Compositions and Antioxidant Activity of Tea Polysaccharides Extracted from Different Tea (Camellia sinensis L.) Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Tea Samples
2.3. TPS Extraction
2.4. Neutral Sugar Content Determination
2.5. Uronic Acid Content Determination
2.6. Protein Content Determination
2.7. Tea Polyphenols Content Determination
2.8. Determination of O2-· Scavenging Ability
2.9. Determination of ·OH Scavenging Ability
2.10. Statistical Analysis
3. Results
3.1. TPS Extraction Efficiency of Different Tea Varieties
3.2. Composition Contents of TPS
3.3. Radical Scavenging Abilities of TPS
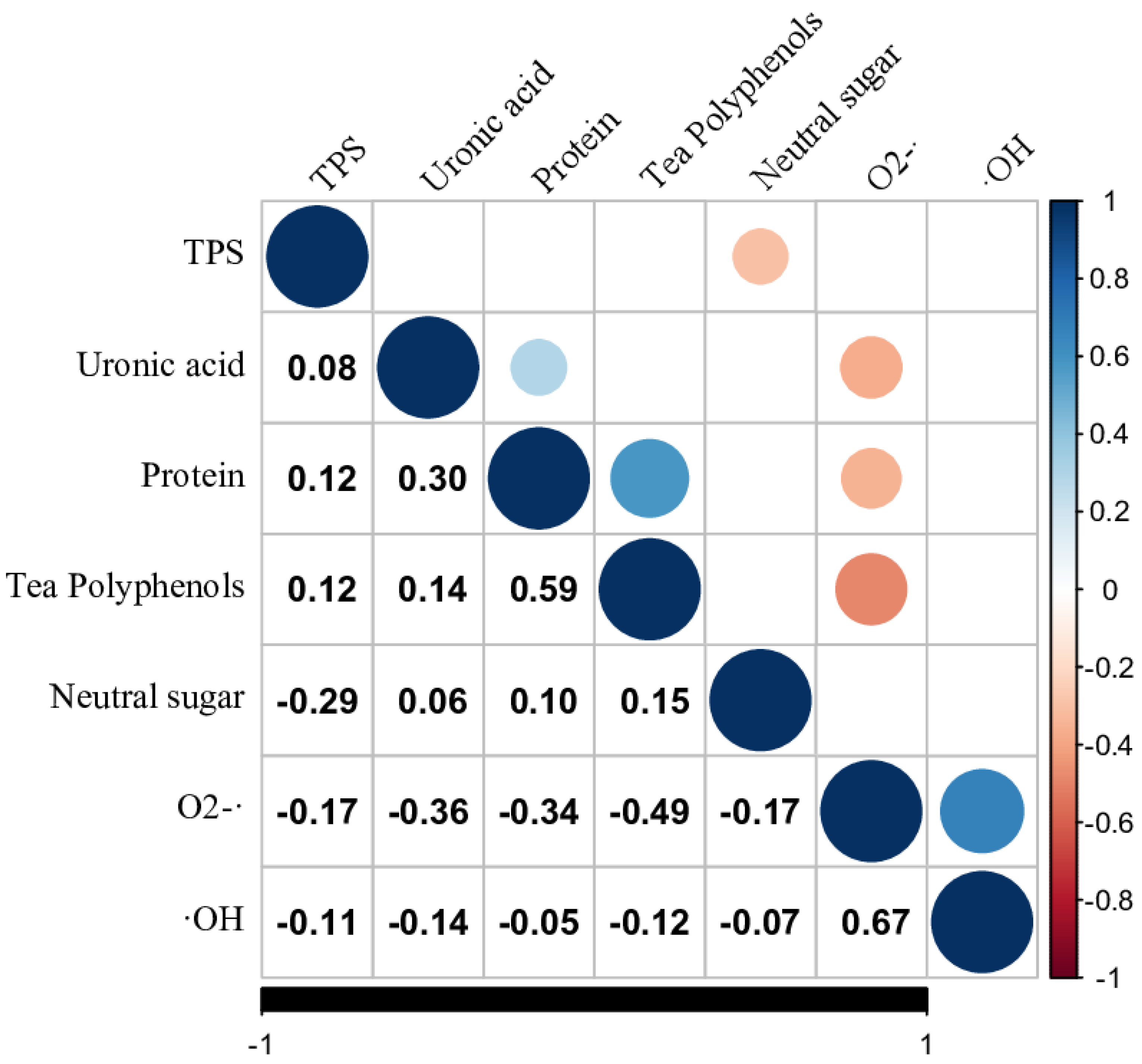
3.4. Correlation Coefficient Analysis
3.5. Analysis of TPSs extracted from Tea Shoots Collected in Different Areas
3.6. Selecting TPSs with High Antioxidant Activity
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, C.; Li, D.; Ma, Y.; Zhang, W.; Lin, C.; Zheng, X.; Liang, Y.; Lu, J. Formation mechanism of the oolong tea characteristic aroma during bruising and withering treatment. Food Chem. 2018, 269, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, J.; Gu, S. Rapid identification of tea quality by E-nose and computer vision combining with a synergetic data fusion strategy. J. Food Eng. 2019, 241, 10–17. [Google Scholar] [CrossRef]
- Liu, S.; Ai, Z.; Meng, Y.; Chen, Y.; Ni, D. Comparative studies on the physicochemical profile and potential hypoglycemic activity of different tea extracts: Effect on sucrase-isomaltase activity and glucose transport in Caco-2 cells. Food Res. Int. 2021, 148, 110604. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Long, L.; Shen, J.; Lin, K.; Yin, L.; Yi, J.; Zhang, L.; Deng, D.Y.B.; Yang, L. Nanoparticles composed of the tea polysaccharide-complexed cationic vitamin B12-conjugated glycogen derivative. Food Funct. 2021, 12, 8522–8534. [Google Scholar] [CrossRef]
- Fan, M.; Qian, Y.; Yue, W.; Yang, Y.; Zhang, X.; Ma, S.; Xu, Y.; Wang, D. Preparation and characterization of metal-tea polysaccharide complexes and their inhibition on alpha-glucosidase. J. Food Biochem. 2021, 45, e13689. [Google Scholar] [CrossRef]
- Fu, Q.; Li, Q.; Lin, X.; Qiao, R.; Yang, R.; Li, X.-M.; Dong, Z.; Xiang, L.; Zheng, X.; Lu, J.; et al. Antidiabetic effects of tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Zhu, C.; Liu, G.; Sun, Y.; Qian, L. Advances in the utilization of tea polysaccharides: Preparation, physicochemical properties, and health benefits. Polymers 2022, 14, 2775. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, X.; Mao, X.; Chen, D.; Yu, B.; Wang, J.; Yan, H. Tea bioactive components prevent carcinogenesis via anti-pathogen, anti-inflammation, and cell survival pathways. IUBMB Life 2021, 73, 328–340. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q.; Saeeduddin, M.; Ou, S.; Zeng, X.; Ye, H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym. 2016, 153, 663–678. [Google Scholar] [CrossRef]
