The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition
Abstract
:1. Introduction
2. Harmful Effects of C. sativa on Health
3. Nutritional Aspects
3.1. Protein
3.1.1. Bioactive Peptides
3.1.2. Structural Modification of Hemp Protein
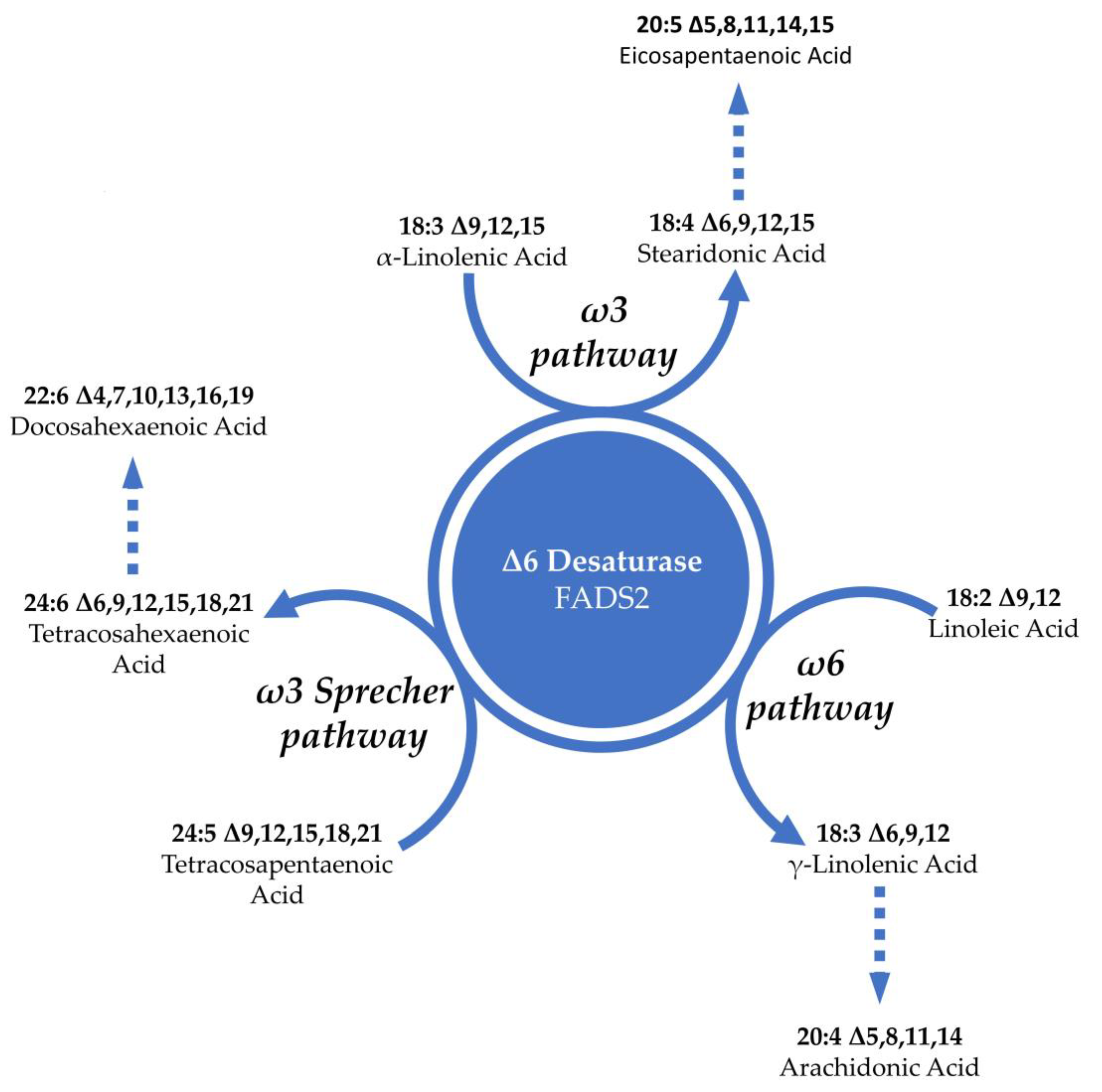
3.2. Lipids
3.3. Dietary Fiber
3.4. Minerals
3.5. Bioactive Molecules
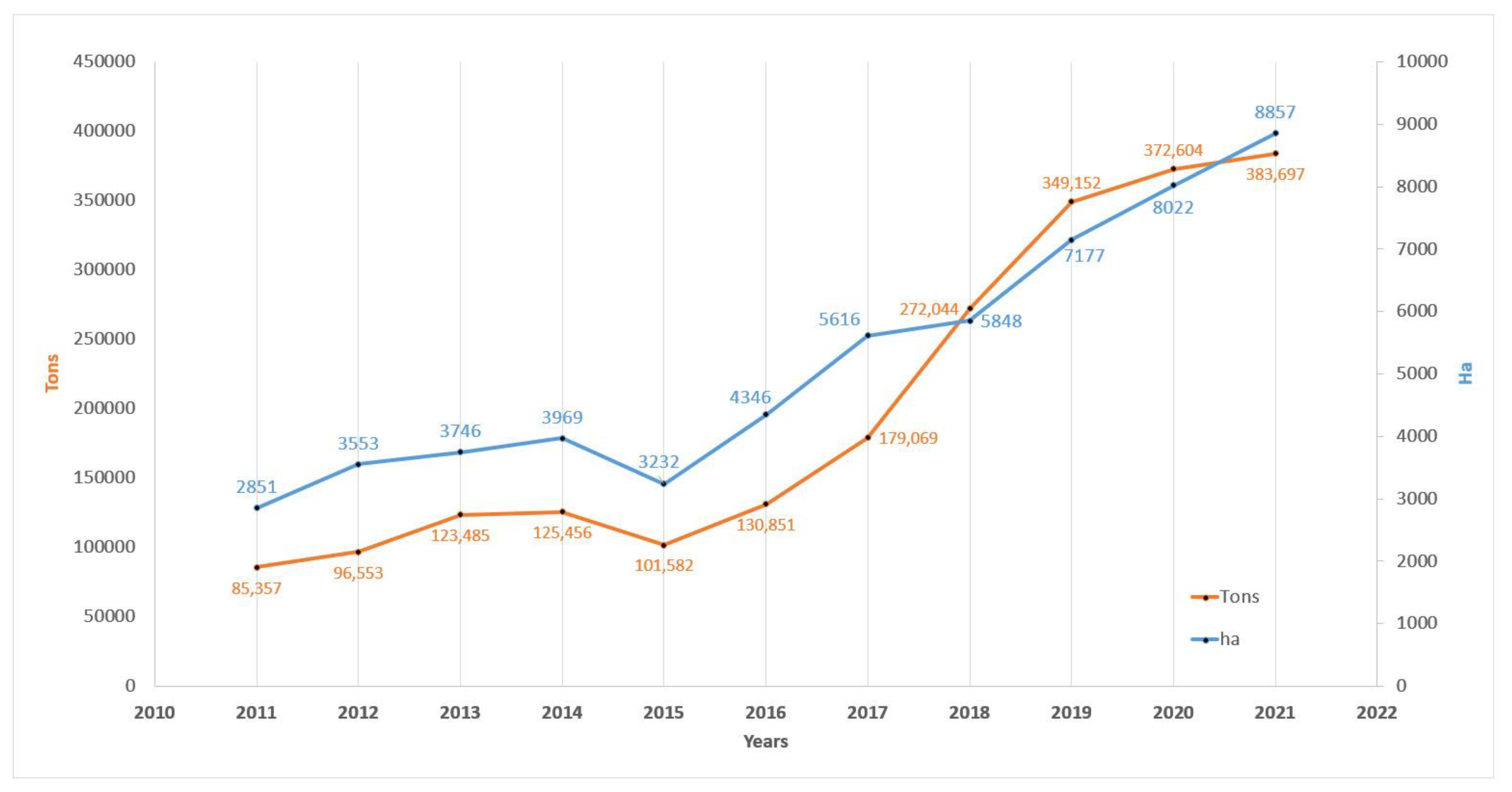
4. Sustainability
5. Hemp Derivatives
- Hemp protein meal (HPM): obtained by crushing hemp seeds after oil extraction.
- Hemp protein concentrate (HPC): obtained from hulled and defatted seeds. It can be obtained from HPM by further processing.
