Abstract
Terfezia arenaria is a desert truffle native to the Mediterranean Basin region, highly appreciated for its nutritional and aromatic properties. Despite the increasing interest in this desert truffle, T. arenaria is not listed as an edible truffle authorized for trade in the European Union. Therefore, our objective was to showcase T. arenaria’s nutritional and chemical composition and volatile profile. The nutritional analysis showed that T. arenaria is a good source of carbohydrates (67%), proteins (14%), and dietary fibre (10%), resulting in a Nutri-Score A. The truffle’s volatile profile was dominated by eight-carbon volatile compounds, with 1-octen-3-ol being the most abundant (64%), and 29 compounds were reported for the first time for T. arenaria. T. arenaria’s nutritional and chemical compositions were similar to those of four commercial mushroom and truffle species, while the aromatic profile was not. An electronic nose corroborated that T. arenaria‘s aromatic profile differs from that of the other four tested mushroom and truffle species. Our data showed that T. arenaria is a valuable food resource with a unique aroma and an analogous composition to meat, which makes it an ideal source for plant-based meat products. Our findings could help promote a sustainable future exploitation of T. arenaria and ensure the quality and authenticity of this delicacy.
1. Introduction
Food production currently faces many challenges []. One of these challenges is climate change, which causes severe health, economic and environmental problems []. Current political actions, such as the United Nation’s Sustainable Development Goals (e.g., SDG 12 Sustainable consumption and production, SDG13 Climate action), aim to mitigate climate change by shifting production and consumption patterns, and the production of mycorrhizal sporocarps (i.e., the fruiting body of macrofungi usually known as mushroom or truffle) is no exception [,]. With a key role in ecosystems [], ectomycorrhizal fungi provide services and goods essential to maintain soil quality, ecosystem functions and food (some species) [].
The global trade of mushrooms and truffles has grown significantly in the last two decades [,]; in 2000, the global production of mushrooms and truffles was 8.78 million tons, and by 2021, this number had grown to 44.20 million tons. This represents a growth of over four times. The average producer price of mushrooms and truffles also increased during this period, by 1.5 times. However, a wider investment in mycorrhizal mushrooms and truffles as a food source, with the associated health, environmental and economic benefits [,], is still hampered by insufficient science to showcase its benefits as a food source, and technology to boost its sustainable production and ensure its reliable identification. In agreement, although the production of mycorrhizal sporocarps has long had a high economic, cultural and environmental impact in the Mediterranean Basin region [], the list of the edible sporocarps authorized for trade in 27 European countries only includes 12 species.
Furthermore, insufficient data make it challenging to provide accurate information on mycorrhizal mushrooms and truffles production and market prices. Taking the desert truffles (i.e., a family of truffles endemic to arid and semiarid areas of the Mediterranean Basin Region, North Africa and the Middle East, which includes several genera, namely Terfezia, Tirmania and Mattirolomyces) as an example, they are among the wild mushrooms and truffles with higher selling prices [,]. Due to strong cultural traditions [], desert truffles have been part of the Mediterranean, North African and Middle Eastern cultures for centuries [], and are widely consumed in these regions [,]. As most of the world’s trade of desert truffles occurs in North African countries [], and most of this regional trade is not official, there is a lack of official data in these countries []. Therefore, the world’s production and annual market of desert truffles is still unknown []. Nevertheless, desert truffle plantation yields can reach approximately 350 kg ha−1, representing an expected average income of 7000 EUR ha−1 []. Besides the economic revenue, desert truffles also constitute an important nourishment source in North African and Arabic countries, being frequently used as a meat substitute [,] or as a powder to increment the nutritional quality of bread and biscuits [,]. Despite its great potential as a food source, only one edible desert truffle species is widely traded: Terfezia claveryi. All other potentially edible desert truffle species are being ignored. Therefore, a wider investment in desert truffles (and other mycorrhizal sporocarps) as a food source, with the associated health, environmental and economic benefits, is being hampered by insufficient science to showcase its benefits as a food source, and technology to boost its sustainable production and ensure its reliable identification.
Terfezia areanaria (Moris) Trappe is another desert truffle that forms a seasonal edible truffle with important ecological and socio-economic relevance []. Some studies have shown that this species, like other desert truffles, is rich in carbohydrates, proteins, and dietary fibre, making it a suitable addition to a balanced diet [,,,,,]. T. arenaria has also been reported to have important biological activities, such as antioxidant, antimicrobial and antitumoral [,,,]. Despite the increasing interest in exploring the nutritional and chemical composition of desert truffles [,,,], T. arenaria’s consumption and trade are still limited to small regions where this species is native. Therefore, one of our objectives was to showcase T. arenaria’s nutritional value by comparing it with other edible mushrooms and truffles, namely species that are well-known by the consumer and are widely available in the market.
Furthermore, ensuring food security and authentication are vital strategies for the sustainable exploitation of this native resource, preserving its long-term viability and conservation. Desert truffles are hypogeous (i.e., mushroom formation occurs belowground), which makes them difficult to detect []. However, as T. arenaria (and other desert truffle species) are mycorrhizal fungi that are associated with a host plant (most frequently from the Cistaceae family []; T. arenaria associates with the annual plant species Tuberaria guttata []), and screening the potential host plants is part of the detection method. Once their potential location is detected, the traditional method of harvesting desert truffles involves a pointed stick to carefully probe the soil []. This potentially destructive technique is time-honoured and traditionally passed from generation to generation, predominantly in Mediterranean regions [,]. However, harvesting desert truffles is a difficult process practiced by specialists [], which are becoming fewer and fewer among the new generations due to the abandonment of rural areas and traditional forestry [,]. Desert truffles’ (and wild mushrooms in general) incorrect harvesting, including excessive harvesting, can lead to the destruction of fungal structures, making future productivity unfeasible []. In the case of incorrect species identification, it can cause poisoning, leading to a feeling of insecurity (mycophobia) among consumers [,]. Like the mushrooms and truffles commonly found in supermarkets, it becomes crucial to establish comprehensive knowledge regarding the safe consumption of desert truffles and other wild mushrooms and truffles. Altogether, developing and implementing guidelines that ensure food safety becomes especially significant for the mushroom trade []. Only by prioritizing the development of sustainable harvesting techniques and tools to assess quality and authenticity can we establish a fair value chain for these endogenous products. These steps are essential to meet consumer’s health and nutritional needs while safeguarding the resource and promoting equitable practices in its utilization.
So far, T. arenaria and other desert truffle species identification has relied on traditional knowledge and morphological identification by experts. However, besides its nutritional value and potential health benefits, T. arenaria has a unique bouquet of volatile organic compounds (VOCs; includes alcohols, aldehydes, ketones, and sulphur compounds [,,]), which is perceived by humans as a subtle, sweet and agreeable flavour [,] and contributes to promote its quality and gastronomic value [,]. Therefore, we consider that T. arenaria’s VOCs bouquet could be explored to develop a robust and efficient analysis method to certify the quality and authenticity of this delicacy.
Studies on the VOCs present in desert truffle species are still scarce [], and only one study included T. arenaria []. Currently, the most common identification and quantification methods for VOCs analysis is gas chromatography–mass spectrometry (GC–MS) [], and it has been widely applied to truffles and desert truffles [,]. GC–MS is a powerful analytical technique with high sensitivity, easy metabolite identification, and has the possibility to couple with separation techniques []. However, it can be time consuming to prepare samples as it is a destructive analysis operated by highly qualified technicians and it is very expensive [,]. On the other hand, the use of electronic nose (e-nose) technology has gained attention in recent years for the identification and analysis of aroma profiles in mushrooms [,,,] and other food products [,]. This methodology has been frequently combined with GC–MS analysis, as a non-destructive and rapid approach to quality control and product authentication []. The e-nose was proven a successful methodology to distinguish between the volatile profile of several filamentous fungi species and/or strains, for health, environmental and food control applications []. In the case of mushrooms, most studies reported the volatile profile in relation with quality analysis in post-harvest processes [,,,,,]. Similarly to what was reported for filamentous fungi, this technology can also be applied for the identification and differentiation of mushroom and truffles species [,,,,]. The use of e-noses in the food industry is widespread, with applications in meat, dairy products, aquatic products, cereals, fruits, and vegetables. Advantages of the e-nose include their rapid response, low cost and a relatively simple operating process []. Therefore, given that T. arenaria has a unique bouquet of volatile organic compounds, we tested if the e-nose was capable of distinguishing T. arenaria from other edible mushroom and truffle species, and therefore guarantee this desert truffle’s authenticity. For that, we used the e-nose Cyranose-320 to analyse T. arenaria’s volatile profile, applying two pre-analysis incubation temperatures to understand if the temperature could affect VOCs emissions and compromise the e-nose’s identification efficiency: (a) T. arenaria samples incubated for one hour at 40 °C (40 °C); and (b) T. arenaria samples incubated for one hour at room temperature (RT). In the identification process, four commercial edible species (Agaricus bisporus, Lentinula edodes, Pleurotus ostreatus and Tuber melanosporum) were also tested to confirm the ability of Cyranose-320 to distinguish T. arenaria from other edible species.
Our review on T. arenaria’s nutritional and health value, and proposal for the first steps in developing a non-destructive and rapid identification method for early detection of Terfezia truffles, their growth stages, and quality are crucial for sustainable resource exploitation. This innovation could promote our understanding and management of desert truffle populations, ensuring their preservation and responsible production and use in the long term.
2. Materials and Methods
2.1. Terfezia arenaria Samples
Desert truffles naturally fruit in the spring from February to May. For three weeks of the 2019 spring season, sixty-three T. arenaria truffles were harvested in Alentejo (south of Portugal) (see Figure S1). The samples were collected in four sampling sites—S1, S2, S3 and S4—with an area of 200 m2 each; the sampling sites were separated by 1 to 2 km distance. In all the sampling sites, the Cistaceae host plants were abundant (Tuberaria guttata), but the forest-dominant species differed: Quercus suber in sites 1 and 2, Pinus pinea in site 3 or mixed in site 4 (Figure S1). Specimens were freed from substrate debris at the site and further cleaned in the laboratory and used to analyse T. arenaria’s nutritional and chemical composition and volatile profile (using 2 techniques). T. arenaria samples were (i) kept at −20 °C until molecular analysis; (ii) dried for nutritional and chemical composition analyses; and (iii) kept at 4 °C until volatile profile analysis in the first 48 h post-harvest.
The specimens were identified by molecular analysis.
