Complement in Human Brain Health: Potential of Dietary Food in Relation to Neurodegenerative Diseases
Abstract
1. Introduction
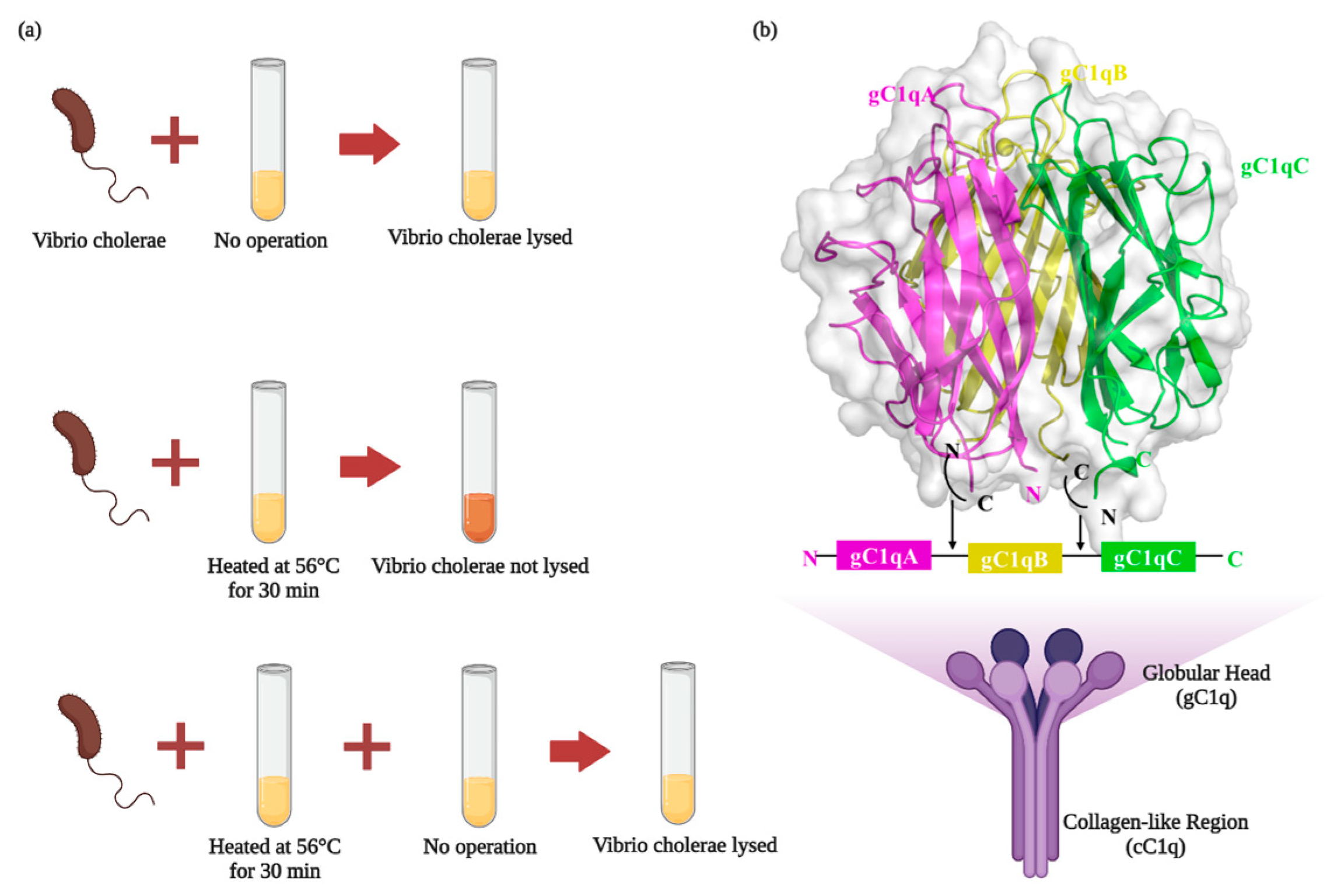
2. C1q Structure and Biological Function
2.1. C1q Structure
| C1q Ligand | C1q Binding Region/Binding Site | Function | Ref. |
|---|---|---|---|
| IgM | C1q globular domain | Activate the classical complement pathway | [24,25] |
| IgG | C1q globular domain | Activate the classical complement pathway | [16] |
| LPS | C1q globular domain | Activate the classical complement pathway | [26] |
| GAPDH | C1q globular domain | Activate the classical complement pathway | [27] |
| Blood platelets | C1q globular domain | Activate the classical complement pathway | [28] |
| CRP | C1q globular domain | Activate the classical complement pathway | [5,19,20] |
| Pentraxin 3 | C1q globular domain | Interacts with C1q and inhibits the classical complement pathway | [41] |
| Fibronectin | C1q globular domain | Activate the classical complement pathway | [29] |
| Calreticulin | C1q globular domain | recognize apoptotic cells | [42,43] |
| Heparin | C1q globular domain | Inhibit the classical complement pathway | [44] |
| ApoE | C1q stalk | Inhibit the classical complement pathway | [45] |
| Adiponectin | Globular domain of the C1q A chain | Activate the classical complement pathway | [17] |
| Von Willebrand factor | N-terminal of the C1q A chain | Inhibit the classical complement pathway | [18] |
| Serum amyloid P | Residues 14–26 and 76–92 of the C1q A chain | Activate the classical complement pathway | [21] |
| DNA | Residues 14–26 of the C1q A chain | Activate the classical complement pathway | [22] |
| Aβ | Residues 14–26 of the C1q A chain | Activate the classical complement pathway | [22] |
| Heme | TyrA122 of the C1q A chain | Inhibit the classical complement pathway | [23] |
| Deoxy-D-ribose | Residues Arg98, Arg111, Asn113 of the C1q C chain | Inhibit C1 activation | [44] |
| Heparan sulfate | Residues Lys129, Tyr155, Trp190 of the C1q C chain | Inhibit C1 activation | [44] |
| PS | Globular domain of the C1q C chain | Efficient apoptotic cell removal determined synaptic vulnerability | [15,46,47] |
2.2. C1q Biological Functions
3. Complement in the Brain
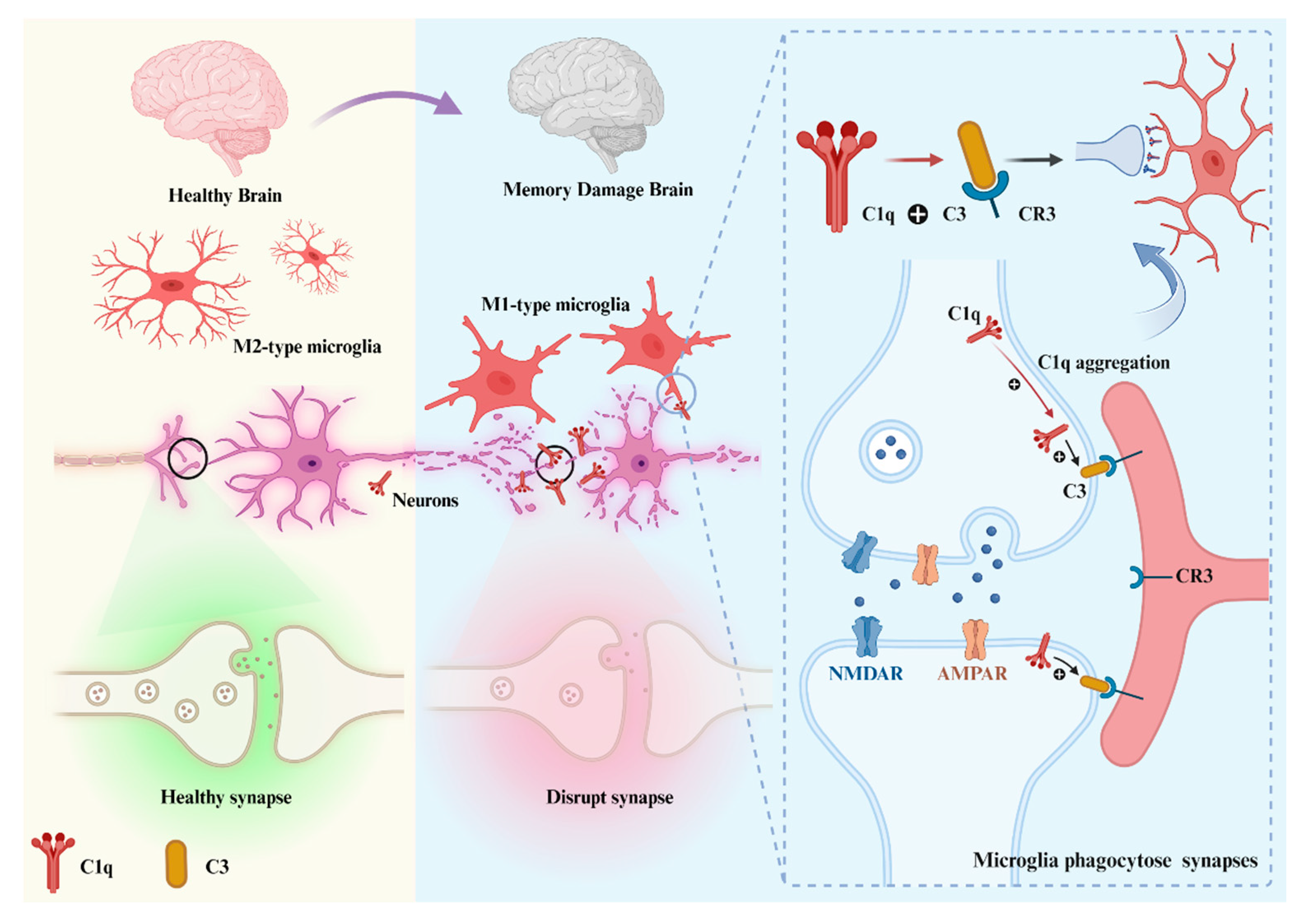
3.1. C1q and the Complement Pathway
3.2. C1q through Classical Pathway Activation Mediated Synapse Pruning
3.2.1. C1q in Developing CNS
3.2.2. C1q in Aging
3.2.3. C1q in Diseases
3.3. The Role of C1q in the Complement-Independent Manner
4. Local Synthesis of C1q in the Brain
4.1. C1q and Microglia
4.2. C1q and Astrocytes
4.3. C1q and Neurons
5. C1q in Neurodegenerative Diseases
5.1. Alzheimer’s Disease
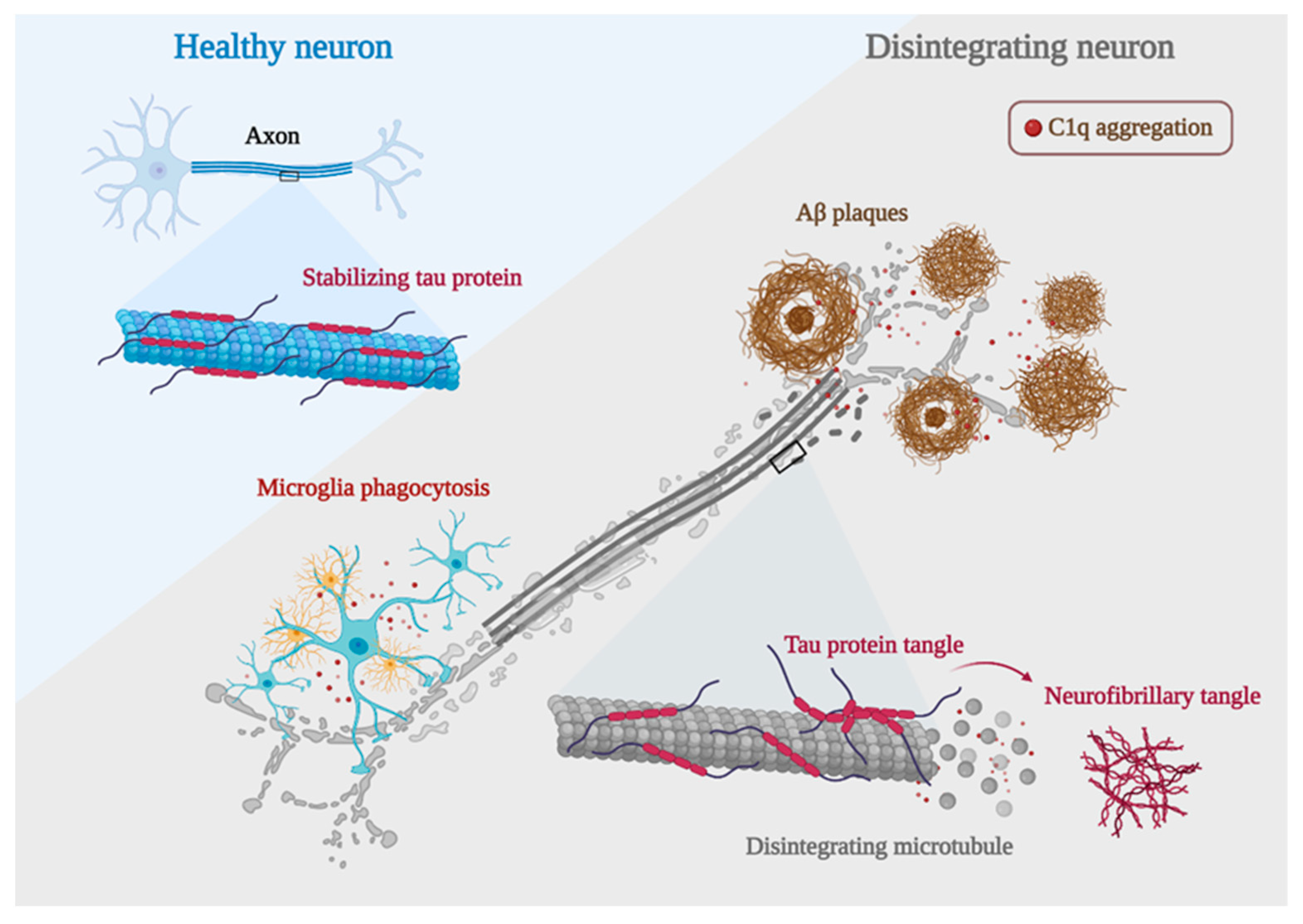
5.1.1. C1q and Aβ in AD
5.1.2. C1q and Tau in AD
5.2. Parkinson Disease
5.3. Huntington’s Disease
5.4. Traumatic Brain Injury
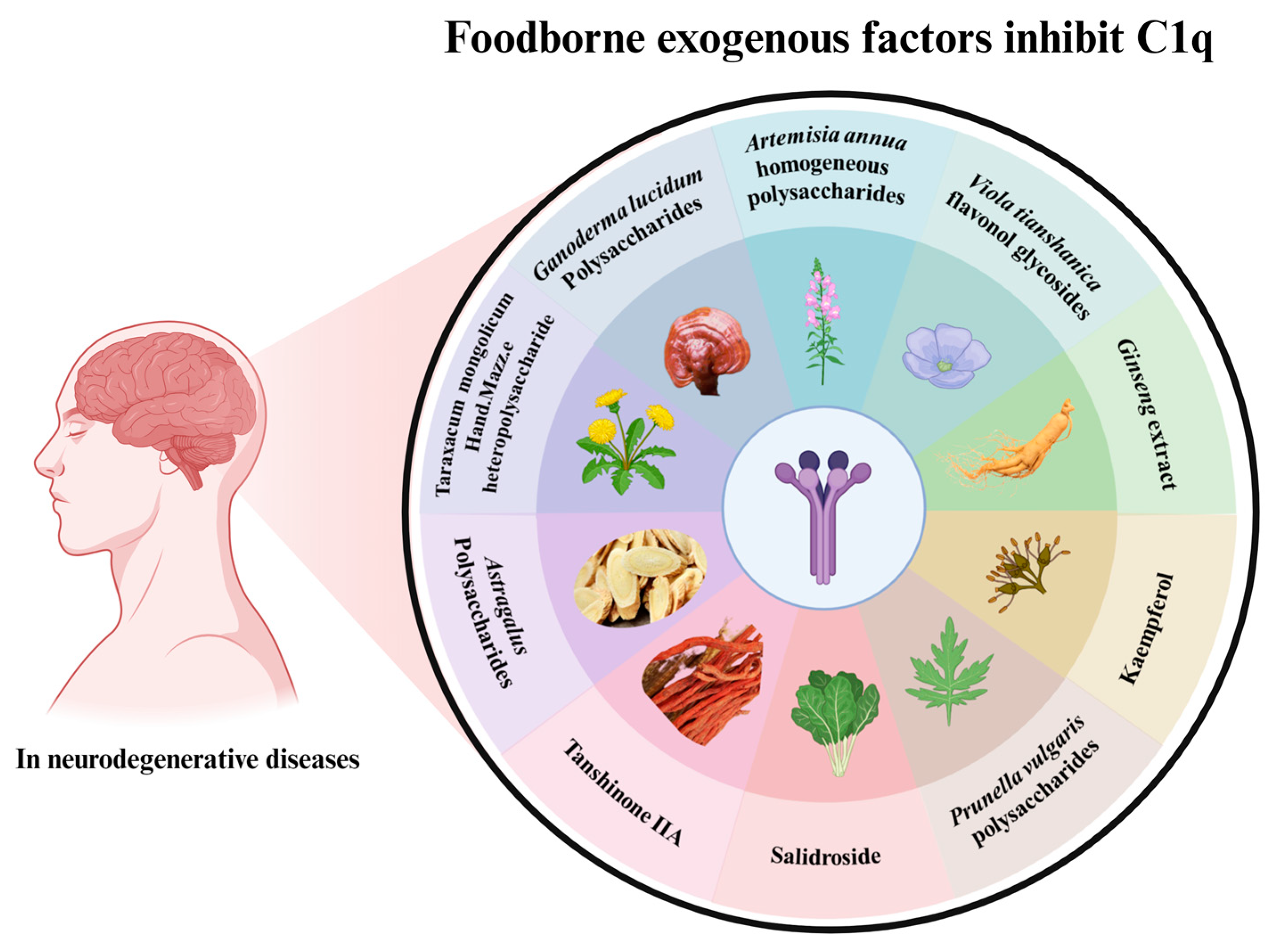
6. Efficacy of Dietary Food Related to C1q for Memory Improvement
7. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Zhou, J.; Botto, M.; Tenner, A.J. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 2004, 24, 6457–6465. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Pisalyaput, K.; Tenner, A.J. Complement component C1q inhibits β-amyloid- and serum amyloid P-induced neurotoxicity via caspase- and calpain-independent mechanisms. J. Neurochem. 2008, 104, 696–707. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich Açai (Euterpe oleracea Mart.) Fruit Pulp Fractions Attenuate Inflammatory Stress Signaling in Mouse Brain BV-2 Microglial Cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Tenner, A.J.; Stevens, B.; Woodruff, T.M. New tricks for an ancient system: Physiological and pathological roles of complement in the CNS. Mol. Immunol. 2018, 102, 3–13. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Depboylu, C.; Schäfer, M.K.H.; Arias-Carrión, O.; Oertel, W.H.; Weihe, E.; Höglinger, G.U. Possible Involvement of Complement Factor C1q in the Clearance of Extracellular Neuromelanin from the Substantia Nigra in Parkinson Disease. J. Neuropathol. Exp. Neurol. 2011, 70, 125–132. [Google Scholar] [CrossRef]
- Ye, J.; Yang, P.; Yang, Y.; Xia, S. Complement C1s as a diagnostic marker and therapeutic target: Progress and propective. Front. Immunol. 2022, 13, 1015128. [Google Scholar] [CrossRef]
- Kishore, U.; Reid, K.B. Modular organization of proteins containing C1q-like globular domain. Immunopharmacology 1999, 42, 15–21. [Google Scholar] [CrossRef]
- Kishore, U.; Ghai, R.; Greenhough, T.J.; Shrive, A.K.; Bonifati, D.M.; Gadjeva, M.G.; Waters, P.; Kojouharova, M.S.; Chakraborty, T.; Agrawal, A. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol. Lett. 2004, 95, 113–128. [Google Scholar] [CrossRef]