- Hemp protein isolate (HPI): this is the most purified form through extraction techniques that provide chemical–physical processing, aimed at optimizing the precipitation of the proteins and removing the non-protein fractions.
6. Regulatory Aspects
7. Future Perspectives and Research Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.-H.; Deng, G. Evaluation of Hemp (Cannabis sativa L.) as an Industrial Crop: A Review. Environ. Sci. Pollut. Res. Int. 2021, 28, 52832–52843. [Google Scholar] [CrossRef]
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- McPartland, J.M.; Guy, G.W.; Hegman, W. Cannabis Is Indigenous to Europe and Cultivation Began during the Copper or Bronze Age: A Probabilistic Synthesis of Fossil Pollen Studies. Veg. Hist. Archaeobot. 2018, 27, 635–648. [Google Scholar] [CrossRef]
- Mukherjee, A.; Roy, S.C.; De Bera, S.; Jiang, H.-E.; Li, X.; Li, C.-S.; Bera, S. Results of Molecular Analysis of an Archaeological Hemp (Cannabis sativa L.) DNA Sample from North West China. Genet. Resour. Crop Evol. 2008, 55, 481–485. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of Hemp in Textiles, Paper Industry, Insulation and Building Materials, Horticulture, Animal Nutrition, Food and Beverages, Nutraceuticals, Cosmetics and Hygiene, Medicine, Agrochemistry, Energy Production and Environment: A Review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Aloo, S.O.; Mwiti, G.; Ngugi, L.W.; Oh, D.-H. Uncovering the Secrets of Industrial Hemp in Food and Nutrition: The Trends, Challenges, and New-Age Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Romano, R.; Aiello, A.; De Luca, L.; Sica, R.; Caprio, E.; Pizzolongo, F.; Blaiotta, G. Characterization of a New Type of Mead Fermented with Cannabis sativa L. (Hemp). J. Food Sci. 2021, 86, 874–880. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a Nutritional Resource: An Overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. A Practical and Natural Taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Y.L. Processing, Nutrition, and Functionality of Hempseed Protein: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Law No. 242 from 2 December 2016 on Provisions for the Promotion of Cultivation and the Agro-Industrial Chain of Hemp. FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC168000/ (accessed on 26 August 2023).
- Johnson, R. Hemp as an Agricultural Commodity. Available online: https://sgp.fas.org/crs/misc/RL32725.pdf (accessed on 25 August 2023).
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial Hemp (Cannabis sativa L.) Agronomy and Utilization: A Review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 25 August 2023).
- Filippin, D.; Sarni, A.R.; Rizzo, G.; Baroni, L. Environmental Impact of Two Plant-Based, Isocaloric and Isoproteic Diets: The Vegan Diet vs. the Mediterranean Diet. Int. J. Environ. Res. Public Health 2023, 20, 3797. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; American Dietetic Association. Position of the American Dietetic Association: Vegetarian Diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Wu, Y.-T.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The Psychotomimetic Effects of Intravenous Delta-9-Tetrahydrocannabinol in Healthy Individuals: Implications for Psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bhattacharyya, S. Does Cannabis Composition Matter? Differential Effects of Delta-9-Tetrahydrocannabinol and Cannabidiol on Human Cognition. Curr. Addict. Rep. 2017, 4, 62–74. [Google Scholar] [CrossRef]
- Weinstein, A.; Livny, A.; Weizman, A. Brain Imaging Studies on the Cognitive, Pharmacological and Neurobiological Effects of Cannabis in Humans: Evidence from Studies of Adult Users. Curr. Pharm. Des. 2016, 22, 6366–6379. [Google Scholar] [CrossRef]
- Sevigny, E.L. Cannabis and Driving Ability. Curr. Opin. Psychol. 2021, 38, 75–79. [Google Scholar] [CrossRef]
- Schulze, H.; Schumacher, M.; Urmeew, R.; Alvarez, J.; Bernhoft, I.M.; de Gier, H.D.G.; Hagenzieker, M.; Houwing, S.; Knoche, A.; Pilgerstorfer, M.; et al. Driving under the Influence of Drugs, Alcohol and Medicines in Europe—Findings from the DRUID Project; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2012. [Google Scholar]
- Hancox, R.J.; Poulton, R.; Ely, M.; Welch, D.; Taylor, D.R.; McLachlan, C.R.; Greene, J.M.; Moffitt, T.E.; Caspi, A.; Sears, M.R. Effects of Cannabis on Lung Function: A Population-Based Cohort Study. Eur. Respir. J. 2010, 35, 42–47. [Google Scholar] [CrossRef]
- Leemaqz, S.Y.; Dekker, G.A.; McCowan, L.M.; Kenny, L.C.; Myers, J.E.; Simpson, N.A.B.; Poston, L.; Roberts, C.T.; SCOPE Consortium. Maternal Marijuana Use Has Independent Effects on Risk for Spontaneous Preterm Birth but Not Other Common Late Pregnancy Complications. Reprod. Toxicol. 2016, 62, 77–86. [Google Scholar] [CrossRef]
- Hall, W. What Has Research over the Past Two Decades Revealed about the Adverse Health Effects of Recreational Cannabis Use? Addiction 2015, 110, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F.; Leson, G.; Pless, P. Evaluating the Impact of THC in Hemp Foods and Cosmetics on Human Health and Workplace Drug Tests. J. Ind. Hemp 2003, 8, 5–36. [Google Scholar] [CrossRef]
- Naraine, S.G.U.; Small, E.; Laursen, A.E.; Campbell, L.G. A Multivariate Analysis of Morphological Divergence of “Seeds” (Achenes) among Ruderal, Fibre, Oilseed, Dioecious/Monoecious and Marijuana Variants of Cannabis sativa L. Genet. Resour. Crop Evol. 2020, 67, 703–714. [Google Scholar] [CrossRef]
- Kwaśnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Kupczyński, R.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Różański, H. Volatile Composition and Sensory Properties as Quality Attributes of Fresh and Dried Hemp Flowers (Cannabis sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in Food Industry: Nutritional Value, Health Benefits, and Industrial Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the Quality of Protein from Hemp Seed (Cannabis sativa L.) Products through the Use of the Protein Digestibility-Corrected Amino Acid Score Method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Malomo, S.A.; He, R.; Aluko, R.E. Structural and Functional Properties of Hemp Seed Protein Products. J. Food Sci. 2014, 79, C1512–C1521. [Google Scholar] [CrossRef]
- Karus, M.; Vogt, D. European Hemp Industry: Cultivation, Processing and Product Lines. Euphytica 2004, 140, 7–12. [Google Scholar] [CrossRef]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannflavins from Hemp Sprouts, a Novel Cannabinoid-Free Hemp Food Product, Target Microsomal Prostaglandin E2 Synthase-1 and 5-Lipoxygenase. PharmaNutrition 2014, 2, 53–60. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/ (accessed on 24 October 2022).
- Aiello, G.; Fasoli, E.; Boschin, G.; Lammi, C.; Zanoni, C.; Citterio, A.; Arnoldi, A. Proteomic Characterization of Hempseed (Cannabis sativa L.). J. Proteom. 2016, 147, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Vastolo, A.; Calabrò, S.; Pacifico, S.; Koura, B.I.; Cutrignelli, M.I. Chemical and Nutritional Characteristics of Cannabis sativa L. Co-Products. J. Anim. Physiol. Anim. Nutr. 2021, 105 (Suppl. S1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Odani, S.; Odani, S. Isolation and Primary Structure of a Methionine- and Cystine-Rich Seed Protein of Cannabis sativa. Biosci. Biotechnol. Biochem. 1998, 62, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Shiraseb, F.; Asbaghi, O.; Bagheri, R.; Wong, A.; Figueroa, A.; Mirzaei, K. Effect of L-Arginine Supplementation on Blood Pressure in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2022, 13, 1226–1242. [Google Scholar] [CrossRef]
- Pahlavani, N.; Entezari, M.H.; Nasiri, M.; Miri, A.; Rezaie, M.; Bagheri-Bidakhavidi, M.; Sadeghi, O. The Effect of L-Arginine Supplementation on Body Composition and Performance in Male Athletes: A Double-Blinded Randomized Clinical Trial. Eur. J. Clin. Nutr. 2017, 71, 544–548. [Google Scholar] [CrossRef]
- Vega-López, S.; Matthan, N.R.; Ausman, L.M.; Harding, S.V.; Rideout, T.C.; Ai, M.; Otokozawa, S.; Freed, A.; Kuvin, J.T.; Jones, P.J.; et al. Altering Dietary Lysine:Arginine Ratio Has Little Effect on Cardiovascular Risk Factors and Vascular Reactivity in Moderately Hypercholesterolemic Adults. Atherosclerosis 2010, 210, 555–562. [Google Scholar] [CrossRef]
- Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps. Front. Plant Sci. 2019, 10, 1265. [Google Scholar] [CrossRef]
- University of Manitoba. Double-Blind, Randomized, Cross-Over Trial of Whole Hemp Seed Protein and Hemp Seed Protein Hydrolysate Derived Bioactive Peptide Consumption for Hypertension; clinicaltrials.gov; 2022. Available online: https://clinicaltrials.gov/study/NCT03508895 (accessed on 24 August 2023).