For the nutritional and chemical analyses, we used three dry samples of T. arenaria truffles collected in three of the sampling sites (sites S1, S2, S3, S4) during the first week of April 2019. From each sampling site, one desert truffle with similar size and appearance was analysed.
For the volatiles profile analysis, we used three fresh samples of T. arenaria truffles collected in three of the sampling sites (sites S2, S3, S4) during the first week of April 2019. From each sampling site, one desert truffle with similar size and appearance was analysed.
To validate T. arenaria’s identification using the e-nose, we used a total of five mushroom and truffle species: T. arenaria, A. bisporus, L. edodes, P. ostreatus and T. melanosporum. Mature T. arenaria truffles were harvested in Alentejo (south of Portugal) as described in Section 2.1. A. bisporus, L. edodes, and P. ostreatus were purchased in a local supermarket, and T. melanosporum was purchased at Espora Gourmet, SL. All fresh mushrooms and truffles samples were kept at 4 °C until analysis in the first 48 h post-harvest.
2.2. Comparing T. arenaria’s Nutritional Value with That of Other Edible Mushrooms and Truffles, and Meat
To showcase its nutritional value, we collected and analysed T. arenaria’s samples for their nutritional and mineral composition. Furthermore, T. arenaria’s data was compared with that reported in the literature for other edible mushroom and truffle species (A. bisporus, L. edodes, P. ostreatus and T. melanosporum), and with meat (cow, pig and chicken). The criteria for selecting the other edible mushroom and truffle species and the types of meat were wide consumption and easy to buy and find in the supermarkets.
Twelve T. arenaria composite samples, three for each sampling site, were prepared. The samples were dried at 40 °C for 72 h to determine their moisture content, and the dry material was powdered in a porcelain mortar and kept in brand-new sealed polyethylene bags under dry conditions at room temperature until analysis. Using the AOAC procedures [], the dry samples were analysed for their (i) crude protein content (applying the conversion factor of N × 4.38), which was estimated by the macro-Kjeldahl method, (ii) crude fat, which was determined by extracting a known weight of powdered sample with petroleum ether, using a Soxhlet apparatus, and (iii) ash concentration, which was determined by incineration at 600 ± 15 °C. A bomb calorimeter (Parr 6200 Isoperibol Calorimeter) was used to estimate the energy of the samples. Total carbohydrates were calculated using the following equation:
The chemical elemental analysis was determined by inductively coupled plasma mass spectrometry (ICP-MS; Agilent Technologies, Bellevue, WA, USA) after digestion with concentrated nitric acid (68% HNO3), and filtered and diluted 20 times with double distilled water (WP750, PG Instruments, Lutterworth, UK) to a total volume of 15 mL. For ICP-MS determinations, external standard calibration curves were performed by serially diluting multi-element standard stock solutions. This protocol was adapted from Mędyk et al., 2016 [].
Additionally, we compared the nutritional and mineral composition of T. arenaria with that of A. bisporus, L. edodes, P. ostreatus (edible mushrooms) and T. melanosporum (edible truffle), and beef, pork and chicken meat. Data used for the other edible mushrooms and truffles were selected from studies published in international peer-review journals reporting the use of methodologies similar to those we used in our study. Therefore, we used one article for T. arenaria, three for A. bisporus, five for L. edodes, six for P. ostreatus and two for T. melanosporum. For beef, pork and chicken meat, one database and one article were consulted for each.
Finally, data on dietary reference intakes of nutrients and elements were compiled from Dietary Reference Intakes Datasets from the USA, Canada [], and the EU [,,,]. The contribution of 100 g of dried and fresh T. arenaria to the daily intake of each nutrient and element was calculated considering the dietary reference intakes values previously compiled. Finally, we determined the Nutri-Score for T. arenaria based on its nutritional composition per 100 g of dry truffles. We used the nutritional content determined in this study, and complemented it with data on sugars and fatty acids from Tejedor-Calvo et al., 2021 []. To determine the Nutri-Score we used the recent algorithm made available by Sante Publique France (https://www.santepubliquefrance.fr/en/nutri-score (accessed on 9 August 2023)) [].
2.3. Volatiles Profile by GC–MS
To showcase its unique bouquet of volatile organic compounds (VOCs), we collected (as previously described in Section 2.1.) and analysed T. arenaria’s samples for their VOCs profile. Furthermore, T. arenaria’s data was compared with that reported in the literature for the same other edible mushroom and truffle species previously described (A. bisporus, L. edodes, P. ostreatus and T. melanosporum).
Analysis of T. arenaria’s VOCs profile was performed using Headspace-Solid Phase Microextraction Gas Chromatography–Mass Spectrometry coupled to GC–MS (HS–SPME/GC–MS), adapted from the protocol reported by Splivallo and Ebeler (2015) []. The three fresh specimens were ground with a clean knife to small cubes of approximately 125,000 mm3, and accurately weighed in 1.5 mL tightly sealed glass vials. A pre-extraction was performed in the vial at 60 °C for 10 min, then the SPME fibre (PDMS/DVB65um) was implanted manually, and the volatile compounds were extracted at 60 °C for 30 min. Afterwards, the SPME fibre was removed and placed manually in the injection port of the GC–MS. The analysis of volatile compounds was conducted on an GC–MS-QP2010 (Shimadzu, Japan), with acquisition mode SCAN (35–600 m/z) and equipped with a TRB-5 MS column (Teknokroma, Barcelona, Spain). The injector and MS interface temperatures were both held at 250 °C. The analytic conditions were the following: the constant flow of helium in the column was kept at 1.0 mL min−1; the oven temperature was held at 40 °C for 10 min, then raised at a rate of 10 °C min−1 to 160 °C, and finally reached 260 °C with a rate of 50 °C min−1 and kept for 2 min. Blank GC–MS runs were performed during the analyses.
Finally, we compared the VOCs profile of T. arenaria with that of A. bisporus, L. edodes, P. ostreatus (edible mushrooms) and T. melanosporum (edible truffle). Data used for these edible mushrooms and truffle were selected from studies published in international peer-review journals reporting the use of methodologies similar to those we used in our study. Therefore, we used one article per each species.
2.4. First Steps in Developing a Non-Destructive and Rapid Identification Method for T. arenaria
To test if T. arenaria’s unique VOCs profile could be applied in developing a non-destructive and rapid identification method for the early detection of T. arenaria, we used the eletronic nose Cyranose-320 (Sensigent, Pasadena, CA, USA). The Cyranose-320 is a portable e-nose equipped with a nanocomposite sensor array (32 nanosensors), an internal air sampling pump, and advanced pattern recognition algorithms. These technologies enable rapid detection and identification of substances based on their chemical profile as visualized by the smellprint []. Therefore, we specifically tested if the e-nose was capable of distinguishing T. arenaria from other edible mushroom and truffle species, and therefore guarantee this desert truffle’s authenticity. This was conducted in the following two phases (Figure 1):
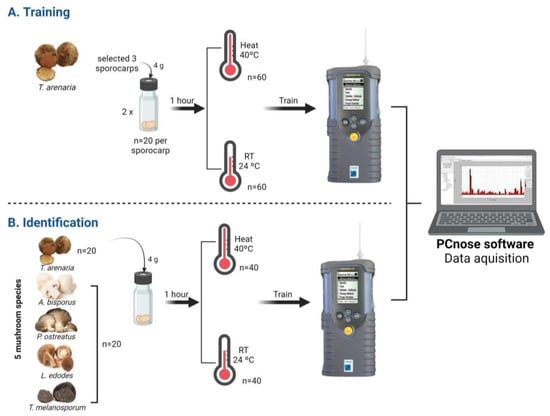
Figure 1.
Schematic representation of the two phases of the non-destructive and rapid identification method for T. arenaria identification using the E-nose Cyranose-320. First, the e-nose was trained with T. arenaria samples (A), and then mushroom and truffle samples belonging to five species were tested for an accurate identification of T. arenaria (B). Two pre-analysis incubation temperatures were tested in both phases: samples were kept at room temperature (RT) or heated at 40 °C.
2.4.1. Phase 1: E-Nose Training
For the training process (Figure 1), T. arenaria’s samples were subjected to one of two pre-analysis incubation temperatures: (a) 40 °C treatment with samples incubated for 1 h at 40 °C; and (b) RT treatment with samples incubated for 1 h at room temperature (i.e., 24 °C). The samples used for analysing T. arenaria’s VOCs profile with the e-nose were clean as previously described, and were kept at 4 °C until analysis in the first 48 h post-harvest. Three fresh T. arenaria truffles were analysed separately. Two replicates with 4 g of T. arenaria were weighed and introduced in a 10 mL vial for each sporocarp and training method. The T. arenaria truffles were identified as: Terf1, Terf2 and Terf3. The Cyranose-320 was mounted on a tripod, which could be adjusted for inserting the e-nose needle into the vials for headspace reading. Ten readings per samples were performed. The e-nose was coupled to the computer and PCnose software was used to set the list of parameter settings of the Cyranose-320 (see Table S1), data acquisition, and analysis. To finish the training phase, an internal data cross-validation was used to assess the accuracy of sample classification in relation to their respective class labels, serving as a measure of effectiveness for the e-nose system [].
2.4.2. Phase 2: E-Nose Identification Accuracy
Similarly to what was conducted in the training phase, four grams of three fresh mushrooms were weighed and introduced in a 10 mL vial, and the two pre-analysis incubation temperatures were applied. Afterwards, each sample headspace was read with Cyranose-320 in the identification mode activated for the respective method trained (40 °C or RT pre-analysis incubation temperatures). Results were displayed in Cyranose-320 and recorded on the PCnose software. The results displayed in the Cyranose-320 are rated with asterisks, between one and five asterisks, accordingly to the identification quality performed. Regarding quality, only samples between three and five asterisks are considered acceptable results (i.e., acceptable, good and excellent, respectively). When the e-nose does not recognize the tested sample, “Confused” or “Unknown” will be displayed (Table S5).
2.5. Statistical Analysis
We used a principal component analysis (PCA) to analyse nutritional and mineral composition (based on fresh weight values) for T. arenaria determined in this study, and A. bisporus, L. edodes and P. ostreatus and fresh beef, pork and chicken meat with values from the literature. T. melanosporum was not included in the PCA because moisture content was not available on the selected literature and thus we were unable to express its nutritional and mineral composition for fresh samples. The PCA explored how T. arenaria’s nutritional and mineral composition compares to reference values for edible mushrooms and meat.
Pie charts and Venn diagram were performed to compare the VOCs profiles of T. arenaria with literature values for A. bisporus, L. edodes, P. ostreatus and T. melanosporum (http://bioinformatics.psb.ugent.be/webtools/Venn/ (accessed on 2 August 2023)).