- Nayak, A.; Ferluga, J.; Tsolaki, A.G.; Kishore, U. The non-classical functions of the classical complement pathway recognition subcomponent C1q. Immunol. Lett. 2010, 131, 139–150. [Google Scholar] [CrossRef]
- Gaboriaud, C.; Teillet, F.; Gregory, L.A.; Thielens, N.M.; Arlaud, G.J. Assembly of C1 and the MBL- and ficolin-MASP complexes: Structural insights. Immunobiology 2007, 212, 279–288. [Google Scholar] [CrossRef]
- Kishore, U.; Gupta, S.K.; Perdikoulis, M.V.; Kojouharova, M.S.; Urban, B.C.; Reid, K.B. Modular organization of the carboxyl-terminal, globular head region of human C1q A, B, and C chains. J. Immunol. 2003, 171, 812–820. [Google Scholar] [CrossRef]
- Paidassi, H.; Tacnet-Delorme, P.; Garlatti, V.; Darnault, C.; Ghebrehiwet, B.; Gaboriaud, C.; Arlaud, G.J.; Frachet, P. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J. Immunol. 2008, 180, 2329–2338. [Google Scholar] [CrossRef]
- Gaboriaud, C.; Juanhuix, J.; Gruez, A.; Lacroix, M.; Darnault, C.; Pignol, D.; Verger, D.; Fontecilla-Camps, J.C.; Arlaud, G.J. The Crystal Structure of the Globular Head of Complement Protein C1q Provides a Basis for Its Versatile Recognition Properties. J. Biol. Chem. 2003, 278, 46974–46982. [Google Scholar] [CrossRef]
- Peake, P.W.; Shen, Y.; Walther, A.; Charlesworth, J.A. Adiponectin binds C1q and activates the classical pathway of complement. Biochem. Biophys. Res. Commun. 2008, 367, 560–565. [Google Scholar] [CrossRef]
- Kölm, R.; Schaller, M.; Roumenina, L.T.; Niemiec, I.; Kremer Hovinga, J.A.; Khanicheh, E.; Kaufmann, B.A.; Hopfer, H.; Trendelenburg, M. Von Willebrand Factor Interacts with Surface-Bound C1q and Induces Platelet Rolling. J. Immunol. 2016, 197, 3669–3679. [Google Scholar] [CrossRef]
- Jiang, H.; Robey, F.A.; Gewurz, H. Localization of sites through which C-reactive protein binds and activates complement to residues 14–26 and 76–92 of the human C1q A chain. J. Exp. Med. 1992, 175, 1373–1379. [Google Scholar] [CrossRef]
- McGrath, F.D.G.; Brouwer, M.C.; Arlaud, G.R.J.; Daha, M.R.; Hack, C.E.; Roos, A. Evidence That Complement Protein C1q Interacts with C-Reactive Protein through Its Globular Head Region. J. Immunol. 2006, 176, 2950–2957. [Google Scholar] [CrossRef]
- Ying, S.C.; Gewurz, A.T.; Jiang, H.; Gewurz, H. Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14–26 and 76–92 of the A chain collagen-like region of C1q. J. Immunol. 1993, 150, 169–176. [Google Scholar] [CrossRef]
- Jiang, H.; Cooper, B.; Robey, F.A.; Gewurz, H. DNA binds and activates complement via residues 14-26 of the human C1q A chain. J. Biol. Chem. 1992, 267, 25597–25601. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Radanova, M.; Atanasov, B.P.; Popov, K.T.; Kaveri, S.V.; Lacroix-Desmazes, S.; Frémeaux-Bacchi, V.; Dimitrov, J.D. Heme Interacts with C1q and Inhibits the Classical Complement Pathway. J. Biol. Chem. 2011, 286, 16459–16469. [Google Scholar] [CrossRef]
- Beurskens, F.J.; van Schaarenburg, R.A.; Trouw, L.A. C1q, antibodies and anti-C1q autoantibodies. Mol. Immunol. 2015, 68, 6–13. [Google Scholar] [CrossRef]
- Duncan, A.R.; Winter, G. The binding site for C1q on IgG. Nature 1988, 332, 738–740. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Popov, K.T.; Bureeva, S.V.; Kojouharova, M.; Gadjeva, M.; Rabheru, S.; Thakrar, R.; Kaplun, A.; Kishore, U. Interaction of the globular domain of human C1q with Salmonella typhimurium lipopolysaccharide. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 1271–1276. [Google Scholar] [CrossRef]
- Terrasse, R.; Tacnet-Delorme, P.; Moriscot, C.; Pérard, J.; Schoehn, G.; Vernet, T.; Thielens, N.M.; Di Guilmi, A.M.; Frachet, P. Human and Pneumococcal Cell Surface Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH) Proteins Are Both Ligands of Human C1q Protein. J. Biol. Chem. 2012, 287, 42620–42633. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Yin, W.; Grigg, S.E.; Ghebrehiwet, B. Blood platelets activate the classical pathway of human complement. J. Thromb. Haemost. 2006, 4, 2035–2042. [Google Scholar] [CrossRef]
- Isliker, H.; Bing, D.H.; Lahan, J.; Hynes, R.O. Fibronectin interacts with Clq, a subcomponent of the first component of complement. Immunol. Lett. 1982, 4, 39–43. [Google Scholar] [CrossRef]