- Schiattarella, A.; Lombardo, M.; Morlando, M.; Rizzo, G. The Impact of a Plant-Based Diet on Gestational Diabetes: A Review. Antioxidants 2021, 10, 557. [Google Scholar] [CrossRef]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 11, 5. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef]
- Wang, X.-S.; Tang, C.-H.; Yang, X.-Q.; Gao, W.-R. Characterization, Amino Acid Composition and in Vitro Digestibility of Hemp (Cannabis sativa L.) Proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Raikos, V.; Duthie, G.; Ranawana, V. Denaturation and Oxidative Stability of Hemp Seed (Cannabis sativa L.) Protein Isolate as Affected by Heat Treatment. Plant Foods Hum. Nutr. 2015, 70, 304–309. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. A Comparative Study of the Structural and Functional Properties of Isolated Hemp Seed (Cannabis sativa L.) Albumin and Globulin Fractions. Food Hydrocoll. 2015, 43, 743–752. [Google Scholar] [CrossRef]
- Storz, M.A.; Ronco, A.L.; Hannibal, L. Observational and Clinical Evidence That Plant-Based Nutrition Reduces Dietary Acid Load. J. Nutr. Sci. 2022, 11, e93. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Cudney, R.; McPherson, A. Crystallographic Characterization and Molecular Symmetry of Edestin, a Legumin from Hemp. J. Mol. Biol. 1994, 235, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, E.; Brambilla, I.M.; Galasso, I. Genome-Wide Identification and Organization of Seed Storage Protein Genes of Cannabis sativa. Biol. Plant 2018, 62, 693–702. [Google Scholar] [CrossRef]
- Semwogerere, F.; Katiyatiya, C.L.F.; Chikwanha, O.C.; Marufu, M.C.; Mapiye, C. Bioavailability and Bioefficacy of Hemp By-Products in Ruminant Meat Production and Preservation: A Review. Front. Vet. Sci. 2020, 7, 572906. [Google Scholar] [CrossRef] [PubMed]
- Pojić, M.; Mišan, A.; Sakač, M.; Dapčević Hadnađev, T.; Šarić, B.; Milovanović, I.; Hadnađev, M. Characterization of Byproducts Originating from Hemp Oil Processing. J. Agric. Food Chem. 2014, 62, 12436–12442. [Google Scholar] [CrossRef]
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in Seed Traits in a Collection of Cannabis sativa L. Genotypes. Front. Plant Sci. 2016, 7, 688. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Reggiani, R. Evaluation of Protein Concentration, Amino Acid Profile and Antinutritional Compounds in Hempseed Meal from Dioecious and Monoecious Varieties. Am. J. Plant Sci. 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Thompson, L.U. Potential Health Benefits and Problems Associated with Antinutrients in Foods. Food Res. Int. 1993, 26, 131–149. [Google Scholar] [CrossRef]
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of Phytochemicals and Antinutritional Factors in Commercial Protein-Rich Plant Products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Kang, J.; Badger, T.M.; Ronis, M.J.J.; Wu, X. Non-Isoflavone Phytochemicals in Soy and Their Health Effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef] [PubMed]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef] [PubMed]
- Occhiuto, C.; Aliberto, G.; Ingegneri, M.; Trombetta, D.; Circosta, C.; Smeriglio, A. Comparative Evaluation of the Nutrients, Phytochemicals, and Antioxidant Activity of Two Hempseed Oils and Their Byproducts after Cold Pressing. Molecules 2022, 27, 3431. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Barbara Różańska, M.; Siger, A.; Zembrzuska, J.; Szwengiel, A. Formation of Maillard Reaction Products in a Model Bread System of Different Gluten-Free Flours. Food Chem. 2023, 429, 136994. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Ten, Z.; Wang, X.-S.; Yang, X.-Q. Physicochemical and Functional Properties of Hemp (Cannabis sativa L.) Protein Isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Orio, L.P.; Boschin, G.; Recca, T.; Morelli, C.F.; Ragona, L.; Francescato, P.; Arnoldi, A.; Speranza, G. New ACE-Inhibitory Peptides from Hemp Seed (Cannabis sativa L.) Proteins. J. Agric. Food Chem. 2017, 65, 10482–10488. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Aluko, R.E. Kinetics and Molecular Docking Studies of the Inhibitions of Angiotensin Converting Enzyme and Renin Activities by Hemp Seed (Cannabis sativa L.) Peptides. J. Agric. Food Chem. 2014, 62, 4135–4144. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. In Vitro Acetylcholinesterase-Inhibitory Properties of Enzymatic Hemp Seed Protein Hydrolysates. J. Am. Oil Chem. Soc. 2016, 93, 411–420. [Google Scholar] [CrossRef]
- Yan, X.; Tang, J.; dos Santos Passos, C.; Nurisso, A.; Simões-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of Lignanamides from Hemp (Cannabis sativa L.) Seed and Their Antioxidant and Acetylcholinesterase Inhibitory Activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.A.; Onuh, J.O.; Girgih, A.T.; Aluko, R.E. Structural and Antihypertensive Properties of Enzymatic Hemp Seed Protein Hydrolysates. Nutrients 2015, 7, 7616–7632. [Google Scholar] [CrossRef] [PubMed]
- Samsamikor, M.; Mackay, D.; Mollard, R.C.; Aluko, R.E. A Double-Blind, Randomized, Crossover Trial Protocol of Whole Hemp Seed Protein and Hemp Seed Protein Hydrolysate Consumption for Hypertension. Trials 2020, 21, 354. [Google Scholar] [CrossRef]
- Wang, X.-S.; Tang, C.-H.; Chen, L.; Yang, X.-Q. Characterization and Antioxidant Properties of Hemp Protein Hydrolysates Obtained with Neutrase®. Food Technol. Biotechnol. 2009, 47, 428–434. [Google Scholar]
- Tang, C.-H.; Wang, X.-S.; Yang, X.-Q. Enzymatic Hydrolysis of Hemp (Cannabis sativa L.) Protein Isolate by Various Proteases and Antioxidant Properties of the Resulting Hydrolysates. Food Chem. 2009, 114, 1484–1490. [Google Scholar] [CrossRef]
- Aiello, G.; Lammi, C.; Boschin, G.; Zanoni, C.; Arnoldi, A. Exploration of Potentially Bioactive Peptides Generated from the Enzymatic Hydrolysis of Hempseed Proteins. J. Agric. Food Chem. 2017, 65, 10174–10184. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, K.; Jin, Y.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and Characterization of Two Novel α-Glucosidase Inhibitory Oligopeptides from Hemp (Cannabis sativa L.) Seed Protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, R.; Zhao, J.; Chen, Q.; Kong, B. Structural and Gel Textural Properties of Soy Protein Isolate When Subjected to Extreme Acid pH-Shifting and Mild Heating Processes. J. Agric. Food Chem. 2015, 63, 4853–4861. [Google Scholar] [CrossRef]
- El-Adawy, T.A. Functional Properties and Nutritional Quality of Acetylated and Succinylated Mung Bean Protein Isolate. Food Chem. 2000, 70, 83–91. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Y.L. Zinc-Binding Behavior of Hemp Protein Hydrolysates: Soluble versus Insoluble Zinc-Peptide Complexes. J. Funct. Foods 2018, 49, 105–112. [Google Scholar] [CrossRef]
- Yin, S.-W.; Tang, C.-H.; Cao, J.-S.; Hu, E.-K.; Wen, Q.-B.; Yang, X.-Q. Effects of Limited Enzymatic Hydrolysis with Trypsin on the Functional Properties of Hemp (Cannabis sativa L.) Protein Isolate. Food Chem. 2008, 106, 1004–1013. [Google Scholar] [CrossRef]
- Yin, S.-W.; Tang, C.-H.; Wen, Q.-B.; Yang, X.-Q. Functional and Structural Properties and in Vitro Digestibility of Acylated Hemp (Cannabis sativa L.) Protein Isolates. Int. J. Food Sci. Technol. 2009, 44, 2653–2661. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, J.; Xiong, Y.L. High Pressure Homogenization Combined with pH Shift Treatment: A Process to Produce Physically and Oxidatively Stable Hemp Milk. Food Res. Int. 2018, 106, 487–494. [Google Scholar] [CrossRef]