- Yao, J.; Liu, H.; Ma, C.; Pu, L.; Yang, W.; Lei, Z. A review on the extraction, bioactivity, and application of tea polysaccharides. Molecules 2022, 27, 4679. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Xie, B. Components and antioxidant activity of polysaccharide conjugate from green tea. Food Chem. 2005, 90, 17–21. [Google Scholar] [CrossRef]
- Chen, X.; Ye, Y.; Cheng, H.; Jiang, Y.; Wu, Y. Thermal effects on the stability and antioxidant activity of an acid polysaccharide conjugate derived from green tea. J. Agric. Food. Chem. 2009, 57, 5795–5798. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Liu, Y.; Chen, X.; Wei, X. Extraction, characterization and antioxidant activities of Se-enriched tea polysaccharides. Int. J. Biol. Macromol. 2015, 77, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, C469–C474. [Google Scholar] [CrossRef]
- Yang, W.; Ren, D.; Zhao, Y.; Liu, L.; Yang, X. Fuzhuan brick tea polysaccharide improved ulcerative colitis in association with gut microbiota-derived tryptophan metabolism. J. Agric. Food. Chem. 2021, 69, 8448–8459. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Ouyang, K.; Wang, W. Effects of polysaccharides from Yingshan Yunwu tea on free amino acids, flavor nucleotides and antioxidant abilities in chickens. Res. Vet. Sci. 2022, 149, 11–20. [Google Scholar] [CrossRef]
- Liu, J.; Lin, J.; Huang, Z.; Zheng, Q.; Lin, F.; Wu, L. Chemical characterization of Tianshan green tea polysaccharides and its protective effects on cell oxidative injury. J. Food Biochem. 2022, 46, e14000. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Du, L.; Fu, Q.; Xiang, L.; Zheng, X.; Lu, J.; Ye, J.; Li, Q.; Polito, C.A.; Liang, Y. Tea Polysaccharides and Their Bioactivities. Molecules 2016, 21, 1449. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tsubaki, S.; Sakamoto, M.; Watanabe, S.; Azuma, J.-I. Growth-dependent chemical and mechanical properties of cuticular membranes from leaves of Sonneratia alba. Plant Cell Environ. 2012, 35, 1201–1210. [Google Scholar] [CrossRef]
- Tsubaki, S.; Sakumoto, S.; Uemura, N.; Azuma, J.-I. Compositional analysis of leaf cuticular membranes isolated from tea plants (Camellia sinensis L.). Food Chem. 2013, 138, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Polyphenol and flavonoid profiles and radical scavenging activity in leafy vegetable Amaranthus gangeticus. BMC Plant Biol. 2020, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; He, J.; Jia, L.; Tu, Y. Optimizing conditions for the extraction of polysaccharides of white tea. Biotechnol. Biotechnol. Equip. 2015, 29, 921–925. [Google Scholar] [CrossRef]
- Li, S.; Cao, X. Extraction of tea polysaccharides (TPS) using anionic reverse micellar system. Sep. Purif. Technol. 2014, 122, 306–314. [Google Scholar] [CrossRef]
- Xia, B.; Liu, Q.; Sun, D.; Wang, Y.; Wang, W.; Liu, D. Ultrasound-assisted deep eutectic solvent extraction of polysaccharides from Anji White tea: Characterization and comparison with the Conventional Method. Foods. 2023, 12, 588. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrates with dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Duan, X.; Hu, X.; Chen, F.; Deng, Z. Bioactive ingredient levels of tea leaves are associated with leaf Al level interactively influenced by acid rain intensity and soil Al supply. J. Food Agric. Environ. 2012, 10, 1197–1204. [Google Scholar]