- Takahashi, K.; Rochford, C.D.P.; Neumann, H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005, 201, 647–657. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, Y.; Wu, M.; Lyu, X.; Wang, H.; Chen, Y.; Wang, H.; Teng, L. Identification of TYROBP and C1QB as Two Novel Key Genes with Prognostic Value in Gastric Cancer by Network Analysis. Front. Oncol. 2020, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Audrain, M.; Haure-Mirande, J.V.; Mleczko, J.; Wang, M.; Griffin, J.K.; St George-Hyslop, P.H.; Fraser, P.; Zhang, B.; Gandy, S.; Ehrlich, M.E. Reactive or transgenic increase in microglial TYROBP reveals a TREM2-independent TYROBP–APOE link in wild-type and Alzheimer’s-related mice. Alzheimer’s Dement. 2020, 17, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Linnartz-Gerlach, B.; Bodea, L.G.; Klaus, C.; Ginolhac, A.; Halder, R.; Sinkkonen, L.; Walter, J.; Colonna, M.; Neumann, H. TREM2 triggers microglial density and age-related neuronal loss. Glia 2018, 67, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Waters, P.; Roumenina, L.T.; Gadjeva, M.; Kojouharova, M.S.; Reid, K.B.; Sim, R.B.; Kishore, U. C1q and its growing family. Immunobiology 2007, 212, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Kojouharova, M.S.; Gadjeva, M.G.; Tsacheva, I.G.; Zlatarova, A.; Roumenina, L.T.; Tchorbadjieva, M.I.; Atanasov, B.P.; Waters, P.; Urban, B.C.; Sim, R.B.; et al. Mutational Analyses of the Recombinant Globular Regions of Human C1q A, B, and C Chains Suggest an Essential Role for Arginine and Histidine Residues in the C1q-IgG Interaction. J. Immunol. 2004, 172, 4351–4358. [Google Scholar] [CrossRef] [PubMed]
- Ghebrehiwet, B.; Kandov, E.; Kishore, U.; Peerschke, E.I.B. Is the A-Chain the Engine That Drives the Diversity of C1q Functions? Revisiting Its Unique Structure. Front. Immunol. 2018, 9, 162. [Google Scholar] [CrossRef]
- Siegelt, R.C.; Schumaker, V.N. Measurement of the association constants of the complexes formed between intact C1q or pepsin-treated C1q stalks and the unactivated or activated C1r2C1s2 tetramers. Mol. Immunol. 1983, 20, 53–66. [Google Scholar] [CrossRef]
- Eggleton, P.; Tenner, A.J.; Reid, K.B.M. C1q receptors. Clin. Exp. Immunol. 2000, 120, 406–412. [Google Scholar] [CrossRef]
- Gaboriaud, C.; Frachet, P.; Thielens, N.M.; Arlaud, G.J. The Human C1q Globular Domain: Structure and Recognition of Non-Immune Self Ligands. Front. Immunol. 2012, 2, 92. [Google Scholar] [CrossRef]
- Ugurlar, D.; Howes, S.C.; de Kreuk, B.-J.; Koning, R.I.; de Jong, R.N.; Beurskens, F.J.; Schuurman, J.; Koster, A.J.; Sharp, T.H.; Parren, P.W.H.I.; et al. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 2018, 359, 794–797. [Google Scholar] [CrossRef]
- Nauta, A.J.; Bottazzi, B.; Mantovani, A.; Salvatori, G.; Kishore, U.; Schwaeble, W.J.; Gingras, A.R.; Tzima, S.; Vivanco, F.; Egido, J.; et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 2003, 33, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ghiran, I. Expression and Function of C1q Receptors and C1q Binding Proteins at the Cell Surface. Immunobiology 2002, 205, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Verneret, M.; Tacnet-Delorme, P.; Osman, R.; Awad, R.; Grichine, A.; Kleman, J.-P.; Frachet, P. Relative Contribution of C1q and Apoptotic Cell-Surface Calreticulin to Macrophage Phagocytosis. J. Innate Immun. 2014, 6, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Garlatti, V.; Chouquet, A.; Lunardi, T.; Vivès, R.; Païdassi, H.; Lortat-Jacob, H.; Thielens, N.M.; Arlaud, G.J.; Gaboriaud, C. Cutting Edge: C1q Binds Deoxyribose and Heparan Sulfate through Neighboring Sites of Its Recognition Domain. J. Immunol. 2010, 185, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Ackermann, S.; Ma, Z.; Mohanta, S.K.; Zhang, C.; Li, Y.; Nietzsche, S.; Westermann, M.; Peng, L.; Hu, D.; et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat. Med. 2019, 25, 496–506. [Google Scholar] [CrossRef]
- Sokolova, D.; Childs, T.; Hong, S. Insight into the role of phosphatidylserine in complement-mediated synapse loss in Alzheimer’s disease. Fac. Rev. 2021, 10, 19. [Google Scholar] [CrossRef]
- Païdassi, H.; Tacnet-Delorme, P.; Verneret, M.; Gaboriaud, C.; Houen, G.; Duus, K.; Ling, W.L.; Arlaud, G.J.; Frachet, P. Investigations on the C1q–Calreticulin–Phosphatidylserine Interactions Yield New Insights into Apoptotic Cell Recognition. J. Mol. Biol. 2011, 408, 277–290. [Google Scholar] [CrossRef]
- van Schaarenburg, R.A.; Schejbel, L.; Truedsson, L.; Topaloglu, R.; Al-Mayouf, S.M.; Riordan, A.; Simon, A.; Kallel-Sellami, M.; Arkwright, P.D.; Åhlin, A.; et al. Marked variability in clinical presentation and outcome of patients with C1q immunodeficiency. J. Autoimmun. 2015, 62, 39–44. [Google Scholar] [CrossRef]
- Walport, M.J.; Mackay, I.R.; Rosen, F.S. Complement. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef]
- Nauta, A.J.; Trouw, L.A.; Daha, M.R.; Tijsma, O.; Nieuwland, R.; Schwaeble, W.J.; Gingras, A.R.; Mantovani, A.; Hack, E.C.; Roos, A. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 2002, 32, 1726–1736. [Google Scholar] [CrossRef]