- Teh, S.-S.; Bekhit, A.E.-D.A.; Carne, A.; Birch, J. Antioxidant and ACE-Inhibitory Activities of Hemp (Cannabis sativa L.) Protein Hydrolysates Produced by the Proteases AFP, HT, Pro-G, Actinidin and Zingibain. Food Chem. 2016, 203, 199–206. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect of Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Vonapartis, E.; Aubin, M.-P.; Seguin, P.; Mustafa, A.F.; Charron, J.-B. Seed Composition of Ten Industrial Hemp Cultivars Approved for Production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Lan, Y.; Zha, F.; Peckrul, A.; Hanson, B.; Johnson, B.; Rao, J.; Chen, B. Genotype x Environmental Effects on Yielding Ability and Seed Chemical Composition of Industrial Hemp (Cannabis sativa L.) Varieties Grown in North Dakota, USA. J. Am. Oil Chem. Soc. 2019, 96, 1417–1425. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids, Olive Oil and Health Status: A Systematic Review and Meta-Analysis of Cohort Studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W.L. Are All N-3 Polyunsaturated Fatty Acids Created Equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I.; Rao, J.S.; Igarashi, M. Brain Metabolism of Nutritionally Essential Polyunsaturated Fatty Acids Depends on Both the Diet and the Liver. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO-FAO Expert Consultation; [Joint WHO-FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, 2002, Geneva, Switzerland]. WHO Technical Report Series; Weltgesundheitsorganisation FAO (Ed.) World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8. [Google Scholar]
- Devi, V.; Khanam, S. Comparative Study of Different Extraction Processes for Hemp (Cannabis sativa) Seed Oil Considering Physical, Chemical and Industrial-Scale Economic Aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Health and Ecological Implications of Fish Consumption: A Deeper Insight. Mediterr. J. Nutr. Metab. 2016, 9, 7–22. [Google Scholar] [CrossRef]
- Davis, B.C.; Kris-Etherton, P.M. Achieving Optimal Essential Fatty Acid Status in Vegetarians: Current Knowledge and Practical Implications. Am. J. Clin. Nutr. 2003, 78, 640S–646S. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L.; Lombardo, M. Promising Sources of Plant-Derived Polyunsaturated Fatty Acids: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 1683. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, Interconversion, and Dose Response of n-3 Fatty Acids in Humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef]
- IOM. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can Agronomic Practices and Cold-Pressing Extraction Parameters Affect Phenols and Polyphenols Content in Hempseed Oils? Ind. Crops Prod. 2019, 130, 511–519. [Google Scholar] [CrossRef]
- Schultz, C.J.; Lim, W.L.; Khor, S.F.; Neumann, K.A.; Schulz, J.M.; Ansari, O.; Skewes, M.A.; Burton, R.A. Consumer and Health-Related Traits of Seed from Selected Commercial and Breeding Lines of Industrial Hemp, Cannabis sativa L. J. Agric. Food Res. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.L.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Volpe, M.G. Comparative Study of Chemical, Biochemical Characteristic and ATR-FTIR Analysis of Seeds, Oil and Flour of the Edible Fedora Cultivar Hemp (Cannabis sativa L.). Molecules 2018, 24, 83. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Potential Oil Yield, Fatty Acid Composition, and Oxidation Stability of the Hempseed Oil from Four Cannabis sativa L. Cultivars. J. Diet. Suppl. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Bennacer, A.F.; Haffaf, E.; Kacimi, G.; Oudjit, B.; Koceir, E.-A. Association of Polyunsaturated/Saturated Fatty Acids to Metabolic Syndrome Cardiovascular Risk Factors and Lipoprotein (a) in Hypertensive Type 2 Diabetic Patients. Ann. Biol. Clin. 2017, 75, 293–304. [Google Scholar] [CrossRef]
- Kapoor, R.; Huang, Y.-S. Gamma Linolenic Acid: An Antiinflammatory Omega-6 Fatty Acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Jiménez-Becker, S.; de Bélair, G. Fatty Acid Profiles and Sn-2 Fatty Acid Distribution of γ-Linolenic Acid-Rich Borago Species. J. Food Compos. Anal. 2018, 66, 74–80. [Google Scholar] [CrossRef]
- Kuhnt, K.; Degen, C.; Jaudszus, A.; Jahreis, G. Searching for Health Beneficial N-3 and n-6 Fatty Acids in Plant Seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 153–160. [Google Scholar] [CrossRef]
- Sprecher, H.; Luthria, D.L.; Mohammed, B.S.; Baykousheva, S.P. Reevaluation of the Pathways for the Biosynthesis of Polyunsaturated Fatty Acids. J. Lipid Res. 1995, 36, 2471–2477. [Google Scholar] [CrossRef]
- James, M.J.; Ursin, V.M.; Cleland, L.G. Metabolism of Stearidonic Acid in Human Subjects: Comparison with the Metabolism of Other N−3 Fatty Acids. Am. J. Clin. Nutr. 2003, 77, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Menotti, A.; Bloemberg, B.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Giampaoli, S.; Jansen, A. Dietary Saturated and Trans Fatty Acids and Cholesterol and 25-Year Mortality from Coronary Heart Disease: The Seven Countries Study. Prev. Med. 1995, 24, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Abuzaytoun, R.; Shahidi, F. Oxidative Stability of Flax and Hemp Oils. J. Am. Oil Chem. Soc. 2006, 83, 855–861. [Google Scholar] [CrossRef]
- Teh, S.-S.; Birch, E.J. Effect of Ultrasonic Treatment on the Polyphenol Content and Antioxidant Capacity of Extract from Defatted Hemp, Flax and Canola Seed Cakes. Ultrason. Sonochemistry 2014, 21, 346–353. [Google Scholar] [CrossRef]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of Dietary Hempseed Oil in Patients with Atopic Dermatitis. J. Dermatolog. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef]
- Rezapour-Firouzi, S.; Arefhosseini, S.R.; Mehdi, F.; Mehrangiz, E.-M.; Baradaran, B.; Sadeghihokmabad, E.; Mostafaei, S.; Fazljou, S.M.B.; Torbati, M.; Sanaie, S.; et al. Immunomodulatory and Therapeutic Effects of Hot-Nature Diet and Co-Supplemented Hemp Seed, Evening Primrose Oils Intervention in Multiple Sclerosis Patients. Complement. Ther. Med. 2013, 21, 473–480. [Google Scholar] [CrossRef]
- Rezapour-Firouzi, S.; Arefhosseini, S.R.; Farhoudi, M.; Ebrahimi-Mamaghani, M.; Rashidi, M.-R.; Torbati, M.-A.; Baradaran, B. Association of Expanded Disability Status Scale and Cytokines after Intervention with Co-Supplemented Hemp Seed, Evening Primrose Oils and Hot-Natured Diet in Multiple Sclerosis Patients(♦). Bioimpacts 2013, 3, 43–47. [Google Scholar] [CrossRef]
- Rezapour-Firouzi, S.; Arefhosseini, S.R.; Ebrahimi-Mamaghani, M.; Baradaran, B.; Sadeghihokmabad, E.; Torbati, M.; Mostafaei, S.; Chehreh, M.; Zamani, F. Activity of Liver Enzymes in Multiple Sclerosis Patients with Hot-Nature Diet and Co-Supplemented Hemp Seed, Evening Primrose Oils Intervention. Complement. Ther. Med. 2014, 22, 986–993. [Google Scholar] [CrossRef]
- Palmieri, B.; Laurino, C.; Vadalà, M. Short-Term Efficacy of CBD-Enriched Hemp Oil in Girls with Dysautonomic Syndrome after Human Papillomavirus Vaccination. Isr. Med. Assoc. J. 2017, 19, 79–84. [Google Scholar]
- Schwab, U.S.; Callaway, J.C.; Erkkilä, A.T.; Gynther, J.; Uusitupa, M.I.J.; Järvinen, T. Effects of Hempseed and Flaxseed Oils on the Profile of Serum Lipids, Serum Total and Lipoprotein Lipid Concentrations and Haemostatic Factors. Eur. J. Nutr. 2006, 45, 470–477. [Google Scholar] [CrossRef]