Standardized data from the 32 sensors were analysed blinded to reference standard results using principal component analysis (PCA) to explore the sensors’ response to the two pre-analysis incubation temperatures (40 °C and RT). Differences between 40 °C and RT pre-analysis incubation temperatures were compared using the Kruskal–Wallis one-way analysis of variance. Multiple pairwise comparisons were performed using Dunn’s test (p < 0.05). All statistical analysis were performed using Microsoft Excel 2019/XLSTAT-Premium (Version 2021.4.1, Addinsoft, Inc., Brooklyn, NY, USA).
3. Results and Discussion
3.1. Showcasing T. arenaria’s Nutritional Value
The diverse array of nutrients found in mushrooms and truffles, including carbohydrates, proteins, lipids, minerals, fibre, and water, contribute to their potential positive effect on the human diet. The average moisture of the Terfezia arenaria samples was 77%, which is within the range reported for other desert truffles (Table 1) [,,]. However, T. arenaria’s lipid concentration was slightly (2 to 8%) lower than that reported for other desert truffles [,,,], but similar to that reported for the commercial edible mushroom and truffle species A. bisporus, L. edodes, P. ostreatus and T. melanosporum [,,,,]. Carbohydrates are the major nutrient category in edible mushrooms and truffles []. However, the concentrations determined in T. arenaria were lower than those reported for other desert truffles [,,]. T. arenaria’s energy potential (387 kcal per 100 g of dry weight) was similar to that reported for the other edible mushrooms and truffles (Table 1) [,,,].
Furthermore, 18 mineral elements were identified in T. arenaria samples (Table 2), with potassium, phosphorus, sulphur, magnesium and calcium being the most abundant. Eight trace elements (iron > zinc > copper > manganese > chromium > molybdenum > selenium > nickel) and two nonessential elements (aluminum and lithium) were also identified. These mineral elements are critical for human health, and their intake must be carefully balanced to avoid health problems. Lithium (Li, 37 µg 100 g−1 dw) and selenium (Se, 50 µg 100 g−1 dw) are of particular importance to human health, due to their proprieties, i.e., antiviral, immunomodulatory, neuroprotective effects, and can be used to treat several mental health conditions [,]. Considering the dietary reference intakes (see Table S2), a balanced consumption of T. arenaria (especially dry) could contribute to a proper intake of these elements. In the case of lithium, this element is higher in T. arenaria than the other edible mushrooms and truffles, which could be an interesting characteristic in the development of new plant-based meat products based on this desert truffle.
Two trace elements with a detrimental health effect were identified in the T. arenaria, Arsenic (As, 10 µg 100 g−1 dw) and Barium (Ba, 32 µg 100 g−1 dw). The As value is similar to the values reported for A. bisporus, L. edodes and Pleurotus ostreatus, while the Ba was lower than that reported for A. bisporus and L. edodes [,] (Table 2), and other edible mushroom species []. Furthermore, according to Siwulski et al. (2021), the estimated daily intakes of these mushrooms, particularly L. edodes, are low and do not pose a health risk []. Also, considering the established dietary reference intakes for Arsenic (15 μg kg−1 body weight per day) and Barium (0.2 mg kg−1 body weight per day), the contribution of T. arenaria is residual when considering a consumption of 100 g of dry or fresh truffles (Table S2).
The nutritional and mineral value of mushrooms is influenced by parameters such as the stage of development, the substrate where they grow, the geographic origins, and their genetic variability intra and interspecies []. Terfezia arenaria samples were harvested from different locations with different dominant forest species and at different times during a three-week harvest season. Although T. arenaria has a wider distribution area and fruits for a longer period, both its distribution area and fruiting period have been severely reduced by lower precipitation in autumn and spring (crucial for desert truffle fructification []) due to climate change and wildfires that destroy productive areas. However, when considering the nutritional values previously reported for other desert truffles, we consider that it is likely that the T. arenaria‘s nutritional and chemical composition we report here could represent this species’ composition. Nonetheless, T. arenaria‘s nutritional and mineral composition was similar to the most commercialized and appreciated species of mushrooms and truffles in the world, such as A. bisporus, L. edodes, P. ostreatus and T. melanosporum [,] (Table 2 and Table 3). Despite their similarities, T. arenaria have a closer resemblance to meat than the other edible mushrooms (Figure 2).
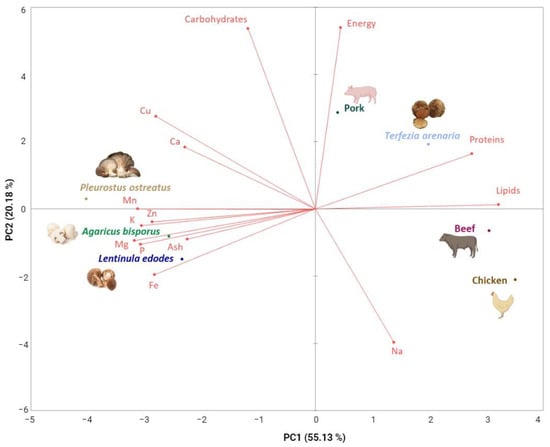
Figure 2.
Fresh T. arenaria’s nutritional and mineral composition in relation to reference values reported for other fresh edible mushrooms and meat. Plot of the first two principal components of the PCA model built with common nutritional and mineral composition values for fresh T. arenaria determined in this study, and fresh A. bisporus, L. edodes, P. ostreatus and beef, pork and chicken meat from the literature.
The Nutri-Score is a promising new front-of-pack nutrition labelling system that has the potential to improve population diets []. It is easy to understand, well-accepted by consumers, and can be effective in encouraging healthier food choices []. The Nutri-score algorithm showed that 100 g of dry T. arenaria has a Nutri-Score of A (i.e., the A score is the best nutritional score when applying the Nutri-Score), which indicates that this product has a very good nutritional profile. Products with an A score are typically low in calories, saturated fat, and sugar, and high in fibre and protein.
Our data on T. arenaria’s nutritional value can help explain why this desert truffle had, and still has, such an important role in the nourishment of rural populations, often serving as a meat substitute [,]. Indeed, poor rural populations in North Africa and Arab countries have used mushrooms as meat substitutes for centuries [,,]. Furthermore, T. arenaria shares equally appealing properties with other edible commercial species (Table 1 and Table 2) as T. arenaria’s protein can range between 14 and 23 g per 100 g−1 dw, its carbohydrates can range between 67 and 77 g/100 dw, and its lipids can range between 2.2 and 5.1 g/100 dw (Table 1 and Table 2) []. T. arenaria’s protein value is similar to the average values for pork and beef meats (pork 13.2 g; beef 19.9 g), while the carbohydrates are higher (pork 2.4 g; beef 2.0 g) and the lipids are lower (pork 37.0 g; beef 4.2 g) []. These characteristics, together with the mineral composition, show that T. arenaria is suitable to be employed in new food products as a potential plant-based meat (Figure 2).
Indeed, as awareness on the adverse effects of meat consumption grows [,], there is a notable shift towards incorporating plant-based ingredients, such as mushrooms and truffles, into meat-based dishes [,]. This increasing acceptance reflects a growing interest in blending plant-based alternatives with traditional meat-based foods []. By incorporating mushrooms and truffles as blenders in meat products, over 7.5 million L of water can be saved per 10,000 portions of this product (made with 70% beef and 30% mushrooms) []. A. bisporus, L. edodes and P. ostreatus are among the most produced mushrooms species worldwide, and are already often used in these products [,].

Table 1.
Nutritional composition and energy values for T. arenaria and other desert truffles, commercial mushrooms and meat (pork, beef and chicken). Values for T. arenaria were determined in the present study while values for the other mushrooms, truffles and meat were determined in other studies.
Table 1.
Nutritional composition and energy values for T. arenaria and other desert truffles, commercial mushrooms and meat (pork, beef and chicken). Values for T. arenaria were determined in the present study while values for the other mushrooms, truffles and meat were determined in other studies.
| Moisture | Ash | Proteins | Lipids | Carbohydrates | Fibre | Energy | References | ||
|---|---|---|---|---|---|---|---|---|---|
| % fw | g/100 g | g/100 g | g/100 g | g/100 g | g/100 g | kcal/100 g | |||
| Desert Truffles | Terfezia arenaria a | 77 | 7.3 | 14 | 2.2 | 67 | 10 | 387 | This study |
| n.a | 4.3 | 23 | 5.1 | 77 | n.a | 394 | [] | ||
| Terfezia claveryi a | 73 | 4.3 | 16 | 7.0 | 65 | 8 | n.a | [] | |
| 83 | 15.3 | 32 | 2.8 | 46 | n.a | 338 | [] | ||
| Terfezia boudieri a | n.a | 12.9 | 17 | 6.4 | 60 | 4 | n.a | [] | |
| 78 | 4.5 | 26 | 8.0 | 62 | n.a | n.a | [] | ||
| Terfezia olbiensis a | 80 | 15.3 | 36 | 3.2 | 48 | n.a | 366 | [] | |
| Commercial mushrooms | Agaricus bisporus a | 91 | 12.7 | 19 | 2.0 | 67 | 10 | 360 | [] |
| 90 | 9.4 | 25 | 2.3 | 64 | n.a | 374 | [] | ||
| Pleurotus ostreatus a | 91 | 7.8 | 18 | 2.6 | 71 | 14 | 382 | [] | |
| n.a | 9.3 | 9 | 1.3 | 70 | 11 | n.a | [] | ||
| 89 | 6.7 | 13 | 2.5 | 78 | n.a | 383 | [] | ||
| Lentinula edodes a | n.a | 3.8 | 18 | 0.9 | 30 | 32 | 264 | [] | |
| 94 | 6.7 | 16 | 1.8 | 74 | 15 | 382 | [] | ||
| 88 | 7.4 | 17 | 2.1 | 73 | n.a | 381 | [] | ||
| Tuber melanosporum a | n.a | 0.0 | 22 | 2.3 | 75 | n.a | 411 | [] | |
| Meat | Pork b | 13 | 37.0 | 2.4 | n.a | 390 | [] | ||
| 64 | 0.9 | 18 | 17.5 | n.a | n.a | 228 | [] | ||
| Beef b | 20 | 4.2 | 2.0 | n.a | 126 | [] | |||
| 63 | 0.8 | 18 | 19.4 | n.a | n.a | 243 | [] | ||
| Chicken b | 19 | 1.3 | 9.4 | n.a | 167 | [] | |||
| 75 | 1.0 | 18 | 7.2 | n.a | n.a | 133 | [] |
a mushroom values presented for dry weight, except for moisture (% fresh weight). b meat values presented for fresh weight; n.a—data not available.