- Botto, M.; Dell’ Agnola, C.; Bygrave, A.E.; Thompson, E.M.; Cook, H.T.; Petry, F.; Loos, M.; Pandolfi, P.P.; Walport, M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998, 19, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.S.; Crawford, G.; Buang, N.; Bartok, I.; Tian, K.; Thielens, N.M.; Bally, I.; Harker, J.A.; Ashton-Rickardt, P.G.; Rutschmann, S.; et al. C1q restrains autoimmunity and viral infection by regulating CD8+T cell metabolism. Science 2018, 360, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chang, C.-Y.; You, R.; Shan, M.; Gu, B.H.; Madison, M.C.; Diehl, G.; Perusich, S.; Song, L.-Z.; Cornwell, L.; et al. Cigarette smoke–induced reduction of C1q promotes emphysema. JCI Insight 2019, 4, e124317. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. Immunotargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef]
- Schartz, N.D.; Tenner, A.J. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J. Neuroinflamm. 2020, 17, 354. [Google Scholar] [CrossRef]
- Luo, L.; O’Leary, D.D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 2005, 28, 127–156. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Gyorffy, B.A.; Kun, J.; Torok, G.; Bulyaki, E.; Borhegyi, Z.; Gulyassy, P.; Kis, V.; Szocsics, P.; Micsonai, A.; Matko, J.; et al. Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proc. Natl. Acad. Sci. USA 2018, 115, 6303–6308. [Google Scholar] [CrossRef]
- Fraser, D.A.; Laust, A.K.; Nelson, E.L.; Tenner, A.J. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J. Immunol. 2009, 183, 6175–6185. [Google Scholar] [CrossRef]
- Fraser, D.A.; Pisalyaput, K.; Tenner, A.J. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J. Neurochem. 2010, 112, 733–743. [Google Scholar] [CrossRef]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Jin, X.; Parada, I.; Pesic, A.; Stevens, B.; Barres, B.; Prince, D.A. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7975–7980. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.K.; Kirsch, W.M. From development to dysfunction: Microglia and the complement cascade in CNS homeostasis. Ageing Res. Rev. 2013, 12, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Reichwald, J.; Danner, S.; Wiederhold, K.-H.; Staufenbiel, M. Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J. Neuroinflamm. 2009, 6, 35. [Google Scholar] [CrossRef]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y.; et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.D.; Cribbs, D.H.; Tenner, A.J.; Shankle, W.R.; Dick, M.; Kesslak, J.P.; Cotman, C.W. Decreased levels of C1q in cerebrospinal fluid of living Alzheimer patients correlate with disease state. Neurobiol. Aging 1994, 15, 609–614. [Google Scholar] [CrossRef]
- Stephan, A.H.; Madison, D.V.; Mateos, J.M.; Fraser, D.A.; Lovelett, E.A.; Coutellier, L.; Kim, L.; Tsai, H.H.; Huang, E.J.; Rowitch, D.H.; et al. A dramatic increase of C1q protein in the CNS during normal aging. J. Neurosci. 2013, 33, 13460–13474. [Google Scholar] [CrossRef]
- Botto, M. C1q, Autoimmunity and Apoptosis. Immunobiology 2002, 205, 395–406. [Google Scholar] [CrossRef]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S.; Almaslamani, M.A.; Thani, A.A.; Yassine, H.M. Antibody-Dependent Enhancement (ADE) and the role of complement system in disease pathogenesis. Mol. Immunol. 2022, 152, 172–182. [Google Scholar] [CrossRef]
- Lynch, N.J.; Willis, C.L.; Nolan, C.C.; Roscher, S.; Fowler, M.J.; Weihe, E.; Ray, D.E.; Schwaeble, W.J. Microglial activation and increased synthesis of complement component C1q precedes blood-brain barrier dysfunction in rats. Mol. Immunol. 2004, 40, 709–716. [Google Scholar] [CrossRef]
- Leigh, L.E.; Ghebrehiwet, B.; Perera, T.P.; Bird, I.N.; Strong, P.; Kishore, U.; Reid, K.B.; Eggleton, P. C1q-mediated chemotaxis by human neutrophils: Involvement of gClqR and G-protein signalling mechanisms. Biochem. J. 1998, 330 Pt 1, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Tenner, A.J.; Fonseca, M.I. The double-edged flower: Roles of complement protein C1q in neurodegenerative diseases. Adv. Exp. Med. Biol. 2006, 586, 153–176. [Google Scholar] [CrossRef]
- Lansita, J.A.; Mease, K.M.; Qiu, H.; Yednock, T.; Sankaranarayanan, S.; Kramer, S. Nonclinical Development of ANX005: A Humanized Anti-C1q Antibody for Treatment of Autoimmune and Neurodegenerative Diseases. Int. J. Toxicol. 2017, 36, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Bensa, J.C.; Reboul, A.; Colomb, M.G. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem. J. 1983, 216, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.E.; Hernandez, M.X.; Dinh, M.L.; Benavente, F.; Vasquez, O.; Tenner, A.J. C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-β neurotoxicity. J. Biol. Chem. 2013, 288, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvo, M.J.; Devous, M.D.; Kennedy, I.; Navitsky, M.; Lu, M.; Galante, N.; Salloway, S.; Doraiswamy, P.M.; Southekal, S.; Arora, A.K.; et al. A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer’s disease dementia. Brain 2019, 142, 1723–1735. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Farber, K.; Cheung, G.; Mitchell, D.; Wallis, R.; Weihe, E.; Schwaeble, W.; Kettenmann, H. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J. Neurosci. Res. 2009, 87, 644–652. [Google Scholar] [CrossRef]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef]