- Kinosian, B.; Glick, H.; Preiss, L.; Puder, K.L. Cholesterol and Coronary Heart Disease: Predicting Risks in Men by Changes in Levels and Ratios. J. Investig. Med. 1995, 43, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Fang, Z.; Wahlqvist, M.L.; Hodgson, J.M.; Johnson, S.K. Extrusion Cooking Increases Soluble Dietary Fibre of Lupin Seed Coat. LWT 2019, 99, 547–554. [Google Scholar] [CrossRef]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.-M.; Hellström, J.; Pihlanto, A. Nutritional Value of Commercial Protein-Rich Plant Products. Plant Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-B.; Hao, Q.-Q.; Hu, H.-R.L. The Relationship between Dietary Fibre and Stroke: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2023, 32, 107144. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, V.V.; Schönenberger, K.A.; Segesser von Brunegg, A.; Reber, E.; Mühlebach, S.; Stanga, Z.; Balmer, M.L. Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 2627. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.; Kumar, S.; Diep Pham, H.T.; Coffey, S.; Mann, J. Dietary Fibre in Hypertension and Cardiovascular Disease Management: Systematic Review and Meta-Analyses. BMC Med. 2022, 20, 139. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Zand, N.; Wang, X. The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 1805. [Google Scholar] [CrossRef]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Mangels, A.R.; Messina, V. Considerations in Planning Vegan Diets: Infants. J. Am. Diet. Assoc. 2001, 101, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R. Content and Bioavailability of Trace Elements in Vegetarian Diets. Am. J. Clin. Nutr. 1994, 59, 1223S–1232S. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Chemistry behind Vegetarianism. J. Agric. Food Chem. 2011, 59, 777–784. [Google Scholar] [CrossRef]
- Jeitler, M.; Storz, M.A.; Steckhan, N.; Matthiae, D.; Dressler, J.; Hanslian, E.; Koppold, D.A.; Kandil, F.I.; Michalsen, A.; Kessler, C.S. Knowledge, Attitudes and Application of Critical Nutrient Supplementation in Vegan Diets among Healthcare Professionals-Survey Results from a Medical Congress on Plant-Based Nutrition. Foods 2022, 11, 4033. [Google Scholar] [CrossRef]
- Mihoc, M.; Pop, G.; Alexa, E.; Radulov, I. Nutritive Quality of Romanian Hemp Varieties (Cannabis sativa L.) with Special Focus on Oil and Metal Contents of Seeds. Chem. Cent. J. 2012, 6, 122. [Google Scholar] [CrossRef]
- Weaver, C.; Plawecki, K. Dietary Calcium: Adequacy of a Vegetarian Diet. Am. J. Clin. Nutr. 1994, 59, 1238S–1241S. [Google Scholar] [CrossRef]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position Paper on Vegetarian Diets from the Working Group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battino, M. VegPlate: A Mediterranean-Based Food Guide for Italian Adult, Pregnant, and Lactating Vegetarians. J. Acad. Nutr. Diet. 2018, 118, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Proulx, W.R.; Heaney, R. Choices for Achieving Adequate Dietary Calcium with a Vegetarian Diet2. Am. J. Clin. Nutr. 1999, 70, 543S–548S. [Google Scholar] [CrossRef]
- Oseyko, M.; Sova, N.; Lutsenko, M.; Kalyna, V. Chemical Aspects of the Composition of Industrial Hemp Seed Products. Ukr. Food J. 2019, 8, 544–559. [Google Scholar] [CrossRef]
- Hunt, J.R.; Roughead, Z.K. Adaptation of Iron Absorption in Men Consuming Diets with High or Low Iron Bioavailability. Am. J. Clin. Nutr. 2000, 71, 94–102. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001; ISBN 978-0-309-07279-3. [Google Scholar]
- Di, Y.; Ding, L.; Gao, L.; Huang, H. Association of Meat Consumption with the Risk of Gastrointestinal Cancers: A Systematic Review and Meta-Analysis. BMC Cancer 2023, 23, 782. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, M.; Long, Z.; Ye, J.; Cao, Y.; Pei, B.; Gao, Y.; Yu, Y.; Han, Z.; Wang, F.; et al. How to Keep the Balance between Red and Processed Meat Intake and Physical Activity Regarding Mortality: A Dose-Response Meta-Analysis. Nutrients 2023, 15, 3373. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. The Association between Red, Processed and White Meat Consumption and Risk of Pancreatic Cancer: A Meta-Analysis of Prospective Cohort Studies. Cancer Causes Control 2023, 34, 569–581. [Google Scholar] [CrossRef]
- Italian Society of Human Nutrition (SINU). Dietary Reference Value of Nutrients and Energy for He Italian Population (LARN); IV REVISION; SICS Editore Srl: Milano, Italy, 2014. [Google Scholar]
- Zhao, M.; Jin, Z.; Xia, C.; Chen, S.; Zeng, L.; Qin, S.; He, Q. Inhibition of Free Heme-Catalyzed Fenton-like Reaction Prevents Non-Alcoholic Fatty Liver Disease by Hepatocyte-Targeted Hydrogen Delivery. Biomaterials 2023, 301, 122230. [Google Scholar] [CrossRef]
- Chang, V.C.; Cotterchio, M.; Khoo, E. Iron Intake, Body Iron Status, and Risk of Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 543. [Google Scholar] [CrossRef]
- Turner, N.D.; Lloyd, S.K. Association between Red Meat Consumption and Colon Cancer: A Systematic Review of Experimental Results. Exp. Biol. Med. 2017, 242, 813–839. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The Isolation and Identification of Two Compounds with Predominant Radical Scavenging Activity in Hempseed (Seed of Cannabis sativa L.). Food Chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Peričin, D.; Krimer, V.; Trivić, S.; Radulović, L. The Distribution of Phenolic Acids in Pumpkin’s Hull-Less Seed, Skin, Oil Cake Meal, Dehulled Kernel and Hull. Food Chem. 2009, 113, 450–456. [Google Scholar] [CrossRef]
- Livadariu, O.; Raiciu, D.; Maximilian, C.; Căpitanu, E. Studies Regarding Treatments of LED-s Emitted Light on Sprouting Hemp (Cannabis sativa L.). Rom Biotechnol Lett. 2019, 24, 485–490. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Giménez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponifiable Fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R. Stresa Workshop Participants Efficacy and Safety of Plant Stanols and Sterols in the Management of Blood Cholesterol Levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar] [CrossRef]
- Vecka, M.; Staňková, B.; Kutová, S.; Tomášová, P.; Tvrzická, E.; Žák, A. Comprehensive Sterol and Fatty Acid Analysis in Nineteen Nuts, Seeds, and Kernel. SN Appl. Sci. 2019, 1, 1531. [Google Scholar] [CrossRef]
- Terao, J. Revisiting Carotenoids as Dietary Antioxidants for Human Health and Disease Prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef]
- Welc-Stanowska, R.; Pietras, R.; Mielecki, B.; Sarewicz, M.; Luchowski, R.; Widomska, J.; Grudzinski, W.; Osyczka, A.; Gruszecki, W.I. How Do Xanthophylls Protect Lipid Membranes from Oxidative Damage? J. Phys. Chem. Lett. 2023, 14, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.-M. Tocopherol, Tocotrienol and Plant Sterol Contents of Vegetable Oils and Industrial Fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The Content and Antioxidant Activity of Phenolic Compounds in Cold-Pressed Plant Oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Zhu, G.-Y.; Yang, J.; Yao, X.-J.; Yang, X.; Fu, J.; Liu, X.; Bai, L.-P.; Liu, L.; Jiang, Z.-H. (±)-Sativamides A and B, Two Pairs of Racemic Nor-Lignanamide Enantiomers from the Fruits of Cannabis sativa. J. Org. Chem. 2018, 83, 2376–2381. [Google Scholar] [CrossRef]
- Bourjot, M.; Zedet, A.; Demange, B.; Pudlo, M.; Girard-Thernier, C. In Vitro Mammalian Arginase Inhibitory and Antioxidant Effects of Amide Derivatives Isolated from the Hempseed Cakes (Cannabis sativa). Planta Med. Int. Open 2016, 3, e64–e67. [Google Scholar] [CrossRef]
- Hempedocle. Available online: https://reica.org/hempedocle/ (accessed on 25 August 2023).