- Chen, X.; Fang, Y.; Nishinari, K.; We, H.; Sun, C.; Li, J.; Jiang, Y. Physicochemical characteristics of polysaccharide conjugates prepared from fresh tea leaves and their improving impaired glucose tolerance. Carbohydr. Polym. 2014, 112, 77–84. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wu, J.; Xu, P.; Wang, Y.; Gao, J.; Hochstetter, D. In vitro antioxidant activity and potential inhibitory action against α-glucosidase of polysaccharides from fruit peel of tea (Camellia sinensis L.). J. Zhejiang Univ. Sci. B 2014, 15, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Yu, Q.; Shi, J.; Xiong, C.; Ling, Z.; He, P. Structural characterization and antioxidant activities of 2 water-soluble polysaccharide fractions purified from tea (Camellia sinensis) flower. J. Food Sci. 2011, 76, C462–C471. [Google Scholar]
- Guo, H.; Fu, M.; Wu, D.; Zhao, Y.; Li, H.; Li, H.; Gan, R. Structural characteristics of crude polysaccharides from 12 Selected Chinese teas, and their antioxidant and anti-diabetic activities. Antioxidants 2021, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Sun, H.; Chen, Y.; Guo, H.; Liu, Y.; Li, Y.; Lu, C.; Wang, X. Antioxidant and hypoglycemic activity of tea polysaccharides with different degrees of fermentation. Int. J. Biol. Macromol. 2023, 228, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Compositional analysis and preliminary toxicological evaluation of a tea polysaccharide conjugate. J. Agric. Food Chem. 2007, 55, 2256–2260. [Google Scholar] [CrossRef]
- Fan, M.; Zhu, J.; Qian, Y.; Yue, W.; Xu, Y.; Zhang, D.; Yang, Y.; Gao, X.; He, H.; Wang, D. Effect of purity of tea polysaccharides on its antioxidant and hypoglycemic activities. J. Food Biochem. 2020, 44, e13277. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, T.; Shi, J.; Xia, X.; Li, J.; Liu, L.; McClements, D.J.; Cao, Y.; Fu, Y.; Han, L.; et al. Physicochemical characterization, emulsifying and antioxidant properties of the polysaccharide conjugates from Chin brick tea (Camellia sinensis). Food Chem. 2022, 395, 133625. [Google Scholar] [CrossRef]
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chem. 2013, 136, 1405–1413. [Google Scholar] [CrossRef]
- Deka, H.; Sarmah, P.P.; Devi, A.; Tamuly, P.; Karak, T. Changes in major catechins, caffeine, and antioxidant activity during CTC processing of black tea from North East India. RSC Adv. 2021, 11, 11457–11467. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Li, J.; Zhao, G. Components and activity of polysaccharides from coarse tea. J. Agric. Food. Chem. 2001, 49, 507–510. [Google Scholar] [CrossRef]


| Composition | Content (%) | ||||
|---|---|---|---|---|---|
| Max | Min | Mean | SD | CV | |
| Neutral sugar | 49.13 | 23.82 | 39.54 | 5.64 | 14.26 |