- Zhou, X.; Chu, X.; Xin, D.; Li, T.; Bai, X.; Qiu, J.; Yuan, H.; Liu, D.; Wang, D.; Wang, Z. L-Cysteine-Derived H2S Promotes Microglia M2 Polarization via Activation of the AMPK Pathway in Hypoxia-Ischemic Neonatal Mice. Front. Mol. Neurosci. 2019, 12, 58. [Google Scholar] [CrossRef]
- Holden, S.S.; Grandi, F.C.; Aboubakr, O.; Higashikubo, B.; Cho, F.S.; Chang, A.H.; Forero, A.O.; Morningstar, A.R.; Mathur, V.; Kuhn, L.J.; et al. Complement factor C1q mediates sleep spindle loss and epileptic spikes after mild brain injury. Science 2021, 373, eabj2685. [Google Scholar] [CrossRef] [PubMed]
- Brachova, L.; Lue, L.F.; Schultz, J.; el Rashidy, T.; Rogers, J. Association cortex, cerebellum, and serum concentrations of C1q and factor B in Alzheimer’s disease. Brain Res. Mol. Brain Res. 1993, 18, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.F.; Rydel, R.; Brigham, E.F.; Yang, L.B.; Hampel, H.; Murphy, G.M., Jr.; Brachova, L.; Yan, S.D.; Walker, D.G.; Shen, Y.; et al. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia 2001, 35, 72–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Barres, B.A. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010, 20, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Munch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, J.; Ding, G.; Gong, Q.; Wang, Y.; Yu, H.; Cheng, X. Microglia Polarization from M1 toward M2 Phenotype Is Promoted by Astragalus Polysaccharides Mediated through Inhibition of miR-155 in Experimental Autoimmune Encephalomyelitis. Oxid. Med. Cell. Longev. 2021, 2021, 5753452. [Google Scholar] [CrossRef]
- Ingram, G.; Loveless, S.; Howell, O.W.; Hakobyan, S.; Dancey, B.; Harris, C.L.; Robertson, N.P.; Neal, J.W.; Morgan, B.P. Complement activation in multiple sclerosis plaques: An immunohistochemical analysis. Acta Neuropathol. Commun. 2014, 2, 53. [Google Scholar] [CrossRef]
- Cho, K.J.; Cheon, S.Y.; Kim, G.W. Apoptosis signal-regulating kinase 1 mediates striatal degeneration via the regulation of C1q. Sci. Rep. 2016, 6, 18840. [Google Scholar] [CrossRef]
- Chen, L.; Yang, N.; Li, Y.; Li, Y.; Hong, J.; Wang, Q.; Liu, K.; Han, D.; Han, Y.; Mi, X.; et al. Cholecystokinin octapeptide improves hippocampal glutamatergic synaptogenesis and postoperative cognition by inhibiting induction of A1 reactive astrocytes in aged mice. CNS Neurosci. Ther. 2021, 27, 1374–1384. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Ho, M.S.; Parpura, V. Evolution of Neuroglia. Adv. Exp. Med. Biol. 2019, 1175, 15–44. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Gasque, P.; Vaudry, D.; Gonzalez, B.; Fontaine, M. Expression of a complete and functional complement system by human neuronal cells in vitro. Int. Immunol. 2000, 12, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Terai, K.; Walker, D.G.; McGeer, E.G.; McGeer, P.L. Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res. 1997, 769, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, T.A.; Angele, B.; Klein, M.; Heesemann, J.; Pfister, H.-W.; Botto, M.; Koedel, U. Complement C1q and C3 Are Critical for the Innate Immune Response to Streptococcus pneumoniae in the Central Nervous System. J. Immunol. 2007, 178, 1861–1869. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Aalinkeel, R.; Parikh, N.U.; Jacob, A.; Cwiklinski, K.; Sandhu, P.; Le, K.; Loftus, A.W.; Schwartz, S.A.; Quigg, R.J.; et al. Immunomodulatory Role of Complement Proteins in the Neuropathology Associated with Opiate Abuse and HIV-1 Co-Morbidity. Immunol. Investig. 2017, 46, 816–832. [Google Scholar] [CrossRef]
- Benoit, M.E.; Tenner, A.J. Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J. Neurosci. 2011, 31, 3459–3469. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar] [CrossRef]