- Tackling Climate Change through Livestock. A Global Assessment of Emissions and Mitigation Opportunities |Policy Support and Governance| Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/1235389/ (accessed on 27 August 2023).
- World Health Organization/Regional Office for Europe. Plant-Based Diets and Their Impact on Health, Sustainability and the Environment: A Review of the Evidence: WHO European Office for the Prevention and Control of Noncommunicable Diseases; World Health Organization/Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Dixon, K.A.; Michelsen, M.K.; Carpenter, C.L. Modern Diets and the Health of Our Planet: An Investigation into the Environmental Impacts of Food Choices. Nutrients 2023, 15, 692. [Google Scholar] [CrossRef]
- Carey, C.N.; Paquette, M.; Sahye-Pudaruth, S.; Dadvar, A.; Dinh, D.; Khodabandehlou, K.; Liang, F.; Mishra, E.; Sidhu, M.; Brown, R.; et al. The Environmental Sustainability of Plant-Based Dietary Patterns: A Scoping Review. J. Nutr. 2023, 153, 857–869. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of Fibre Hemp (Cannabis sativa L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Ranalli, P.; Venturi, G. Hemp as a Raw Material for Industrial Applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa Subsp. Sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Laate, E. Industrial Hemp Production in Canada. Available online: https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/econ9631/$file/Final%20-%20Industrial%20Hemp%20Production%20in%20Canada%20-%20June%2025%202012.pdf?OpenElement (accessed on 25 August 2023).
- Pojić, M.; Dapčević Hadnađev, T.; Hadnađev, M.; Rakita, S.; Brlek, T. Bread Supplementation with Hemp Seed Cake: A By-Product of Hemp Oil Processing. J. Food Qual. 2015, 38, 431–440. [Google Scholar] [CrossRef]
- Ruban, A.; Hrivna, L.; Kong, J.L.H.; Dostalova, Y.; Machalkova, L.; Mullerova, M.; Sottnikova, V.; Mrkvicova, E.; Vyhnanek, T.; Trojan, V.; et al. The Use of Hemp and Color Wheat Flour as Baking Ingredients. Ph.D. Thesis, Mendel University in Brno (Mendelova Univerzita v Brně), Brno, Czechia, 2016. [Google Scholar]
- Korus, A.; Gumul, D.; Krystyjan, M.; Juszczak, L.; Korus, J. Evaluation of the Quality, Nutritional Value and Antioxidant Activity of Gluten-Free Biscuits Made from Corn-Acorn Flour or Corn-Hemp Flour Composites. Eur. Food Res. Technol. 2017, 243, 1429–1438. [Google Scholar] [CrossRef]
- Grace, M.H.; Guzman, I.; Roopchand, D.E.; Moskal, K.; Cheng, D.M.; Pogrebnyak, N.; Raskin, I.; Howell, A.; Lila, M.A. Stable Binding of Alternative Protein-Enriched Food Matrices with Concentrated Cranberry Bioflavonoids for Functional Food Applications. J. Agric. Food Chem. 2013, 61, 6856–6864. [Google Scholar] [CrossRef] [PubMed]
- Hemp-Based Infant Formula and Methods of Making Same. Patent US-2015079235-A1, 16 March 2012. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US2015079235 (accessed on 25 August 2023).
- Švec, I.; Hrušková, M. Properties and Nutritional Value of Wheat Bread Enriched by Hemp Products. Potravin. Slovak J. Food Sci. 2015, 9, 304–308. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Norajit, K.; Ryu, G.-H. Influence of Extruded Hemp-Rice Flour Addition on the Physical Properties of Wheat Bread. J. Food Sci. Nutr. 2011, 16, 62–66. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Norajit, K.; Kim, M.-H.; Kim, Y.-H.; Ryu, G.-H. Influence of Extrusion Condition and Hemp Addition on Wheat Dough and Bread Properties. Food Sci. Biotechnol. 2013, 22, 89–97. [Google Scholar] [CrossRef]
- Mikulec, A.; Kowalski, S.; Sabat, R.; Skoczylas, Ł.; Tabaszewska, M.; Wywrocka-Gurgul, A. Hemp Flour as a Valuable Component for Enriching Physicochemical and Antioxidant Properties of Wheat Bread. LWT 2019, 102, 164–172. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa Subsp. Sativa) Flour and Protein Preparation as Natural Nutrients and Structure Forming Agents in Starch Based Gluten-Free Bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Latif, S.; Anwar, F. Physicochemical Studies of Hemp (Cannabis sativa) Seed Oil Using Enzyme-Assisted Cold-Pressing. Eur. J. Lipid Sci. Technol. 2009, 111, 1042–1048. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Zeng, Q.-X.; An, Q.; Zeng, Q.-Z.; Jian, L.-X.; Zhu, Z.-W. Ultrasonic Extraction of Hempseed Oil. J. Food Process Eng. 2012, 35, 76–90. [Google Scholar] [CrossRef]
- Teh, S.-S.; Bekhit, A.E.-D.; Birch, J. Antioxidative Polyphenols from Defatted Oilseed Cakes: Effect of Solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef]
- Teh, S.-S.; Bekhit, A.E.-D.; Carne, A.; Birch, J. Effect of the Defatting Process, Acid and Alkali Extraction on the Physicochemical and Functional Properties of Hemp, Flax and Canola Seed Cake Protein Isolates. Food Meas. 2014, 8, 92–104. [Google Scholar] [CrossRef]
- Lu, R.-R.; Qian, P.; Sun, Z.; Zhou, X.-H.; Chen, T.-P.; He, J.-F.; Zhang, H.; Wu, J. Hempseed Protein Derived Antioxidative Peptides: Purification, Identification and Protection from Hydrogen Peroxide-Induced Apoptosis in PC12 Cells. Food Chem. 2010, 123, 1210–1218. [Google Scholar] [CrossRef]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.-M.; Popović, S.; Biliaderis, C.G. Hempseed Meal Protein Isolates Prepared by Different Isolation Techniques. Part I. Physicochemical Properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. Conversion of a Low Protein Hemp Seed Meal into a Functional Protein Concentrate through Enzymatic Digestion of Fibre Coupled with Membrane Ultrafiltration. Innov. Food Sci. Emerg. Technol. 2015, 31, 151–159. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Vahanvaty, U.S. Hemp Seed and Hemp Milk: The New Super Foods? Infant Child Adolesc. Nutr. 2009, 1, 232–234. [Google Scholar] [CrossRef]
- FDA Reopens Comment Period for the Draft Guidance on Labeling of Plant-Based Milk Alternatives; FDA. 2023. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-reopens-comment-period-draft-guidance-labeling-plant-based-milk-alternatives (accessed on 27 August 2023).
- Brusati, M.; Baroni, L.; Rizzo, G.; Giampieri, F.; Battino, M. Plant-Based Milk Alternatives in Child Nutrition. Foods 2023, 12, 1544. [Google Scholar] [CrossRef]
- Maurotti, S.; Mare, R.; Pujia, R.; Ferro, Y.; Mazza, E.; Romeo, S.; Pujia, A.; Montalcini, T. Hemp Seeds in Post-Arthroplasty Rehabilitation: A Pilot Clinical Study and an In Vitro Investigation. Nutrients 2021, 13, 4330. [Google Scholar] [CrossRef] [PubMed]
- Mohammadrezaei, A.; Kavakeb, A.; Abbasalizad-Farhangi, M.; Mesgari-Abbasi, M. Effects of Hemp Seed Alone and Combined with Aerobic Exercise on Metabolic Parameters, Oxidative Stress, and Neurotrophic Factors in Young Sedentary Men. J. Food Biochem. 2022, 46, e14417. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Selg-Mann, K. Production of a Food Seasoning, e.g., Useful as a Substitute for Soy Sauce, by Two-Stage Fermentation of Hemp Seeds 2001. Available online: https://patents.google.com/patent/DE10002389A1/en (accessed on 25 August 2023).
