0.1470 | 281.7500 | 1.0600 | 0.4586 |
| R2 | 0.9794 | 0.9900 | 0.9787 | 0.9928 | 0.9919 | ||||||||||
| Adj R2 | 0.9530 | 0.9771 | 0.9513 | 0.9834 | 0.9814 | ||||||||||
| Solvent | Extraction Yields (μg/g) | Total (μg/g) | ||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | ||
| Petroleum ether | 60.93 ± 0.70 a | 420.73 ± 0.45 a | 48.75 ± 1.43 a | 593.99 ± 3.36 a | 776.32 ± 12.46 a | 1900.72 ± 9.64 a |
| DES-7 | 79.27 ± 1.21 b | 608.53 ± 2.47 b | 56.67 ± 1.54 b | 708.61 ± 4.85 b | 1212.89 ± 15.96 b | 2665.97 ± 11.26 b |
| Increment (%) | 30.1 | 44.6 | 16.2 | 19.3 | 56.2 | 40.3 |
| Macroporous Resins | Yields (%) | ||||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | |
| AB-8 | 82.75 | 79.26 | 75.40 | 85.32 | 87.88 |
| DM-130 | 86.12 | 80.74 | 78.87 | 65.08 | 81.23 |
| D-101 | 78.38 | 81.86 | 72.41 | 76.33 | 86.04 |
| NKA | – a | – | – | 9.72 | 6.58 |
| HPD-100 | 90.31 | 87.69 | 85.62 | 90.48 | 92.25 |
| HP-20 | – | – | – | – | 69.41 |
| D4020 | 77.56 | – | 79.68 | – | – |
| DM-301 | 78.46 | 81.26 | 80.52 | 85.71 | 87.36 |
| Group | Cell Viability (%) | Compared Group | p | Compared Group | p |
|---|---|---|---|---|---|
| Normal | 100 ± 0.10 | - | - | - | - |
| Model | 52.14 ± 1.44 | Normal | ** | - | - |
| Nimodipine | 87.98 ± 2.68 | Model | *** | - | - |
| DES–UAE (5 μg/mL) | 76.51 ± 2.10 | Model | *** | - | - |
| PE-UAE (5 μg/mL) | 68.38 ± 3.04 | Model | ** | DES–UAE (5 μg/mL) | * |
| DES-Heating (5 μg/mL) | 58.16 ± 1.66 | Model | ** | DES–UAE (5 μg/mL) | *** |
| DES-Heating + Stirring (5 μg/mL) | 62.16 ± 1.32 | Model | *** | DES–UAE (5 μg/mL) | ** |
| DES–UAE (10 μg/mL) | 82.84 ± 1.68 | Model | **** | - | - |
| PE-UAE (10 μg/mL) | 72.66 ± 1.87 | Model | *** | DES–UAE (10 μg/mL) | ** |
| DES-Heating (10 μg/mL) | 62.44 ± 1.72 | Model | ** | DES–UAE (10 μg/mL) | *** |
| DES-Heating + Stirring (10 μg/mL) | 68.75 ± 2.04 | Model | *** | DES–UAE (10 μg/mL) | *** |
| DES–UAE (20 μg/mL) | 90.67 ± 2.16 | Model | **** | - | - |
| PE-UAE (20 μg/mL) | 86.12 ± 1.96 | Model | **** | DES–UAE (20 μg/mL) | ns |
| DES-Heating (20 μg/mL) | 65.72 ± 1.88 | Model | *** | DES–UAE (20 μg/mL) | *** |
| DES-Heating + Stirring (20 μg/mL) | 74.89 ± 2.26 | Model | *** | DES–UAE (20 μg/mL) | *** |
| Group | NO Inhibition (%) | Compared Group | p | Compared Group | p |
|---|---|---|---|---|---|
| L-Name | 76.35 ± 0.86 | - | - | - | - |
| DES–UAE (5 μg/mL) | 50.46 ± 0.78 | L-Name | **** | - | - |
| PE-UAE (5 μg/mL) | 38.16 ± 1.06 | L-Name | **** | DES–UAE (5 μg/mL) | *** |
| DES-Heating (5 μg/mL) | 18.65 ± 1.62 | L-Name | **** | DES–UAE (5 μg/mL) | *** |
| DES-Heating + Stirring (5 μg/mL) | 28.66 ± 1.25 | L-Name | **** | DES–UAE (5 μg/mL) | **** |
| DES–UAE (10 μg/mL) | 52.68 ± 1.39 | L-Name | *** | - | - |
| PE-UAE (10 μg/mL) | 41.55 ± 1.46 | L-Name | *** | DES–UAE (10 μg/mL) | *** |
| DES-Heating (10 μg/mL) | 19.86 ± 1.53 | L-Name | **** | DES–UAE (10 μg/mL) | **** |
| DES-Heating + Stirring (10 μg/mL) | 30.58 ± 0.96 | L-Name | **** | DES–UAE (10 μg/mL) | **** |
| DES–UAE (20 μg/mL) | 55.73 ± 1.66 | L-Name | ** | - | - |
| PE-UAE (20 μg/mL) | 43.81 ± 1.96 | L-Name | *** | DES–UAE (20 μg/mL) | ** |
| DES-Heating (20 μg/mL) | 21.33 ± 1.71 | L-Name | **** | DES–UAE (20 μg/mL) | **** |
| DES-Heating + Stirring (20 μg/mL) | 32.06 ± 1.64 | L-Name | *** | DES–UAE (20 μg/mL) | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, Z.; Men, L.; Li, J.; Gong, X. Deep Eutectic Solvent-Based Ultrasound-Assisted Strategy for Simultaneous Extraction of Five Macamides from Lepidium meyenii Walp and In Vitro Bioactivities. Foods 2023, 12, 248. https://doi.org/10.3390/foods12020248
Li K, Li Z, Men L, Li J, Gong X. Deep Eutectic Solvent-Based Ultrasound-Assisted Strategy for Simultaneous Extraction of Five Macamides from Lepidium meyenii Walp and In Vitro Bioactivities. Foods. 2023; 12(2):248. https://doi.org/10.3390/foods12020248
Chicago/Turabian StyleLi, Keke, Zhongyu Li, Lei Men, Jiwen Li, and Xiaojie Gong. 2023. "Deep Eutectic Solvent-Based Ultrasound-Assisted Strategy for Simultaneous Extraction of Five Macamides from Lepidium meyenii Walp and In Vitro Bioactivities" Foods 12, no. 2: 248. https://doi.org/10.3390/foods12020248
APA StyleLi, K., Li, Z., Men, L., Li, J., & Gong, X. (2023). Deep Eutectic Solvent-Based Ultrasound-Assisted Strategy for Simultaneous Extraction of Five Macamides from Lepidium meyenii Walp and In Vitro Bioactivities. Foods, 12(2), 248. https://doi.org/10.3390/foods12020248