| Uronic acid | 45.38 | 13.54 | 26.65 | 5.53 | 20.75 |
| Protein | 9.21 | 1.76 | 5.21 | 1.5 | 28.79 |
| Tea polyphenols | 16.12 | 2.45 | 6.70 | 2.05 | 30.60 |
| Analysis Items | SSM | SSE | F Value | p-Value |
|---|---|---|---|---|
| Neutral sugar content (%) | 2.0172 | 0.0774 | 52.51 | ** |
| Uronic acid content (%) | 1.6777 | 0.2734 | 12.36 | ** |
| Protein content (%) | 0.7545 | 0.4362 | 3.48 | ** |
| Polyphenol content (%) | 0.6330 | 0.0200 | 63.68 | ** |
| scavenging capacity of ·OH (%) | 0.4570 | 0.3060 | 3.01 | ** |
| scavenging capacity of O2-· (nU/mL) | 2.3528 | 0.2533 | 18.71 | ** |
| TPS | Composition Content (%) | Scavenging Ability | ||||||
|---|---|---|---|---|---|---|---|---|
| Neutral Sugar | Protein | Uronic Acid | Tea Polyphenols | Total | ·OH (%) | O2-· (nU/mL) | ||
| Varieties | FADB | 34.72 ± 5.69 a | 5.87 ± 0.81 a | 26.06 ± 0.89 b | 6.75 ± 0.96 a | 73.98 ± 5.63 ab | 65.2 ± 3.73 b | 15.41 + 2.86 bc |
| FDDH | 36.08 ± 4.09 a | 4.87 ± 1.22 a | 27.22 ± 2.38 ab | 6.03 ± 0.92 abc | 74.82 ± 4.18 ab | 62.77 ± 0.89 b | 11.37 + 0.66 c | |
| FY6 | 33.00 ± 2.54 a | 4.45 ± 0.40 a | 24.43 ± 2.17 b | 6.59 ± 1.02 c | 67.34 ± 4.31 b | 67.38 ± 4.33 b | 16.06 + 2.13 bc | |
| MZ | 37.42 ± 4.94 a | 5.34 ± 1.13 a | 23.30 ± 4.79 b | 7.32 ± 0.48 ab | 73.13 ± 8.85 ab | 63.66 ± 2.45 b | 18.35 + 3.35 ab | |
| WNZ | 37.52 ± 3.68 a | 4.58 ± 1.61 a | 34.35 ± 6.71 a | 6.64 ± 0.54 bc | 82.29 ± 11.17 a | 66.69 ± 2.93 b | 15.18 + 2.83 bc | |
| Ysh | 36.11 ± 2.21 a | 4.85 ± 0.66 a | 26.29 ± 5.57 ab | 5.46 ± 0.65 abc | 73.64 ± 4.61 ab | 77.95 ± 3.69 a | 22.44 + 5.82 a | |
| Origins | Hubei | 4.18 ± 4.18 a | 4.84 ± 1.58 a | 26.63 ± 2.90 a | 7.07 ± 0.81 a | 73.61 ± 3.94 a | 66.10 ± 4.71 a | 17.37 + 3.83 a |
| Jiangxi | 3.77 ± 3.77 a | 4.88 ± 0.51 a | 26.00 ± 8.06 a | 5.85 ± 0.85 a | 72.25 ± 10.7 a | 66.33 ± 6.44 a | 16.46 + 6.44 a | |
| Yunnan | 3.89 ± 3.89 a | 5.26 ± 0.78 a | 28.2 ± 3.59 a | 6.39 ± 1.33 a | 76.74 ± 6.53 a | 69.40 ± 6.63 a | 15.57 + 3.03 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, K.; Shi, Y.; Liu, S.; Chen, Y.; Ni, D.; Yu, Z. Compositions and Antioxidant Activity of Tea Polysaccharides Extracted from Different Tea (Camellia sinensis L.) Varieties. Foods 2023, 12, 3584. https://doi.org/10.3390/foods12193584
Xiao K, Shi Y, Liu S, Chen Y, Ni D, Yu Z. Compositions and Antioxidant Activity of Tea Polysaccharides Extracted from Different Tea (Camellia sinensis L.) Varieties. Foods. 2023; 12(19):3584. https://doi.org/10.3390/foods12193584
Chicago/Turabian StyleXiao, Kunyue, Yutao Shi, Sisi Liu, Yuqiong Chen, Dejiang Ni, and Zhi Yu. 2023. "Compositions and Antioxidant Activity of Tea Polysaccharides Extracted from Different Tea (Camellia sinensis L.) Varieties" Foods 12, no. 19: 3584. https://doi.org/10.3390/foods12193584
APA StyleXiao, K., Shi, Y., Liu, S., Chen, Y., Ni, D., & Yu, Z. (2023). Compositions and Antioxidant Activity of Tea Polysaccharides Extracted from Different Tea (Camellia sinensis L.) Varieties. Foods, 12(19), 3584. https://doi.org/10.3390/foods12193584