- Hemp-Based Foods Market Overview, Industry Trends, Segmentation, Scenario, and Forecast by 2029. Available online: https://www.databridgemarketresearch.com/reports/global-hemp-based-foods-market (accessed on 25 August 2023).
- Kolodinsky, J.; Lacasse, H. Consumer Response to Hemp: A Case Study of Vermont Residents from 2019 to 2020. GCB Bioenergy 2021, 13, 537–545. [Google Scholar] [CrossRef]
- Research and Markets ltd.Hemp Based Foods Global Market Report 2023—Research and Markets. Available online: https://www.researchandmarkets.com/reports/5744168/hemp-based-foods-global-market-report (accessed on 25 August 2023).
- Cannuse. Available online: https://cannusedb.csic.es/ (accessed on 25 August 2023).
- Balant, M.; Gras, A.; Gálvez, F.; Garnatje, T.; Vallès, J.; Vitales, D. CANNUSE, a Database of Traditional Cannabis Uses-an Opportunity for New Research. Database 2021, 2021, baab024. [Google Scholar] [CrossRef] [PubMed]
- Staginnus, C.; Zörntlein, S.; de Meijer, E. A PCR Marker Linked to a THCA Synthase Polymorphism Is a Reliable Tool to Discriminate Potentially THC-Rich Plants of Cannabis sativa L. J. Forensic Sci. 2014, 59, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, D.; Miselli, F.; Micheler, M.; Carboni, A.; Ranalli, P.; Mandolino, G. Genetics and Marker-Assisted Selection of the Chemotype in Cannabis sativa L. Mol. Breed. 2006, 17, 257–268. [Google Scholar] [CrossRef]
- Broséus, J.; Anglada, F.; Esseiva, P. The Differentiation of Fibre- and Drug Type Cannabis Seedlings by Gas Chromatography/Mass Spectrometry and Chemometric Tools. Forensic Sci. Int. 2010, 200, 87–92. [Google Scholar] [CrossRef]
- de Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- Grof, C.P.L. Cannabis, from Plant to Pill. Br. J. Clin. Pharmacol. 2018, 84, 2463–2467. [Google Scholar] [CrossRef]
- Www.Emcdda.Europa.Eu. New Report Describes Growing Complexity and Change in Cannabis Laws in Europe. Available online: https://www.emcdda.europa.eu/news/2023/cannabis-laws-europe-questions-and-answers-policymaking_en (accessed on 27 August 2023).
- European Monitoring Centre for Drugs and Drug Addiction (EU Body or Agency); Hughes, B. Cannabis Legislation in Europe: An Overview; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-9497-328-3. [Google Scholar]
- EUPVP—COMMON CATALOGUE—Varieties of Agricultural Plant and Vegetable Species. Available online: https://ec.europa.eu/food/plant-variety-portal/ (accessed on 25 August 2023).
- Ondřej Hanuš, L.; Martin Meyer, S.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of Cannabinoids in Commercial Hemp Seed Oil and Decarboxylation Kinetics Studies of Cannabidiolic Acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Linciano, P.; Panseri, S.; Vezzalini, F.; Forni, F.; Vandelli, M.A.; Cannazza, G. Cannabinoid Profiling of Hemp Seed Oil by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Front. Plant Sci. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.; Bast, A. Demanding Safe Foods—Safety Testing under the Novel Food Regulation (2015/2283). Trends Food Sci. Technol. 2018, 72, 125–133. [Google Scholar] [CrossRef]
- FDA Responds to Three GRAS Notices for Hemp Seed-Derived Ingredients for Use in Human Food; FDA. 2019. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-responds-three-gras-notices-hemp-seed-derived-ingredients-use-human-food (accessed on 25 August 2023).
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Advances in Microencapsulation of Polyunsaturated Fatty Acids (PUFAs)-Rich Plant Oils Using Complex Coacervation: A Review. Food Hydrocoll. 2017, 69, 369–381. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An Efficient Technology to Boost the Antimicrobial Potential of Plant Essential Oils in Food System. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Vojvodić, A.; Bušić, A.; Keppler, J.; Steffen-Heins, A.; Komes, D. Encapsulation Templated Approach to Valorization of Cocoa Husk, Poppy and Hemp Macrostructural and Bioactive Constituents. Ind. Crops Prod. 2018, 112, 402–411. [Google Scholar] [CrossRef]
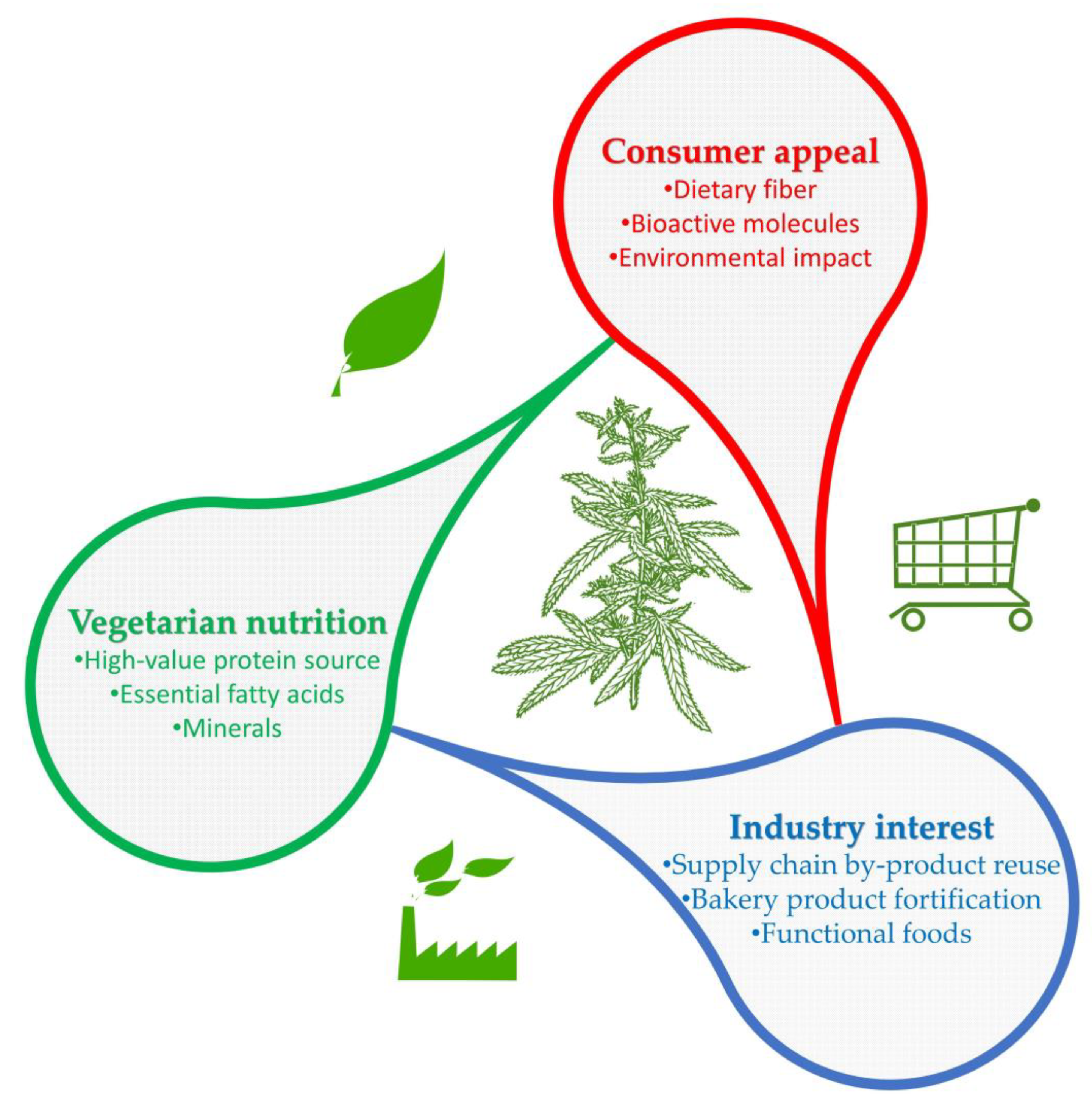
| Hemp Feature | Benefits |
|---|---|
| Protein | The high nutritional value of hemp protein can help one to reach the adequate intake of protein in a vegetarian diet without animal sources. |
| Essential fatty Acids | A vegetarian diet has limited sources of EFAs, especially omega 3. The advantageous ratio of n6/n3 in hemp oil can help to obtain a balanced intake of polyunsaturated fatty acids. |
| Calcium | There are limited sources of calcium in a vegetarian diet without milk and dairy usage. Using various plant foods including hemp can help to reach the RDA for calcium. |
| Iron | In a vegetarian diet, there are only low-bioavailable sources of iron so different iron-rich foods must be used, and hemp can have a high content of this mineral. |
| Fiber | Even if all plant-based foods are rich in dietary fibers, the functional properties of insoluble fiber from hemp can stimulate its consumption. |
| Phytochemicals | The high content of bioactive molecules with health effects can stimulate the consumption of hemp in a plant-based context. |