- Davies, C.A.; Mann, D.M.; Sumpter, P.Q.; Yates, P.O. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J. Neurol. Sci. 1987, 78, 151–164. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Scheff, S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 1990, 27, 457–464. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Dejanovic, B.; Huntley, M.A.; De Maziere, A.; Meilandt, W.J.; Wu, T.; Srinivasan, K.; Jiang, Z.; Gandham, V.; Friedman, B.A.; Ngu, H.; et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 2018, 100, 1322–1336.e1327. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, G.; Liu, L.; Xu, S. Suppressive effects of melatonin on amyloid-β-induced glial activation in rat hippocampus. Arch. Med. Res. 2007, 38, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Rogers, J.; Cooper, N.R.; Webster, S.; Schultz, J.; McGeer, P.L.; Styren, S.D.; Civin, W.H.; Brachova, L.; Bradt, B.; Ward, P. Complement activation by beta-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10016–10020. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.t.; Kotwal, G.J. Pro-inflammatory complement activation by the Aβ peptide of Alzheimer’s disease is biologically significant and can be blocked by vaccinia virus complement control protein. Neurobiol. Aging 1998, 19, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Tacnet-Delorme, P.; Chevallier, S.; Arlaud, G.J. β-amyloid fibrils activate the C1 complex of complement under physiological conditions: Evidence for a binding site for Aβ on the C1q globular regions. J. Immunol. 2001, 167, 6374–6381. [Google Scholar] [CrossRef]
- Jiang, H.; Burdick, D.; Glabe, C.G.; Cotman, C.W.; Tenner, A.J. β-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J. Immunol. 1994, 152, 5050–5059. [Google Scholar] [CrossRef]
- Lui, H.; Zhang, J.; Makinson, S.R.; Cahill, M.K.; Kelley, K.W.; Huang, H.-Y.; Shang, Y.; Oldham, M.C.; Martens, L.H.; Gao, F.; et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell 2016, 165, 921–935. [Google Scholar] [CrossRef]
- Fan, R.; Tenner, A.J. Complement C1q expression induced by Aβ in rat hippocampal organotypic slice cultures. Exp. Neurol. 2004, 185, 241–253. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Chu, S.H.; Hernandez, M.X.; Fang, M.J.; Modarresi, L.; Selvan, P.; MacGregor, G.R.; Tenner, A.J. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J. Neuroinflamm. 2017, 14, 48. [Google Scholar] [CrossRef]
- Ghebrehiwet, B.; Hosszu, K.H.; Peerschke, E.I. C1q as an autocrine and paracrine regulator of cellular functions. Mol. Immunol. 2017, 84, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Biernat, J.; Gustke, N.; Drewes, G.; Mandelkow, E.M.; Mandelkow, E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: Distinction between PHF-like immunoreactivity and microtubule binding. Neuron 1993, 11, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Bennecib, M.; Grignon, Y.; Uchihara, T.; He, Y.; Piette, F.; Hauw, J.J. Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol. Aging 1997, 18, 267–273. [Google Scholar] [CrossRef]
- Brody, A.H.; Nies, S.H.; Guan, F.; Smith, L.M.; Mukherjee, B.; Salazar, S.A.; Lee, S.; Lam, T.K.T.; Strittmatter, S.M. Alzheimer risk gene product Pyk2 suppresses tau phosphorylation and phenotypic effects of tauopathy. Mol. Neurodegener. 2022, 17, 32. [Google Scholar] [CrossRef]
- Takahashi, H.; Klein, Z.A.; Bhagat, S.M.; Kaufman, A.C.; Kostylev, M.A.; Ikezu, T.; Strittmatter, S.M.; Alzheimer’s Disease Neuroimaging, I. Opposing effects of progranulin deficiency on amyloid and tau pathologies via microglial TYROBP network. Acta Neuropathol. 2017, 133, 785–807. [Google Scholar] [CrossRef]
- Audrain, M.; Haure-Mirande, J.V.; Wang, M.; Kim, S.H.; Fanutza, T.; Chakrabarty, P.; Fraser, P.; St George-Hyslop, P.H.; Golde, T.E.; Blitzer, R.D.; et al. Integrative approach to sporadic Alzheimer’s disease: Deficiency of TYROBP in a tauopathy mouse model reduces C1q and normalizes clinical phenotype while increasing spread and state of phosphorylation of tau. Mol. Psychiatry 2019, 24, 1383–1397. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Rozemuller, A.J.M.; Eikelenboom, P.; Theeuwes, J.W.; Jansen Steur, E.N.H.; de Vos, R.A.I. Activated microglial cells and complement factors are unrelated to cortical Lewy bodies. Acta Neuropathol. 2000, 100, 701–708. [Google Scholar] [CrossRef]
- Depboylu, C.; Schorlemmer, K.; Klietz, M.; Oertel, W.H.; Weihe, E.; Höglinger, G.U.; Schäfer, M.K.H. Upregulation of microglial C1q expression has no effects on nigrostriatal dopaminergic injury in the MPTP mouse model of Parkinson disease. J. Neuroimmunol. 2011, 236, 39–46. [Google Scholar] [CrossRef]
- Carbutt, S.; Duff, J.; Yarnall, A.; Burn, D.J.; Hudson, G. Variation in complement protein C1q is not a major contributor to cognitive impairment in Parkinson’s disease. Neurosci. Lett. 2015, 594, 66–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Crotti, A.; Glass, C.K. The choreography of neuroinflammation in Huntington’s disease. Trends Immunol. 2015, 36, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Singhrao, S.K.; Neal, J.W.; Morgan, B.P.; Gasque, P. Increased Complement Biosynthesis by Microglia and Complement Activation on Neurons in Huntington’s Disease. Exp. Neurol. 1999, 159, 362–376. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Lopez-Sanchez, C.; Garcia-Martinez, V.; Poejo, J.; Garcia-Lopez, V.; Salazar, J.; Gutierrez-Merino, C. Early Reactive A1 Astrocytes Induction by the Neurotoxin 3-Nitropropionic Acid in Rat Brain. Int. J. Mol. Sci. 2020, 21, 3609. [Google Scholar] [CrossRef]