Table 2.
Mineral content [mg kg−1 dw] for T. arenaria and other desert truffles, commercial mushrooms and meat (pork, beef and chicken). Values for T. arenaria were determined in the present study while values for the other mushrooms, truffles and meat were determined in other studies.
Table 2.
Mineral content [mg kg−1 dw] for T. arenaria and other desert truffles, commercial mushrooms and meat (pork, beef and chicken). Values for T. arenaria were determined in the present study while values for the other mushrooms, truffles and meat were determined in other studies.
| Minerals | Terfezia arenaria | Agaricus bisporus | Lentinula edodes | Pleurotus ostreatus | Tuber melanosporum | Pork | Beef | Chicken |
|---|---|---|---|---|---|---|---|---|
| Major essential elements | ||||||||
| Ca | 26 | 580 | 438 | 730 | 817 | 60 | 70 | 60 |
| K | 3695 | 38,400 | 21,700 | 14,244 | 7356 | 3180 | 2730 | 3020 |
| Mg | 128 | 1300 | 1330 | 2800 | 241 | 190 | 164 | 205 |
| Na | 23 | 491 | 144 | 35 | 67 | 540 | 550 | 630 |
| P | 1407 | 8210 | 4080 | 6204 | 2678 | 1730 | 1440 | 1660 |
| S | 299 | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Essential trace elements | ||||||||
| Cr | 0.9 | 7.0 | 0.3 | n.a | n.a | n.a | n.a | n.a |
| Cu | 6.6 | 34.2 | 7.1 | 39 | 18 | 0.7 | 0.6 | 0.4 |
| Fe | 19.6 | 49.4 | 35.7 | 130 | 12 | 7.9 | 19.6 | 5.9 |
| Mn | 1.4 | 6.6 | 19.3 | 14 | 1 | <0.125 | <0.125 | 0.1 |
| Mo | 0.6 | 0.3 | 0.2 | <0.01 | n.a | n.a | n.a | n.a |
| Ni | 0.1 | 0.7 | 0.1 | 0.7 | n.a | n.a | n.a | n.a |
| Se | 0.5 | 1.7 | 1.1 | 0.3 | n.a | n.a | n.a | n.a |
| Zn | 11.0 | 51.5 | 76.3 | 110.4 | 37 | 22.3 | 38.5 | 11.8 |
| Non-essential elements | ||||||||
| Al | 10.7 | 17.9 | 5.8 | n.a | n.a | n.a | n.a | n.a |
| Li | 0.4 | <0.1 | 0.1 | 0.3 | n.a | n.a | n.a | n.a |
| Elements with detrimental health effects | ||||||||
| As | 0.1 | 0.3 | 0.5 | <0.1 | n.a | n.a | n.a | n.a |
| Ba | 0.3 | 2.8 | 1.7 | n.a | n.a | n.a | n.a | n.a |
| References | This study | [] | [] | [,,] | [] | [] | [] | [] |
n.a—data not available.

Table 3.
List of the volatile organic compounds (VOCs) detected in T. arenaria using GC–MS. The VOCs are listed according to their abundance (i.e., area). The values represent the mean relative peak areas (expressed as % from total peak areas) and retention times (RT). Information on each VOC’s classification, metabolic pathway and odor is also presented (n = 3).
Table 3.
List of the volatile organic compounds (VOCs) detected in T. arenaria using GC–MS. The VOCs are listed according to their abundance (i.e., area). The values represent the mean relative peak areas (expressed as % from total peak areas) and retention times (RT). Information on each VOC’s classification, metabolic pathway and odor is also presented (n = 3).
| Formula | RT | Area (%) | Name of Compounds | Funtional Groups | Pathway | Odor |
|---|---|---|---|---|---|---|
| C8H16O | 15.847 | 57.21 | 1-Octen-3-ol | Alcohols | Lipoxygenase–linoleic acid | Mushroom like |
| C8H16O | 16.007 | 12.86 | 3-Octanone | Ketones | Lipoxygenase–linoleic acid | Green apple-like |
| C6H12O | 7.412 | 4.29 | Hexanal | Aldehydes | Lipoxygenase–linoleic acid | Green, grassy |
| C8 H16 O | 17.952 | 3.22 | (Z)-2-Octen-1-ol | Alcohols | Lipoxygenase–linoleic acid | Green, citrus |
| C10H16 | 14.379 | 1.87 | α-Pinene | Terpenes | Monoterpenoid biosynthesis | Woody, resinous |
| C8H18O | 16.256 | 1.54 | 3-Octanol | Alcohols | Lipoxygenase–linoleic acid | Floral, fatty |
| C8H14O | 15.658 | 1.38 | (5Z)-Octa-1,5-dien-3-ol | Alcohols | Lipid metabolism | Sweet or floral |
| C8 H16 O2 | 16.549 | 0.98 | Pentyl propanoate | Ester | n.a | Fruity, sweet |
| C8 H14 O | 17.735 | 0.86 | I-2-Octenal | Aldehydes | Lipoxygenase–linoleic acid | Fatty, nutty |
| C21H41IO2 | 24.933 | 0.39 | Propionic acid, 3-iodo-, octadecyl ester | Ester | n.a | n.a |
| C10 H16 | 17.086 | 0.35 | Limonene | Terpenes | Monoterpenoid biosynthesis | Citrus |
| C8H9N | 18.411 | 0.32 | Pyridine, 5-ethenyl-2-methyl- | Other compounds | n.a | Pungent, fish-like |
| C18H37ClO2S | 25.156 | 0.3 | 1-Octadecanesulphonyl chloride | Other compounds | n.a | Strong and pungent |
| C21H42O2 | 25.127 | 0.29 | Henicosanoic acid | Other compounds | n.a | Odorless |
| C32H66 | 25.155 | 0.28 | Dotriacontane | Hydrocarbons | n.a | Odourless |
| C8 H16 | 17.479 | 0.26 | Caprylene (1-octene) | Hydrocarbons | n.a | Petroleum-like |
| C8H15NO3 | 20.904 | 0.23 | 2-Octanone, 1-nitro- | Ketones | n.a | Sweet |
| C12H24O3 | 23.046 | 0.22 | Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | Ester | n.a | Mild, fruity or sweet |
| C8H8O | 17.417 | 0.21 | Benzeneacetaldehyde | Aldehydes | Phenylalanine metabolism | Sweet, floral |
| C14 H30 | 24.692 | 0.21 | Tetradecane | Hydrocarbons | n.a | Gasoline-like to odorless |
| C14 H30 | 24.692 | 0.21 | Eicosane-7-hexyl | Hydrocarbons | n.a | n.a |
| C13 H22 O3 Si2 | 18.831 | 0.19 | Benzaldehyde, 2,5-bis[(trimethylsilyl)oxy] | Aldehydes | n.a | n.a |
| C16H34 | 24.134 | 0.15 | Hexadecane | Hydrocarbons | Fatty acid degradation | Odourless |
| C6H13ClO | 23.201 | 0.14 | Chlorohexanol | Alcohols | n.a | Odorless |
| C10H22 | 24.654 | 0.13 | 3,3,5-Trimethylheptane | Hydrocarbons | n.a | Gasoline-like |
| C7H7NO2 | 24.979 | 0.13 | Anthranilic acid | Other compounds | L-tryptophan-kynurenine | Odorless |
| C9H18O | 20.273 | 0.12 | Nonanal | Aldehydes | n.a | Fruity, waxy |
| C8H10O2 | 23.461 | 0.11 | Tyrosol | Other compounds | Tyrosine metabolism | Floral, phenolic |
| C16H32 | 23.812 | 0.1 | 1-Dodecanol | Alcohols | n.a | Waxy, fatty |
| C20H41Cl | 25.336 | 0.1 | 1-chloroeicosane | Hydrocarbons | n.a | n.a |
| C13H22O | 23.68 | 0.09 | Geranylacetone | Ketones | Ketone Body Metabolism | Sweet, floral, fruity |
| C20 H42 | 23.201 | 0.08 | Eicosane | Hydrocarbons | n.a | Odourless |
n.a—data not available.
3.2. Volatiles Profile by GC–MS
Volatile profiles, particularly in mushrooms and truffles, are crucial in determining their characteristic odours and strongly influence consumers’ preferences. T. arenaria’s distinct volatile profile serves as a key characteristic and significantly impacts consumers’ preferences.
Thirty-two VOCs were identified in T. arenaria fresh samples, i.e., eight hydrocarbons, six alcohols, five aldehydes, three ketones, three esters, two terpenes, and five other compounds. Among them, the most abundant were the eight carbon (C-8) compounds and Hexanal, with 1-octen-3-ol being the main volatile (64%) in T. arenaria (see Tables S3 and S4). 1-Octen-3-ol is generally referred to as the mushroom alcohol and is one of the most abundant VOCs produced by fungi [,]. This is consistent with the fact that C-8 compounds are the main volatile components found in several edible mushrooms and truffles [,,,] (Tables S3 and S4). Despite using similar methodologies, Harki et al. (2010) identified 27 VOCs in T. arenaria, but only three compounds were common with our study (nonanal, 3-octanone and 2-octenal) []. Both internal factors (e.g., maturity stage, tissue specificity and postharvest storage []) and external factors (e.g., place of origin, interaction with microorganisms []), result in distinct metabolic processes within the fungi, which alters their VOCs profile. The main volatiles identified in T. arenaria were C-8 compounds resulting from the breakdown of fatty acids (such as linoleic acid) by lipoxygenase and related enzymes (Table 3), which is in agreement with the evidence that the umami taste, so characteristic of mushrooms, is associated with the fatty acid metabolism []. Lipoxygenases are pivotal in the biosynthesis of leukotrienes, associated with various inflammatory conditions such as cancer, arthritis, asthma, and allergies []. Given their role in these disease processes, lipoxygenase inhibitors are being explored as potential therapeutic options for preventing and managing these inflammatory disorders [,]. Notably, certain mushroom extracts have demonstrated the ability to inhibit these enzymes, offering potential health benefits [,,]. These extracts have been used to enhance the nutritional value of pasta, contributing to healthier products [,]. Exploring the potential inhibitor of Terfezia may be necessary for incorporating this product in food formulations.