| Environmental impact | Considering the wide motivation for a vegetarian diet adoption, the low impact of hemp cultivation can prompt the use of hemp as an eco-friendly plant-based source. |
| Versatility | Hemp can have promising features, and the seeds can be employed in various industrial productions including some products of interest for a vegetarian diet such as plant-based milk and meat alternatives. Moreover, employed as a fortifier, hemp derivatives can be used in supplement and bakery products. |
| Food | Energy (Kcal) | Protein (g) | Arg (g) | Fat (g) | SFA (g) | MUFAs (g) | OA (g) | PUFAs (g) | LA (g) | ALA (g) | SDA (g) | Ca (mg) | Fe (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemp seeds | 553 | 31.56 | 4.55 | 48.75 | 4.6 | 5.4 | 5.276 | 38.1 | 27.459 | 10.024 | 0.617 | 70 | 7.95 |
| Adzuki beans | 329 | 19.87 | 1.284 | 0.53 | 0.191 | 0.05 | 0.05 | 0.113 | 0.113 | NA | NA | 66 | 4.98 |
| Almonds | 579 | 21.15 | 2.465 | 49.93 | 3.802 | 31.551 | 31.294 | 12.329 | 12.324 | 0.003 | 0 | 269 | 3.71 |
| Brazil nuts | 659 | 14.32 | 2.14 | 67.1 | 16.134 | 23.879 | 23.594 | 24.399 | 24.363 | 0.036 | 0 | 160 | 2.43 |
| Cashew nuts | 553 | 18.22 | 2.123 | 43.85 | 7.783 | 23.797 | 23.523 | 7.845 | 7.782 | 0.062 | 0 | 37 | 6.68 |
| Chestnuts | 213 | 2.42 | 0.173 | 2.26 | 0.425 | 0.78 | 0.749 | 0.894 | 0.798 | 0.095 | NA | 27 | 1.01 |
| Chia seeds | 486 | 16.54 | 2.143 | 30.74 | 3.33 | 2.309 | 2.203 | 23.665 | 5.835 | 17.83 | NA | 631 | 7.72 |
| Chickpeas | 378 | 20.47 | 1.939 | 6.04 | 0.603 | 1.377 | 1.365 | 2.731 | 2.629 | 0.102 | 0 | 57 | 4.31 |
| Fava beans | 88 | 7.92 | NA | 0.73 | 0.118 | 0.104 | 0.097 | 0.342 | 0.312 | 0.03 | NA | 37 | 1.55 |
| Flaxseed | 534 | 18.29 | 1.925 | 42.16 | 3.663 | 7.527 | 7.359 | 28.73 | 5.903 | 22.813 | 0 | 255 | 5.73 |
| Kidney beans | 333 | 23.58 | 1.46 | 0.83 | 0.12 | 0.064 | 0.064 | 0.457 | 0.178 | 0.279 | 0 | 143 | 8.2 |
| Lentils | 352 | 24.63 | 1.903 | 1.06 | 0.154 | 0.193 | 0.184 | 0.526 | 0.414 | 0.112 | 0 | 35 | 6.51 |
| Lima beans | 338 | 21.46 | 1.315 | 0.69 | 0.161 | 0.062 | 0.052 | 0.309 | 0.215 | 0.095 | 0 | 81 | 7.51 |
| Lupins | 371 | 36.17 | 3.877 | 9.74 | 1.156 | 3.94 | 3.558 | 2.439 | 1.995 | 0.446 | NA | 176 | 4.36 |
| macadamia nuts | 718 | 7.91 | 1.402 | 75.77 | 12.061 | 58.877 | 43.755 | 1.502 | 1.296 | 0.206 | 0 | 85 | 3.69 |
| Mungo beans | 341 | 25.21 | 1.642 | 1.64 | 0.114 | 0.085 | 0.085 | 1.071 | 0.072 | 0.999 | NA | 138 | 7.57 |
| Navy beans | 337 | 22.33 | 1.02 | 1.5 | 0.17 | 0.128 | 0.117 | 0.873 | 0.335 | 0.538 | NA | 147 | 5.49 |
| Peanuts | 567 | 25.8 | 3.085 | 49.24 | 6.279 | 24.426 | 23.756 | 15.558 | 15.555 | 0.003 | 0 | 92 | 4.58 |
| pecan nuts | 691 | 9.17 | 1.177 | 71.97 | 6.18 | 40.801 | 40.594 | 21.614 | 20.628 | 0.986 | 0 | 70 | 2.53 |
| Pine nuts | 673 | 13.69 | 2.413 | 68.37 | 4.899 | 18.764 | 17.947 | 34.071 | 33.15 | 0.164 | 0 | 16 | 5.53 |
| Pistachio | 560 | 20.16 | 2.134 | 45.32 | 5.907 | 23.257 | 22.674 | 14.38 | 14.091 | 0.289 | 0 | 105 | 3.92 |
| Soybeans | 446 | 36.49 | 3.153 | 19.94 | 2.884 | 4.404 | 4.348 | 11.255 | 9.925 | 1.33 | NA | 277 | 15.7 |
| Walnuts | 654 | 15.23 | 2.278 | 65.21 | 6.126 | 8.933 | 8.799 | 47.174 | 38.093 | 9.08 | 0 | 98 | 2.91 |
| Nutrient | Fraction | Whole | Dehulled | Meal | Hull | Oil |
|---|---|---|---|---|---|---|
| Protein | Total (%) | 21–28 | 36 | 41 | 13 | |
| Arginine (%) | 2.28–3.10 | 4.55 | 3.91 | 0.94 | ||
| Lipids | Total (%) | 24–36 | 47 | 10 | 10 | 100 |
| PUFA (%) LA (%) ALA (%) SDA (%) n6:n3 | 72–84 52–59 10–22 0.2–2 2.5–5.5 | |||||
| Fiber | Total (%) | 28–34 | 8 | 30 | 65 | |
| Insoluble (%) | 22–31 | |||||
| Soluble (%) | 3–5 | |||||
| Sterols | Total (mg/100 g) β-Sitosterol | 124 54–80 | 279 190 | |||
| Tocopherols | Total (mg/100 g) γ-Tocopherol (mg/100 g) | 61–135 1–295 | 14–97 15–89 | |||
| Minerals | Ca (mg/100 g) | 90–955 | ||||
| Fe (mg/100 g) | 4–240 |
| Hemp Derivative | Application | Main Features |
|---|---|---|
| Protein isolate | Juice fortification, infant formula, plant-based-milk and supplement manufacture | Increasing oxidative stability and protein-rich source |
| Extruded product or seed flour | Production of bakery products, pasta, energy bars and meat analogues | Increasing dough volume and color Increasing mineral, protein, fat, fiber and antioxidant content Application to gluten-free products |
| Seeds | As food for salads and soups, or seasoning production | Nutrient source |
| Seed oil | Table oil (not for cooking) | Polyunsaturated fatty acid and antioxidant nutritional source |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, G.; Storz, M.A.; Calapai, G. The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition. Foods 2023, 12, 3505. https://doi.org/10.3390/foods12183505
Rizzo G, Storz MA, Calapai G. The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition. Foods. 2023; 12(18):3505. https://doi.org/10.3390/foods12183505
Chicago/Turabian StyleRizzo, Gianluca, Maximilian Andreas Storz, and Gioacchino Calapai. 2023. "The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition" Foods 12, no. 18: 3505. https://doi.org/10.3390/foods12183505
APA StyleRizzo, G., Storz, M. A., & Calapai, G. (2023). The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition. Foods, 12(18), 3505. https://doi.org/10.3390/foods12183505