- Lopez-Sanchez, C.; Poejo, J.; Garcia-Lopez, V.; Salazar, J.; Garcia-Martinez, V.; Gutierrez-Merino, C. Kaempferol prevents the activation of complement C3 protein and the generation of reactive A1 astrocytes that mediate rat brain degeneration induced by 3-nitropropionic acid. Food Chem. Toxicol. 2022, 164, 113017. [Google Scholar] [CrossRef]
- Beschorner, R.; Nguyen, T.D.; Gözalan, F.; Pedal, I.; Mattern, R.; Schluesener, H.J.; Meyermann, R.; Schwab, J.M. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 2002, 103, 541–549. [Google Scholar] [CrossRef]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef]
- Pearn, M.L.; Niesman, I.R.; Egawa, J.; Sawada, A.; Almenar-Queralt, A.; Shah, S.B.; Duckworth, J.L.; Head, B.P. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell. Mol. Neurobiol. 2016, 37, 571–585. [Google Scholar] [CrossRef]
- Bellander, B.-M.; Singhrao, S.K.; Ohlsson, M.; Mattsson, P.; Svensson, M. Complement Activation in the Human Brain after Traumatic Head Injury. J. Neurotrauma 2001, 18, 1295–1311. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Chou, A.; Feng, X.; Tiret, B.; Paladini, M.-S.; Riparip, L.-K.; Chaumeil, M.; Lemere, C.; Rosi, S. Traumatic Brain Injury in Aged Mice Induces Chronic Microglia Activation, Synapse Loss, and Complement-Dependent Memory Deficits. Int. J. Mol. Sci. 2018, 19, 3753. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.M.; Doran, S.J.; Glaser, E.P.; Meadows, V.E.; Faden, A.I.; Stoica, B.A.; Loane, D.J. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol. Aging 2019, 77, 194–206. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Scahill, R.I.; Durr, A.; Roos, R.A.C.; Leavitt, B.R.; Jones, R.; Landwehrmeyer, G.B.; Fox, N.C.; Johnson, H.; Hicks, S.L.; et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: The 12-month longitudinal analysis. Lancet Neurol. 2011, 10, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Lu, Y.; Xia, L.; Chen, D. Structural characterization and anticomplement activities of three acidic homogeneous polysaccharides from Artemisia annua. J. Ethnopharmacol. 2020, 247, 112281. [Google Scholar] [CrossRef]
- Du, D.; Lu, Y.; Cheng, Z.; Chen, D. Structure characterization of two novel polysaccharides isolated from the spikes of Prunella vulgaris and their anticomplement activities. J. Ethnopharmacol. 2016, 193, 345–353. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, Q.; Cao, J.; Yin, C.; Chen, D.; Cheng, Z. Flavonol glycosides and other phenolic compounds from Viola tianshanica and their anti-complement activities. Pharm. Biol. 2016, 54, 1140–1147. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Shi, S.; Chen, Y.; Wang, H.; Fan, H.; Wang, S. Structure analysis of a heteropolysaccharide from Taraxacum mongolicum Hand.-Mazz. and anticomplementary activity of its sulfated derivatives. Carbohydr. Polym. 2016, 152, 241–252. [Google Scholar] [CrossRef]
- Lai, C.S.; Yu, M.S.; Yuen, W.H.; So, K.F.; Zee, S.Y.; Chang, R.C. Antagonizing β-amyloid peptide neurotoxicity of the anti-aging fungus Ganoderma lucidum. Brain Res. 2008, 1190, 215–224. [Google Scholar] [CrossRef]
- Cai, Q.; Li, Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflamm. 2017, 14, 63. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.Y.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Black Ginseng Ameliorates Cellular Senescence via p53-p21/p16 Pathway in Aged Mice. Biology 2022, 11, 1108. [Google Scholar] [CrossRef]
- Lu, B.L.; Li, J.; Zhou, J.; Li, W.W.; Wu, H.F. Tanshinone IIA decreases the levels of inflammation induced by Aβ1–42 in brain tissues of Alzheimer’s disease model rats. Neuroreport 2016, 27, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hong, H.; Zhang, X.; Lai, W.; Wang, Y.; Chu, K.; Brown, J.; Hong, G.; Chen, L. Salidroside Inhibits Inflammation through PI3K/Akt/HIF Signaling after Focal Cerebral Ischemia in Rats. Inflammation 2017, 40, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Xie, X.; Zhang, X.; Wang, Y.; Chu, K.; Brown, J.; Chen, L.; Hong, G. Inhibition of Complement Drives Increase in Early Growth Response Proteins and Neuroprotection Mediated by Salidroside After Cerebral Ischemia. Inflammation 2018, 41, 449–463. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Lai, W.; Huang, X.; Chu, K.; Brown, J.; Hong, G. Salidroside Restores an Anti-inflammatory Endothelial Phenotype by Selectively Inhibiting Endothelial Complement After Oxidative Stress. Inflammation 2020, 43, 310–325. [Google Scholar] [CrossRef] [PubMed]