Nine VOCs identified in T. arenaria (i.e., 1-octen-3-ol, 3-octanol, 3-octanone, 2-octenal, hexanal, nonanal, benzeneacetaldehyde, eicosane, limonene and α-pinene) had been previously reported for the desert truffles T. boudieri and T. claveryi [,,]. These compounds are also prevalent in commercial edible mushrooms and truffles such as A. bisporus, L. edodes, P. ostreatus and T. melanosporum [,,,]. On the other hand, 18 VOCs detected in T. arenaria, had not been reported for other Terfezia spp. or A. bisporus, L. edodes, P. ostreatus and T. melanosporum (Table S4). Additionally, a review of the reported VOCs composition of these species revealed that only three compounds are common between T. arenaria and these four commercial species (Figure 3). These VOCs, which are C-8 compounds (i.e., 1-octen-3-ol, 3-octanol, 3-octanone), are abundant in A. bisporus, L. edodes, P. ostreatus and T. arenaria and greatly contribute to their floral and green aromas [] (Table 2 and Table S3). They are also present in T. melanosporum, but in lower quantities [,].
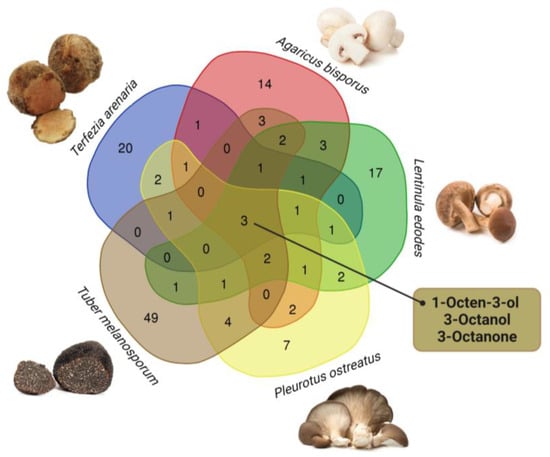
Figure 3.
A Venn diagram comparing T. arenaria’s VOCs profile with that reported in the literature for A. bisporus, L. edodes, P. ostreatus and T. melanosporum (http://bioinformatics.psb.ugent.be/webtools/Venn/ (accessed on 2 August 2023)).
When comparing T. arenaria’s aroma profile with that from other edible mushrooms and truffles (A. bisporus, L. edodes, P. ostreatus, and T. melanosporum) (Figure 3, Table S4), T. arenaria exhibits a distinct composition. Figure 4 highlights potential differences between T. arenaria and the other edible mushrooms and truffles in terms of the number and quantity of compounds per main functional group. In T. arenaria, there is a higher presence of alcohol compounds compared to A. bisporus and T. melanosporum, although it is less diverse in terms of the number of compounds. Unlike L. edodes and T. melanosporum, T. arenaria does not contain detectable acids or sulphur compounds, making it stand apart in its aromatic profile. The latter two species have a greater variety of compounds, indicating a more complex volatile profile than T. arenaria.
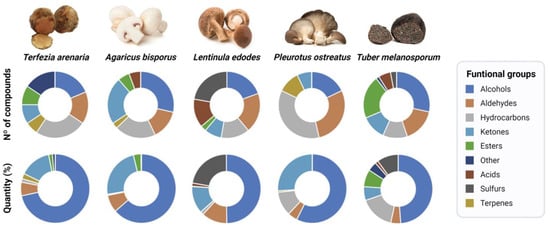
Figure 4.
Aromatic profiles of the five edible mushroom and truffles species as shown by the number and identity of the functional groups of compounds and their relative proportion. The pie charts present the number of identified compounds and their relative proportion (%) per group in each mushroom and truffle species.
On the other hand, A. bisporus and P. ostreatus demonstrate simpler volatile profiles, with only five main functional groups identified. Among all the analysed edible mushrooms and truffles, A. bisporus appears to have the most similar aromatic profile to T. arenaria. Despite the high similarity between the VOCs profiles of T. arenaria and A. bisporus (Figure 4), the e-nose successfully distinguished between the volatile profiles these fungal species [,,,,].
3.3. First Steps in Developing a Non-Destructive and Rapid Identification Method for T. arenaria
Electronic noses have been coupled with gas chromatography–mass spectrometry (GC–MS) to analyse aromas in various food products []. This technique has gained prominence due to its versatility and speed of response. Although GC–MS is a more precise and accurate technique, it is also more complex, expensive, and time-consuming. For example, in this work, analysing only three samples using GC–MS required one week, including sample preparation, sample reading, and data processing. In contrast, using an e-nose, after the initial equipment training (which takes about 4 h), a quick response can be obtained within one to two hours (including sample preparation, incubation, and equipment reading).
3.3.1. Phase 1: Electronic Nose Training
As the Cyranose-320 e-nose showed sensitivity to the pre-analysis incubation temperature (40 °C or 24 °C), temperature influenced the volatile compounds emitted by T. arenaria fresh samples (Figure 5). The Cyranose-320 correctly classified 73% of the T. areanaria samples incubated at room temperature, and 81% of the T. areanaria samples incubated at 40 °C. All 32 sensors of the Cyranose-320 e-nose signalled the emitted VOCs for pre-analysis incubation temperatures (40 °C and 24 °C). From those, five sensors had major responses in the two pre-analysis incubation temperatures (i.e., S5, S6, S23, S28, and S31), and sensor number 31 showed the highest sensitivity (Figure 5a). The pre-analysis incubation temperature influenced these sensors (i.e., S5, S6, S23, S28, and S31; p < 0.001), with higher responses at the higher temperature. The first two principal components (PC1 and PC2) explained 95.1% of the total variance (92.5% and 2.6%, respectively), which means that the incubation temperature has a significant effect on the volatile compounds emitted by T. arenaria (Figure 5b). Similar results were reported for A. bisporus (92% and 99%) stored at different temperatures []; Tuber indicum (100%) under different drying processes []; and Thricholoma matsutake (90%) from different provenience regions. This confirms the importance of the pre-analysis temperature (i.e., storage temperature) and the sensitivity of the e-nose methodology to detect the volatile profile of different mushroom and truffle species. The score plot (Figure 5b) showed that the aroma profile of T. arenaria subjected to different pre-analysis incubation temperatures can be discriminated and the two clusters were very similar in terms of their volatile compound content (Figure 5a).
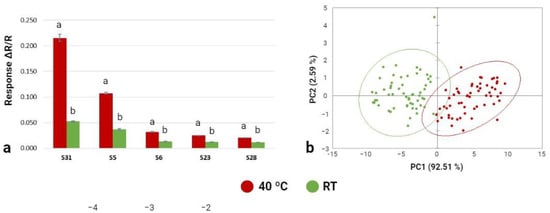
Figure 5.
Effect of the pre-analysis incubation temperature (40 °C and RT) on the Cyranose-320 sensor’s response to T. arenaria fresh samples. (a) Histogram of the 5 sensors showing larger responses to T. arenaria samples. Different letters show a significant effect (p < 0.05) of the pre-analysis incubation temperature on the sensors responses. (b) Plot of the first two principal components of the PCA model built with the Cyranose-320 data related to T. arenaria samples at 40 °C and RT.
3.3.2. Phase 2: E-Nose Identification Accuracy
Based on their volatile profile, the e-nose Cyranose-320 accurately recognized the T. arenaria samples (Terf1, Terf2 or Terf3) and rated their result with stars (Table S5). Although all T. arenaria samples were identified as one of the trained volatile profiles in both pre-analysis incubation temperatures, the pre-analysis incubation temperature influenced the identification accuracy. So, when the pre-analysis incubation was performed at room temperature, the identification of 80% of the samples was acceptable or excellent, while when the pre-analysis incubation was performed at 40 °C, only 45% of the samples were acceptable or excellent, and considered accurately identified (Table S5). Despite this trend, the pre-analysis incubation temperature did not affect the T. arenaria samples’ identification accuracy (p < 0.05) (Figure 6). Nevertheless, pre-analysis incubation at room temperature improved the e-nose’s capacity to distinguish T. arenaria samples from those of the other edible mushrooms and truffles (A. bisporus, L. edodes, P. ostreatus and T. melanosporum) (Figure 6).
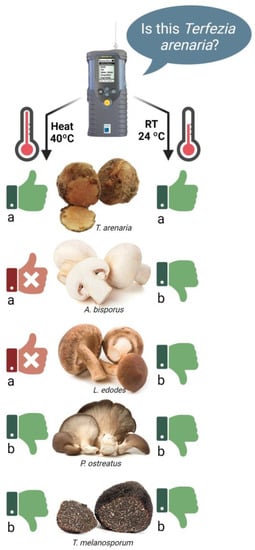
Figure 6.
Effect of the pre-analysis incubation temperature (40 °C and RT) on the e-nose Cyranose-320 capacity to accurately identify T. arenaria, and distinguish it from A. bisporus, L. edodes, P. ostreatus and T. melanosporum. The “thumbs up” symbol represents the cases when the e-nose identified the sample as being T. arenaria, while the “thumbs down” symbol represents the cases when the e-nose identified the sample as not being T. arenaria. Green thumbs indicate an accurate identification, while red thumbs indicate an inaccurate identification by the e-nose. Different letters show a significant effect (p < 0.05) of the pre-analysis incubation temperature on the identification accuracy for each species.
Indeed, when the pre-analysis incubation was performed at 40 °C, some samples were misclassified as T. arenaria (Figure 6), specifically some A. bisporus and L. edodes samples were identified with a 100% probability of being T. arenaria (5 stars). The pre-analysis incubation temperature affected the e-nose’s capacity to distinguish between T. arenaria and A. bisporus and L. edodes but not P. ostreatus and T. melanosporum (p < 0.05).
Most of the studies reporting the use of the e-nose technology on mushrooms and truffles focused on sample quality as influenced by dehydration [,,], shelf life and packaging [,], and maturation process []. Some studies also explored the differentiation of species by analysing their volatile profiles using the e-nose [,,,,]. By applying the e-nose methodology for a rapid and non-destructive accurate identification of T. arenaria samples, we demonstrate our approach’s potential for mushrooms and truffles identification during harvest. It is important to keep in mind that T. arenaria is traditionally harvested near the host plant (Tuberaria guttata), with the collector using a pointed stick to repeatedly explore the soil until a truffle is detected and extracted []. Therefore, the development of a tool based on our non-destructive and rapid methodology could contribute to the early detection of Terfezia truffles in the field, potentially discriminating between maturity stages and sample quality, which would contribute to the much-needed technology to boost Terfezia’s sustainable production and ensure its reliable identification. Furthermore, this could be useful for other truffles whose belowground development makes it difficult to detect them and distinguish maturity stages and quality []. As the VOCs emitted by mushrooms and truffles are important to the food industry, especially for developing new food products or even for new tools that could contribute to food security, our study contributes to unlock many possibilities for using this delicacy (T. arenaria) in the food industry worldwide.
4. Conclusions
From a nutritional standpoint, T. arenaria is a well-balanced food, rich in carbohydrates, fibres, and proteins, while containing a low-fat content. It is also a good source of minerals, including lithium, selenium, and iron. Furthermore, it has a unique aroma dominated by the C8-compounds produced in the lipoxygenase pathway. Twenty nine new volatile organic compounds (VOCs) were identified for T. arenaria, from which the C8-compounds produced in the lipoxygenase pathway were predominant. Further analysis is required to understand the variations attributed to the specific internal and external factors and how these regulate fatty acid metabolism. In addition to the importance of defining T. arenaria’s aromatic profile, this metabolic pathway is also related to the umami taste, essential for the development of plant-based meat.
The e-nose Cyranose-320 accurately identified T. arenaria samples (especially when samples were incubated at room temperature before analysis) and was able to distinguish T. arenaria from other edible mushrooms and truffles (A. bisporus, L. edodes, P. ostreatus and T. melanosporum). The E-nose Cyranose-320 was more accurate when mushroom and truffle samples were pre-incubated at room temperature than at 40 °C. Our data point the e-nose’s great potential as a rapid and non-destructive detection tool for identifying T. arenaria and possibly other mushroom and truffle species. However, larger datasets (more samples and in different maturity stages) are necessary to fine-tune the parameter settings of the Cyranose-320 and optimize the identification process.
Altogether, T. arenaria is a nutritious and sustainable food that has the potential to be used in a variety of new food products. It is a good source of protein and minerals, and it has a Nutri-Score of A. T. arenaria could be used as a meat substitute or as an ingredient in plant-based meat products. Studying the nutritional composition and volatile profile of T. arenaria provides valuable insights into its potential as a nutritious food source. The application of the electronic nose technology enhances our ability to identify and authenticate the unique aroma profiles of these desert truffles. Moreover, this study unveils the e-nose’s potential for the early detection of T. arenaria in the field, which could contribute to the sustainable production of this delicacy. The electronic nose could also be used to distinguish between maturity stages and quality of T. arenaria, which would be valuable for ensuring the quality of this food product. This knowledge contributes to advancements in food science and technology, supporting the development of quality control measures and ensuring the authenticity of food products in the market.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12193527/s1, Figure S1: Map of the sampling areas of Tefezia arenaria collected during this study, and forest-dominant species per area. Table S1: Parameters settings for using the Cyranose-320; Table S2: Dietary Reference Intakes for nutrients and elements; Table S3: Comparison of the abundance of the main VOCs identified in T. arenaria and in the other edible mushroom and truffle species; Table S4: Characterization of the VOCs identified in T. arenaria, and in the other edible mushroom and truffle species (A. bisporus, L. edodes, P. ostreatus and T. melanosporum); Table S5: Results and rates of Cyranose-320 identification of T. arenaria, A. bisporus, L. edodes, P. ostreatus and T. melanosporum with 40 °C and RT pre-analysis incubation temperatures.
Author Contributions
Conceptualization, I.F. and C.C.; methodology, I.F.; formal analysis, I.F.; investigation, I.F., T.D. and C.C.; resources, C.C.; writing—original draft preparation, I.F.; writing—review and editing, T.D., A.M.M. and C.C.; visualization, T.D.; supervision, A.M.M. and C.C.; project administration, T.D., A.M.M. and C.C.; funding acquisition, I.F. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by: (i) Portuguese funds through Fundação para a Ciência e a Tecnologia (project UIDB/00329/2020, PhD Grant SFRH/BD/131232/2017 to IF, and researcher contract to TD); and (ii) European Union’s Horizon 2020 Research and Innovation programme SOILdarity under grant agreement No. 952051.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Acknowledgments
The authors would like to acknowledge the technical support provided by Juliana Melo. The authors would like to thank the two anonymous reviewers for taking the time to assess our manuscript, for their attentive analysis and for the positive comments that helped improve this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Köberle, A.C. Food Security in Climate Mitigation Scenarios. Nat. Food 2022, 3, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Corrêa, A.; Cruz, C. Sustainable Production of Ectomycorrhizal Fungi in the Mediterranean Region to Support the European Green Deal. Plants People Planet 2023, 5, 14–26. [Google Scholar] [CrossRef]
- Boa, E. Wild Edible Fungi. A Global Overview of Their Use and Importance to People. In Non-Wood Forest Products; FAO: Rome, Italy, 2004; Volume 17, ISBN 9251051577. [Google Scholar]
- Ferreira, I.; Dias, T.; Cruz, C. The Potential of Ectomycorrhizal Fungi to Modulate below and Aboveground Communities May Be Mediated by 1-Octen-3-Ol. J. Fungi 2023, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- de Frutos, P. Changes in World Patterns of Wild Edible Mushrooms Use Measured through International Trade Flows. For. Policy Econ. 2020, 112, 102093. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 4 August 2023).
- Pérez-Moreno, J.; Guerin-Laguette, A.; Rinaldi, A.C.; Yu, F.; Verbeken, A.; Hernández-Santiago, F.; Martínez-Reyes, M. Edible Mycorrhizal Fungi of the World: What is Their Role in Forest Sustainability, Food Security, Biocultural Conservation and Climate Change? Plants People Planet 2021, 3, 471–490. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.; Mortimer, P.; Xu, J.; Karunarathna, S.; Li, H.; Pérez-Moreno, J.; Mortimer, P.; Xu, J.; Karunarathna, S.; Li, H. Global Perspectives on the Ecological, Cultural and Socioeconomic Relevance of Wild Edible Fungi. Stud. Fungi 2021, 6, 408–424. [Google Scholar] [CrossRef]
- Andrino, A.; Navarro-Ródenas, A.; Marqués-Gálvez, J.E.; Morte, A. The Crop of Desert Truffle Depends on Agroclimatic Parameters during Two Key Annual Periods. Agron. Sustain. Dev. 2019, 39, 51. [Google Scholar] [CrossRef]
- Oliach, D.; Morte, A.; Sánchez, S.; Navarro-Ródenas, A.; Marco, P.; Gutiérrez, A.; Martín-Santafé, M.; Fischer, C.; Albisu, L.M.; García-Barreda, S.; et al. Las Trufas y Las Turmas. In Los Productos Forestales no Madereros en España: Del Monte a la Industria; Sánchez-González, M., Calama, R., Bonet, J.A., Eds.; INIA, Ministerio de Economía Industria y Competitividad: Madrid, Spain, 2020; pp. 283–324. ISBN 9788474985856. [Google Scholar]
- Bradai, L.; Neffar, S.; Amrani, K.; Bissati, S.; Chenchouni, H. Ethnomycological Survey of Traditional Usage and Indigenous Knowledge on Desert Truffles among the Native Sahara Desert People of Algeria. J. Ethnopharmacol. 2015, 162, 31–38. [Google Scholar] [CrossRef]
- Shavit, E. The History of Desert Truffle Use; Springer: Berlin/Heidelberg, Germany, 2014; pp. 217–241. [Google Scholar]
- Chevalier, G. The European Desert Truffles. In Desert Truffles. Soil Biology; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 121–141. [Google Scholar] [CrossRef]
- Morte, A.; Arenas, F.; Marqués-Gálvez, J.E.; Andrino, A.; Guarnizo, Á.L.; Gutiérrez, A.; Berná, L.M.; Pérez-Gilabert, M.; Rodríguez, A.; Navarro-Ródenas, A. Desert Truffles (Terfezia spp.) Breeding. In Advances in Plant Breeding Strategies: Vegetable Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 10, pp. 479–504. [Google Scholar] [CrossRef]
- Shavit, E. Truffles Roasting in the Evening Fires. Pages Hist. Desert Truffles Fungi 2008, 1, 18–23. [Google Scholar]
- Gadallah, M.G.E.; Ashoush, I.S. Value Addition on Nutritional and Sensory Properties of Biscuit Using Desert Truffle (Terfezia claveryi) Powder. Food Nutr. Sci. 2016, 7, 1171–1181. [Google Scholar] [CrossRef][Green Version]
- Najjaa, H.; Abdelkbir, R.; Ben Arfa, A.; Doria, E.; Tlili, H.; Zouari, N.; Neffati, M. Improved Sensory Quality and Antioxidant Capacity of Wheat Bread Supplemented with the Desert Truffle Terfezia boudieri Flour. Anal. Lett. 2021, 54, 867–883. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Farag, M.A.; Yosri, N.; Sabir, J.S.M.; Saeed, A.; Al-Mousawi, S.M.; Taha, W.; Musharraf, S.G.; Patel, S.; El-Seedi, H.R. Truffles: From Islamic Culture to Chemistry, Pharmacology, and Food Trends in Recent Times. Trends Food Sci. Technol. 2019, 91, 193–218. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Maggi, L.; Jiménez-Monreal, A.M.; Murcia, M.A.; Marí, J.A.T. Nutritional and Antioxidant Properties of Terfezia and Picoa; Springer: Berlin/Heidelberg, Germany, 2014; pp. 261–273. [Google Scholar]
- Hamza, A.; Zouari, N.; Zouari, S.; Jdir, H.; Zaidi, S.; Gtari, M.; Neffati, M. Nutraceutical Potential, Antioxidant and Antibacterial Activities of Terfezia boudieri Chatin, a Wild Edible Desert Truffle from Tunisia Arid Zone. Arab. J. Chem. 2016, 9, 383–389. [Google Scholar] [CrossRef]
- Kıvrak, İ. Analytical Methods Applied to Assess Chemical Composition, Nutritional Value and In Vitro Bioactivities of Terfezia olbiensis and Terfezia claveryi from Turkey. Food Anal. Methods 2015, 8, 1279–1293. [Google Scholar] [CrossRef]