| Neurodegenerative Diseases | Animal/Cellular Model | Characteristics | C1q Effects | Ref. |
|---|---|---|---|---|
| AD | Tg2576 animals (APP) with C1q-deficient mice | Aβ plaques | Aβ co-localizes with C1q | [2,108,109] |
| PS19 mice overexpressing the P301S mutant of human Tau/Hek cells | Tau protein misfolding and assembly | Tau protein co-localizes with C1q | [113,114,115] | |
| Tg2576 animals (APP) with C1q-deficient mice | Synapse elimination | Synapse co-localizes with C1q | [2,3,62] | |
| PD | - | Depigmentation of the substantia nigra and locus coeruleus | C1q was restricted to microglia throughout the brain | [118] |
| Autopsies from PD patients | Neuronal loss in the pars compacta of the substantia nigra | [8,120] | ||
| HD | Early HD patients | CAG trinucleotide repeat expansion in the huntingtin gene on chromosome 4 | C1q produced locally by M1-type microglia is activated on the membranes of neurons | [124,134] |
| TBI | Sections of brains obtained at autopsy from 25 cases following closed TBI | Traumatic brain injury disrupts the BBB | C1q prompts the transformation of M2-type microglia into M1-type microglia and enhances complement system activation | [78,131] |
| Sources | Main Active Ingredients | Mechanism of Action | Function | Ref. |
|---|---|---|---|---|
| Artemisia annua L. | Acidic homogeneous polysaccharides | Inhibited the classical pathway and the alternative pathway | Anti-complement activity | [135] |
| Prunella vulgaris | Homogeneous acidic polysaccharides | Reduced excessive activation of the complement system | Anti-complement activity | [136] |
| Viola tianshanica flavonol glycosides | Flavonol glycosides and other phenolic compounds | Inhibited the classical pathway and the alternative pathway | Anti-complement activity | [137] |
| Taraxacum mongolicum Hand.-Mazz. heteropolysaccharide | Heteropolysaccharide | Inhibited excessive activation of the complement system | Anti-complement activity | [138] |
| Ganoderma lcidum | Polysaccharides | Down-regulates LPS- or Aβ-induced pro-inflammatory cytokines, promotes anti-inflammatory cytokine expressions in BV-2 and primary microglia and reduces C1q expression | Neuroprotective | [140] |
| Black Ginseng | Panax ginseng | Downregulated age-related inflammatory genes, included in the complement system | Ameliorates cellular senescence | [141] |
| Astragalus | Polysaccharides | Regulates the polarization of microglia from M1 to M2 phenotype by inhibiting the miR-155, reduces the secretion of inflammatory factors, and inhibits the activation of neurotoxic astrocytes | Inhibit neuroinflammation and demyelination in experimental autoimmune encephalomyelitis | [87] |
| Salvia miltiorrhiza | Tanshinone IIA | Reduced the number of astrocytes and microglial cells and induced C1q decreased in the brain of Alzheimer’s disease model rats | Reduced inflammation levels of AD rats | [142] |
| Rhodiola Rosea | Salidroside | Reducing early activation of the lectin pathway on the cerebral endothelium and inhibiting the gradual activation of the classical pathway after cerebral IR | Neuroprotective | [144] |
| Rhodiola Rosea | Salidroside | Inhibited classical complement activation and increased CD46 and CD59 | The protection afforded in cerebral ischemia-reperfusion injury | [145] |
| Vegetables and fruits | Kaempferol | Blocked the NPA-induced increase of NF-κB expression and enhanced secretion of cytokines IL-1α, TNFα, and C1q | Prevents the activation of complement C3 protein and the generation of reactive A1 astrocytes | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Zhang, D.; Fang, L.; Wang, J.; Liu, C.; Wu, D.; Liu, X.; Wang, X.; Min, W. Complement in Human Brain Health: Potential of Dietary Food in Relation to Neurodegenerative Diseases. Foods 2023, 12, 3580. https://doi.org/10.3390/foods12193580
Xing Y, Zhang D, Fang L, Wang J, Liu C, Wu D, Liu X, Wang X, Min W. Complement in Human Brain Health: Potential of Dietary Food in Relation to Neurodegenerative Diseases. Foods. 2023; 12(19):3580. https://doi.org/10.3390/foods12193580
Chicago/Turabian StyleXing, Yihang, Dingwen Zhang, Li Fang, Ji Wang, Chunlei Liu, Dan Wu, Xiaoting Liu, Xiyan Wang, and Weihong Min. 2023. "Complement in Human Brain Health: Potential of Dietary Food in Relation to Neurodegenerative Diseases" Foods 12, no. 19: 3580. https://doi.org/10.3390/foods12193580
APA StyleXing, Y., Zhang, D., Fang, L., Wang, J., Liu, C., Wu, D., Liu, X., Wang, X., & Min, W. (2023). Complement in Human Brain Health: Potential of Dietary Food in Relation to Neurodegenerative Diseases. Foods, 12(19), 3580. https://doi.org/10.3390/foods12193580