- Al Obaydi, M.F.; Hamed, W.M.; Al Kury, L.T.; Talib, W.H. Terfezia boudieri: A Desert Truffle With Anticancer and Immunomodulatory Activities. Front. Nutr. 2020, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Calvo, E.; Amara, K.; Reis, F.S.; Barros, L.; Martins, A.; Calhelha, R.C.; Venturini, M.E.; Blanco, D.; Redondo, D.; Marco, P.; et al. Chemical Composition and Evaluation of Antioxidant, Antimicrobial and Antiproliferative Activities of Tuber and Terfezia Truffles. Food Res. Int. 2021, 140, 110071. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Mohamed, M.A.; Hami, M.A. Libyan Truffles Terfezia boudieri Chatin: Chemical Composition and Toxicity. J. Food Sci. 1981, 46, 927–929. [Google Scholar] [CrossRef]
- Amara, K.; Reis, F.S.; Barros, L.; Skhiri, F.; Martins, A.; Ferreira, I.C.F.R. Nutritional Values, Chemical Characterization and Cytotoxicity in Human Tumor Cell Lines of Desert Truffles. In Proceedings of the 8th Journées Scientifiques Internationales sur la Valorisation des Bioressources, Monastir, Tunisia, 5–7 May 2017. [Google Scholar]
- Benaceur, F.; Chaibi, R.; Berrabah, F.; Neifar, A.; Leboukh, M.; Benaceur, K.; Nouioua, W.; Rezzoug, A.; Bouazzara, H.; Gouzi, H.; et al. Purification and Characterization of Latent Polyphenol Oxidase from Truffles (Terfezia arenaria). Int. J. Biol. Macromol. 2020, 145, 885–893. [Google Scholar] [CrossRef]
- Harir, M.; Bendif, H.; Yahiaoui, M.; Bellahcene, M.; Zohra, F.; Rodríguez-Couto, S. Evaluation of Antimicrobial Activity of Terfezia arenaria Extracts Collected from Saharan Desert against Bacteria and Filamentous Fungi. 3 Biotech 2019, 9, 281. [Google Scholar] [CrossRef]
- Moreno, G.; Alvarado, P.; Manjón, J.L. Hypogeous Desert Fungi. In Desert Truffles: Phylogeny, Physiology, Distribution and Domestication; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–20. [Google Scholar]
- Ammarellou, A.; Wang, Y.; Nematzadeh, G.; Tajick, M. Non-Mediterranean Asian Desert Countries; Springer: Berlin/Heidelberg, Germany, 2014; pp. 173–192. [Google Scholar]
- Dafri, A.; Beddiar, A. Morphological Characterisation of the Mycorrhizal Symbiosis between Tuberaria guttata (L.) Fourr and Terfezia arenaria (Moris) Trappe. Symbiosis 2018, 75, 149–154. [Google Scholar] [CrossRef]
- Brenko, A.; Vidale, E.; Oliach, D.; Marois, O.; Andrighetto, N.; Stara, K.; de Aragón, J.M.; Bonet, J.A. Short Communication: Edible Wild Mushrooms of the Northern Mediterranean Area–Sectorial Analysis and Future Perspectives. For. Syst. 2022, 31, eSC05. [Google Scholar] [CrossRef]
- Egli, S.; Peter, M.; Buser, C.; Stahel, W.; Ayer, F. Mushroom Picking Does Not Impair Future Harvests–Results of a Long-Term Study in Switzerland. Biol. Conserv. 2006, 129, 271–276. [Google Scholar] [CrossRef]
- Peintner, U.; Schwarz, S.; Mešić, A.; Moreau, P.-A.; Moreno, G.; Saviuc, P. Mycophilic or Mycophobic? Legislation and Guidelines on Wild Mushroom Commerce Reveal Different Consumption Behaviour in European Countries. PLoS ONE 2013, 8, 63926. [Google Scholar] [CrossRef] [PubMed]
- Harki, E.; Farah, A.; Bouseta, A. Volatile Compounds from Four Species of Moroccan Truffles. Vice Ed. Chief Vice Redacteur Chef 2010, 12, 10. [Google Scholar]
- Farag, M.A.; Fathi, D.; Shamma, S.; Shawkat, M.S.A.; Shalabi, S.M.; El Seedi, H.R.; Afifi, S.M. Comparative Metabolome Classification of Desert Truffles Terfezia claveryi and Terfezia boudieri via Its Aroma and Nutrients Profile. LWT 2021, 142, 111046. [Google Scholar] [CrossRef]
- Kamle, M.; Bar, E.; Lewinsohn, D.; Shavit, E.; Roth-Bejerano, N.; Kagan-Zur, V.; Barak, Z.; Guy, O.; Zaady, E.; Lewinsohn, E.; et al. Characterization of Morphology, Volatile Profiles, and Molecular Markers in Edible Desert Truffles from the Negev Desert. J. Agric. Food Chem. 2017, 65, 2977–2983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wen, Y.; Wu, W.; Zhang, L.; Salman Farid, M.; Shan, S.; Wen, J.; Farag, M.A.; Zhang, Y.; Zhao, C. The Flavors of Edible Mushrooms: A Comprehensive Review of Volatile Organic Compounds and Their Analytical Methods. Crc Cr Rev Food Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Angeloni, S.; Nzekoue, F.K.; Abouelenein, D.; Sagratini, G.; Caprioli, G.; Torregiani, E. An Overview on Truffle Aroma and Main Volatile Compounds. Molecules 2020, 25, 5948. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. GC–MS Based Metabolomics Used for the Identification of Cancer Volatile Organic Compounds as Biomarkers. J. Pharm. Biomed. Anal. 2018, 147, 313–322. [Google Scholar] [CrossRef]
- Zhou, Y.; Abbas, F.; Wang, Z.; Yu, Y.; Yue, Y.; Li, X.; Yu, R.; Fan, Y. HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules 2021, 26, 5425. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, T.; Ye, R. Differentiation of Eight Commercial Mushrooms by Electronic Nose and Gas Chromatography-Mass Spectrometry. J. Sens. 2015, 2015, 374013. [Google Scholar] [CrossRef]
- Guo, Q.; Adelina, N.M.; Hu, J.; Zhang, L.; Zhao, Y. Comparative Analysis of Volatile Profiles in Four Pine-Mushrooms Using HS-SPME/GC-MS and E-Nose. Food Control 2022, 134, 108711. [Google Scholar] [CrossRef]
- Gholami, R.; Aghili Nategh, N.; Rabbani, H. Evaluation the Effects of Temperature and Packaging Conditions on the Quality of Button Mushroom during Storage Using E-Nose System. J. Food Sci. Technol. 2023, 60, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hu, Z.; Liang, M.; Song, L.; Wu, W.; Li, R.; Li, Z.; Zhang, J. Evaluation of the Flavor Compounds of Pleurotus eryngii as Affected by Baking Temperatures Using HS-SPME-GC–MS and Electronic Nose. J. Food Process. Preserv. 2022, 46, e17056. [Google Scholar] [CrossRef]
- Chilo, J.; Pelegri-Sebastia, J.; Cupane, M.; Sogorb, T. E-Nose Application to Food Industry Production. IEEE Instrum. Meas. Mag. 2016, 19, 27–33. [Google Scholar] [CrossRef]
- Falasconi, M.; Concina, I.; Gobbi, E.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Electronic Nose for Microbiological Quality Control of Food Products. Int. J. Electrochem. 2012, 2012, 715763. [Google Scholar] [CrossRef]
- Mota, I.; Teixeira-Santos, R.; Cavaleiro Rufo, J. Detection and Identification of Fungal Species by Electronic Nose Technology: A Systematic Review. Fungal Biol. Rev. 2021, 37, 59–70. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the Two Drying Approaches on the Volatile Profiles of Button Mushroom (Agaricus bisporus) by Headspace GC–MS and Electronic Nose. LWT Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Li, M.; Hao, T.; Lin, S. The Dynamic Changes in Product Attributes of Shiitake Mushroom Pilei and Stipes during Dehydration by Hot Air Drying. J. Food Process. Preserv. 2021, 45, e15648. [Google Scholar] [CrossRef]
- Ma, N.; Pei, F.; Yu, J.; Wang, S.; Ho, C.T.; Su, K.; Hu, Q. Valid Evaluation of Volatile Flavor Composition of Fresh and Dehydrated Tuber indicum with Different Drying Methods. CyTA J. Food 2018, 16, 413–421. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Q.; Wu, Y.; Pei, F.; Kimatu, B.M.; Su, A.; Yang, W. Storage Time Assessment and Shelf-Life Prediction Models for Postharvest Agaricus bisporus. LWT 2019, 101, 360–365. [Google Scholar] [CrossRef]
- Portalo-Calero, F.; Arroyo, P.; Suárez, J.I.; Lozano, J. Triangular Test of Amanita Mushrooms by Using Electronic Nose and Sensory Panel. Foods 2019, 8, 414. [Google Scholar] [CrossRef]
- Portalo-Calero, F.; Lozano, J.; Meléndez, F.; Arroyo, P.; Suárez, J.I. Identification of Poisonous Mushrooms by Means of a Hand-Held Electronic Nose. Proceeding 2019, 14, 33. [Google Scholar] [CrossRef]
- Keshri, G.; Challen, M.; Elliott, T.; Magan, N. Differentiation of Agaricus Species and Other Homobasidiomycetes Based on Volatile Production Patterns Using an Electronic Nose System. Mycol. Res. 2003, 107, 609–613. [Google Scholar] [CrossRef]
- Gómez, I.; Lavega González, R.; Tejedor-Calvo, E.; Pérez Clavijo, M.; Carrasco, J. Odor Profile of Four Cultivated and Freeze-Dried Edible Mushrooms by Using Sensory Panel, Electronic Nose and GC-MS. J. Fungi 2022, 8, 953. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of Electronic Nose and Its Application in Fresh Foods: A Review. Crit. Rev. Food Sci. Nutr. 2017, 58, 2700–2710. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC Official Methods of Analysis, 15th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 1990; pp. 136–138. [Google Scholar]
- Mędyk, M.; Chudzińska, M.; Barałkiewicz, D.; Falandysz, J. Specific Accumulation of Cadmium and Other Trace Elements in Sarcodon imbricatus Using ICP-MS with a Chemometric Approach. J Environ Sci Heal B 2017, 52, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Medicine, I. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar]
- SCHER Assessment of the Tolerable Daily Intake of Barium; European Commission Director General Health and Consumer: Brussels, Belgium, 2012.
- Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [CrossRef]
- Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14, e15121E. [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Peláez, C.; et al. Scientific Opinion on the Tolerable Upper Intake Level for Selenium. EFSA J. 2023, 21, e07704. [Google Scholar] [CrossRef]
- Nutri-Score. Available online: https://www.santepubliquefrance.fr/en/nutri-score (accessed on 9 August 2023).
- Splivallo, R.; Ebeler, S.E. Sulfur Volatiles of Microbial Origin Are Key Contributors to Human-Sensed Truffle Aroma. Appl. Microbiol. Biotechnol. 2015, 99, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Sensigents. Available online: https://www.sensigent.com/products/cyranose.html (accessed on 5 June 2023).
- Santos, J.P.; Lozano, J.L.; Aleixandre, M.; Sayago, I.; Fernández, M.J.; Arés, L.; Gutiérrez, J.; Horrillo, M.D.C. Discrimination of Different Aromatic Compounds in Water, Ethanol and Wine with a Thin Film Sensor Array. Sens. Actuators B Chem. 2004, 103, 98–103. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martínez-Tomé, M.; Vera, A.; Morte, A.; Gutierrez, A.; Honrubia, M.; Jiménez, A.M. Effect of Industrial Processing on Desert Truffles Terfezia claveryi Chatin and Picoa juniperi Vittadini): Proximate Composition and Fatty Acids. J. Sci. Food Agric. 2003, 83, 535–541. [Google Scholar] [CrossRef]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional Value and Biological Properties of Chilean Wild and Commercial Edible Mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, I.; Mendiola-Lanao, M.; Pérez-Clavijo, M.; Delgado-Andrade, C. Effect of Different Cooking Methods on Nutritional Value and Antioxidant Activity of Cultivated Mushrooms. Int J Food Sci Nutr. 2016, 68, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ekute, B. Nutritional Profile of Two Nigerian Edible Mushrooms: Pleurotus ostreatus and Pleurotus pulmonarius. J. Appl. Sci. Environ. Manag. 2019, 22, 1745–1747. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, M.; Zhang, B.; Wu, H.; Zhang, Y.; Zhang, L. Analysis of Nutritional Composition in 23 Kinds of Edible Fungi. J. Food Qual. 2020, 2020, 8821315. [Google Scholar] [CrossRef]
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Rybakowski, J.K.; Ferensztajn-Rochowiak, E. Mini-Review: Anomalous Association between Lithium Data and Lithium Use. Neurosci. Lett. 2022, 777, 136590. [Google Scholar] [CrossRef]
- Siwulski, M.; Niedzielski, P.; Budka, A.; Budzyńska, S.; Kuczyńska-Kippen, N.; Kalač, P.; Sobieralski, K.; Mleczek, M. Patterns of Changes in the Mineral Composition of Agaricus Bisporus Cultivated in Poland between 1977 and 2020. J. Food Compos. Anal. 2022, 112, 104660. [Google Scholar] [CrossRef]
- Siwulski, M.; Budka, A.; Budzyńska, S.; Gąsecka, M.; Kalač, P.; Niedzielski, P.; Mleczek, M. Mineral Composition of Traditional and Organic-Cultivated Mushroom Lentinula edodes in Europe and Asia–Similar or Different? LWT 2021, 147, 111570. [Google Scholar] [CrossRef]
- Kalac, P. Mineral Composition and Radioactivity of Edible Mushrooms; Academic Press: Cambridge, MA, USA, 2019; ISBN 0128176067. [Google Scholar]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in Edible Mushrooms: An Inter-Species Comparative Study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 5–13. [Google Scholar]
- Reyna, S.; Garcia-Barreda, S. Black Truffle Cultivation: A Global Reality. For. Syst. 2014, 23, 317–328. [Google Scholar] [CrossRef]
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013; p. 102. ISBN 9789241506236.
- van den Akker, K.; Bartelet, D.; Brouwer, L.; Luijpers, S.; Nap, T.; Havermans, R. The Impact of the Nutri-Score on Food Choice: A Choice Experiment in a Dutch Supermarket. Appetite 2022, 168, 105664. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, R. A Review on Nutritional Advantages of Edible Mushrooms and Its Industrialization Development Situation in Protein Meat Analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Kwasny, T.; Dobernig, K.; Riefler, P. Towards Reduced Meat Consumption: A Systematic Literature Review of Intervention Effectiveness, 2001–2019. Appetite 2022, 168, 105739. [Google Scholar] [CrossRef] [PubMed]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Guinard, J.X.; Myrdal Miller, A.; Mills, K.; Wong, T.; Lee, S.M.; Sirimuangmoon, C.; Schaefer, S.E.; Drescher, G. Consumer Acceptance of Dishes in Which Beef Has Been Partially Substituted with Mushrooms and Sodium has been Reduced. Appetite 2016, 105, 449–459. [Google Scholar] [CrossRef]
- Li, J.; Silver, C.; Gómez, M.I.; Milstein, M.; Sogari, G. Factors Influencing Consumer Purchase Intent for Meat and Meat Substitutes. Future Foods 2023, 7, 100236. [Google Scholar] [CrossRef]
- Lang, M. Consumer Acceptance of Blending Plant-Based Ingredients into Traditional Meat-Based Foods: Evidence from the Meat-Mushroom Blend. Food Qual. Prefer. 2020, 79, 103758. [Google Scholar] [CrossRef]
- De Cianni, R.; Pippinato, L.; Mancuso, T. A Systematic Review on Drivers Influencing Consumption of Edible Mushrooms and Innovative Mushroom-Containing Products. Appetite 2023, 182, 106454. [Google Scholar] [CrossRef] [PubMed]
- Food Data Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1999627/nutrients (accessed on 11 May 2023).
- Koutrotsios, G.; Danezis, G.; Georgiou, C.; Zervakis, G.I. Elemental Content in Pleurotus Ostreatus and Cyclocybe cylindracea Mushrooms: Correlations with Concentrations in Cultivation Substrates and Effects on the Production Process. Molecules 2020, 25, 2179. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Zakil, F.; Xuan, L.H.; Zaman, N.; Alan, N.I.; Salahutheen, N.A.A.; Sueb, M.S.M.; Isha, R. Growth Performance and Mineral Analysis of Pleurotus Ostreatus from Various Agricultural Wastes Mixed with Rubber Tree Sawdust in Malaysia. Bioresour. Technol. Rep. 2022, 17, 100873. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2018, 43, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, T.; Kinoshita, A.; Kusumoto, N.; Nakano, S.; Nakamura, N.; Yamanaka, T. Component Features, Odor-Active Volatiles, and Acute Oral Toxicity of Novel White-Colored Truffle Tuber japonicum Native to Japan. Food Sci. Nutr. 2020, 8, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Rodriguez, E.; Rivera-Macias, L.E.; Adame-Alvarez, R.M.; Torres, J.M.; Heil, M. Shared Weapons in Fungus-Fungus and Fungus-Plant Interactions? Volatile Organic Compounds of Plant or Fungal Origin Exert Direct Antifungal Activity In Vitro. Fungal Ecol. 2018, 33, 115–121. [Google Scholar] [CrossRef]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Eight-Carbon Volatiles in Mushrooms and Fungi: Properties, Analysis, and Biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Maga, J.A. Mushroom Flavor. J. Agric. Food Chem. 1981, 29, 1–4. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, J.; Zhang, Y.R.; Liu, Q.; Pan, L.Q.; Tu, K. Discrimination of Volatiles of Shiitakes (Lentinula Edodes) Produced during Drying Process by Electronic Nose. Int. J. Food Eng. 2020, 16, 20190233. [Google Scholar] [CrossRef]
- Feng, T.; Yang, M.; Ma, B.; Zhao, Y.; Zhuang, H.; Zhang, J.; Chen, D. Volatile Profiles of Two Genotype Agaricus Bisporus Species at Different Growth Stages. Food Res. Int. 2021, 140, 109761. [Google Scholar] [CrossRef]
- Tagkouli, D.; Bekiaris, G.; Pantazi, S.; Anastasopoulou, M.E.; Koutrotsios, G.; Mallouchos, A.; Zervakis, G.I.; Kalogeropoulos, N. Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Cultivated on Agricultural and Agro-Industrial by-Products. Foods 2021, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, Y.; Kobayashi, D.; Sato, R.; Hayashi, S.; Joh, T. Variations in 1-Octen-3-Ol and Lipoxygenase Gene Expression in the Oyster Mushroom Pleurotus Ostreatus According to Fruiting Body Development, Tissue Specificity, Maturity, and Postharvest Storage. Mycoscience 2019, 60, 170–176. [Google Scholar] [CrossRef]
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle Volatiles: From Chemical Ecology to Aroma Biosynthesis. New Phytol. 2011, 189, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.B.; Zhang, Z.Y.; Xin, G.; Sun, B.X.; Bao, X.J.; Wei, Y.Y.; Zhao, X.M.; Xu, H.R. Advances in Umami Taste and Aroma of Edible Mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, R.P. Evaluation of the Antioxidative Properties of Lipoxygenase Inhibitors. Pharmacol. Rep. 2012, 64, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Anti-Inflammatory Effects of Four Psilocybin-Containing Magic Mushroom Water Extracts in Vitro on 15-Lipoxygenase Activity and on Lipopolysaccharide-Induced Cyclooxygenase-2 and Inflammatory Cytokines in Human U937 Macrophage Cells. J. Inflamm. Res. 2021, 14, 3729. [Google Scholar] [CrossRef] [PubMed]
- Mashima, R.; Okuyama, T. The Role of Lipoxygenases in Pathophysiology; New Insights and Future Perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Szymanowska, U.; Tutaj, K.; Domagała, D.; Złotek, U. The Addition of Reishi and Lion’s Mane Mushroom Powder to Pasta Influences the Content of Bioactive Compounds and the Antioxidant, Potential Anti-Inflammatory, and Anticancer Properties of Pasta. Antioxidants 2023, 12, 738. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Szymanowska, U.; Tutaj, K.; Domagała, D.; Złotek, U. Influence of Addition of Dried Maitake and Enoki Mushrooms on Antioxidant, Potentially Anti-Inflammatory, and Anti-Cancer Properties of Enriched Pasta. Appl. Sci. 2023, 13, 8183. [Google Scholar] [CrossRef]
- Darwish, R.S.; Shawky, E.; Nassar, K.M.; Rashad ElSayed, R.M.; Hussein, D.E.; Ghareeb, D.A.; El Sohafy, S.M. Differential Anti-Inflammatory Biomarkers of the Desert Truffles Terfezia claveryi and Tirmania nivea Revealed via UPLC-QqQ-MS-Based Metabolomics Combined to Chemometrics. LWT 2021, 150, 111965. [Google Scholar] [CrossRef]
- Choo, K.S.O.; Bollen, M.; Dykes, G.A.; Coorey, R. Aroma-Volatile Profile and Its Changes in Australian Grown Black Périgord Truffle (Tuber melanosporum) during Storage. Int. J. Food Sci. Technol. 2021, 56, 5762–5776. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Principles and Recent Advances in Electronic Nose for Quality Inspection of Agricultural and Food Products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).