Peptidomics Analysis of Soy Protein Hydrolysates—Antioxidant Properties and Mechanism of their Inhibition of the Oxidation of Palm Olein during Frying Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Soybean Meal
2.3. Extraction of Proteins from Soybean Meal and Their Hydrolysis
2.4. Determination of Protein Content and Gel Electrophoresis (SDS-PAGE)
2.5. Mass Spectrometry Analysis
2.6. Evaluation of Antioxidant Properties
2.6.1. Ability to Scavenge 2, 2-Diphenyl-1-picrylhydrazyl (DPPH•)
2.6.2. Evaluation of Ferric Reducing Antioxidant Power (FRAP)
2.6.3. Oxygen Radical Absorbance Capacity Assay
2.7. Palm Olein Stabilization and Frying of Plantain Banana Chips
2.8. Chemical Characterization of Frying Oils
2.9. Statistical Analysis
3. Results
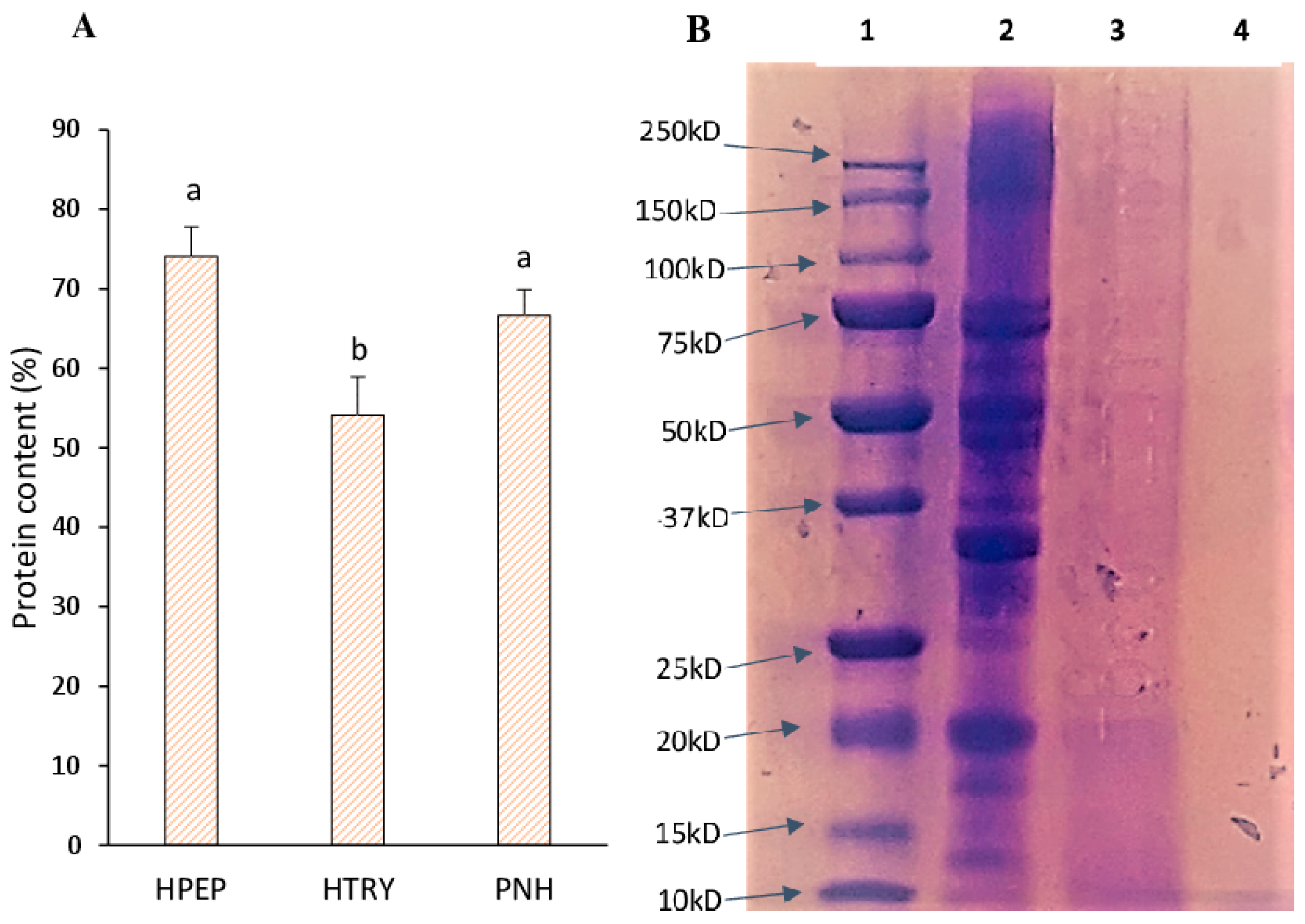
3.1. Soluble Protein Content of Soybean Protein Isolate, Hydrolysates, and Molecular Weights
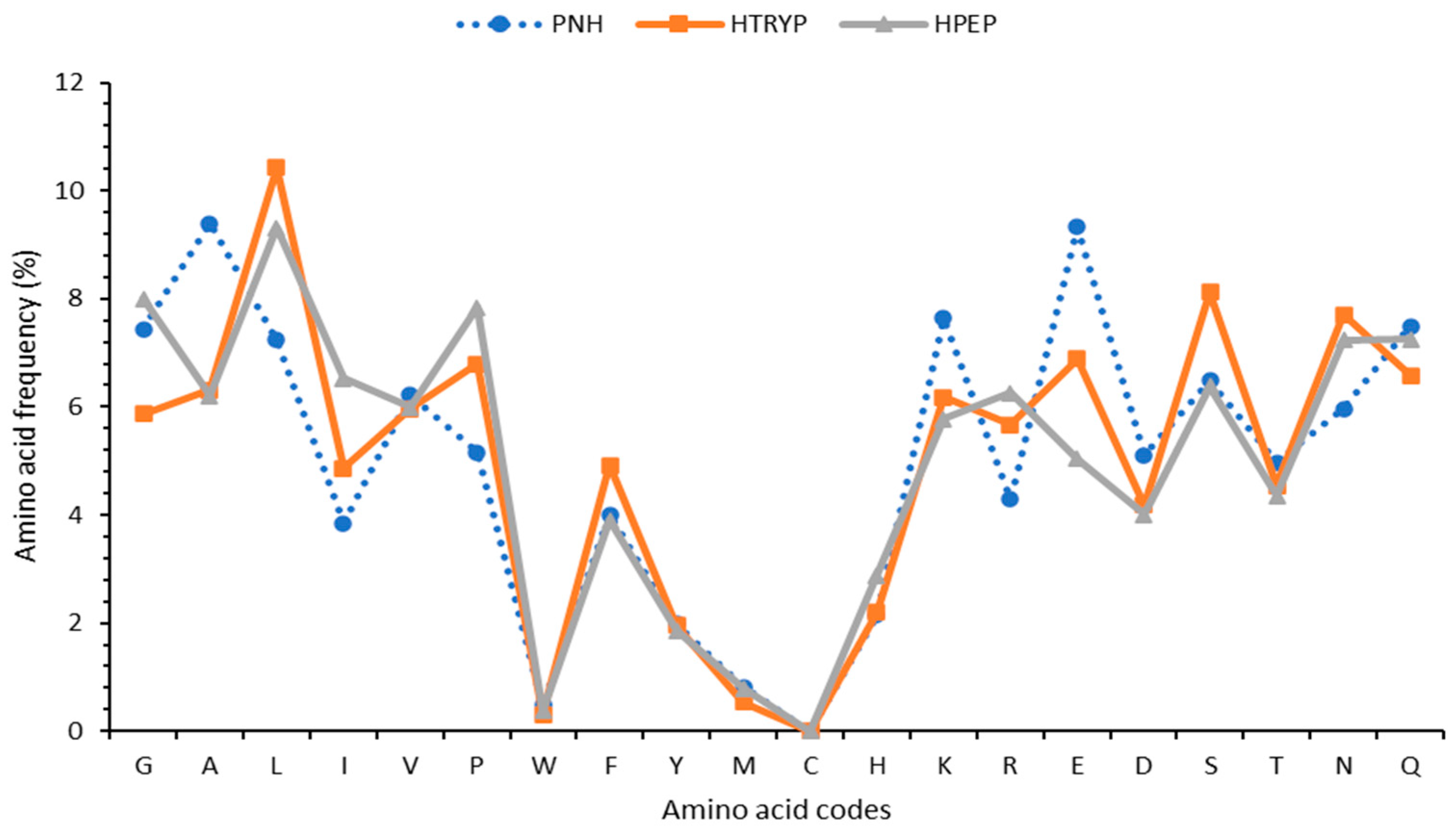
3.2. Mass Spectrometry Identification of Proteins and Peptides
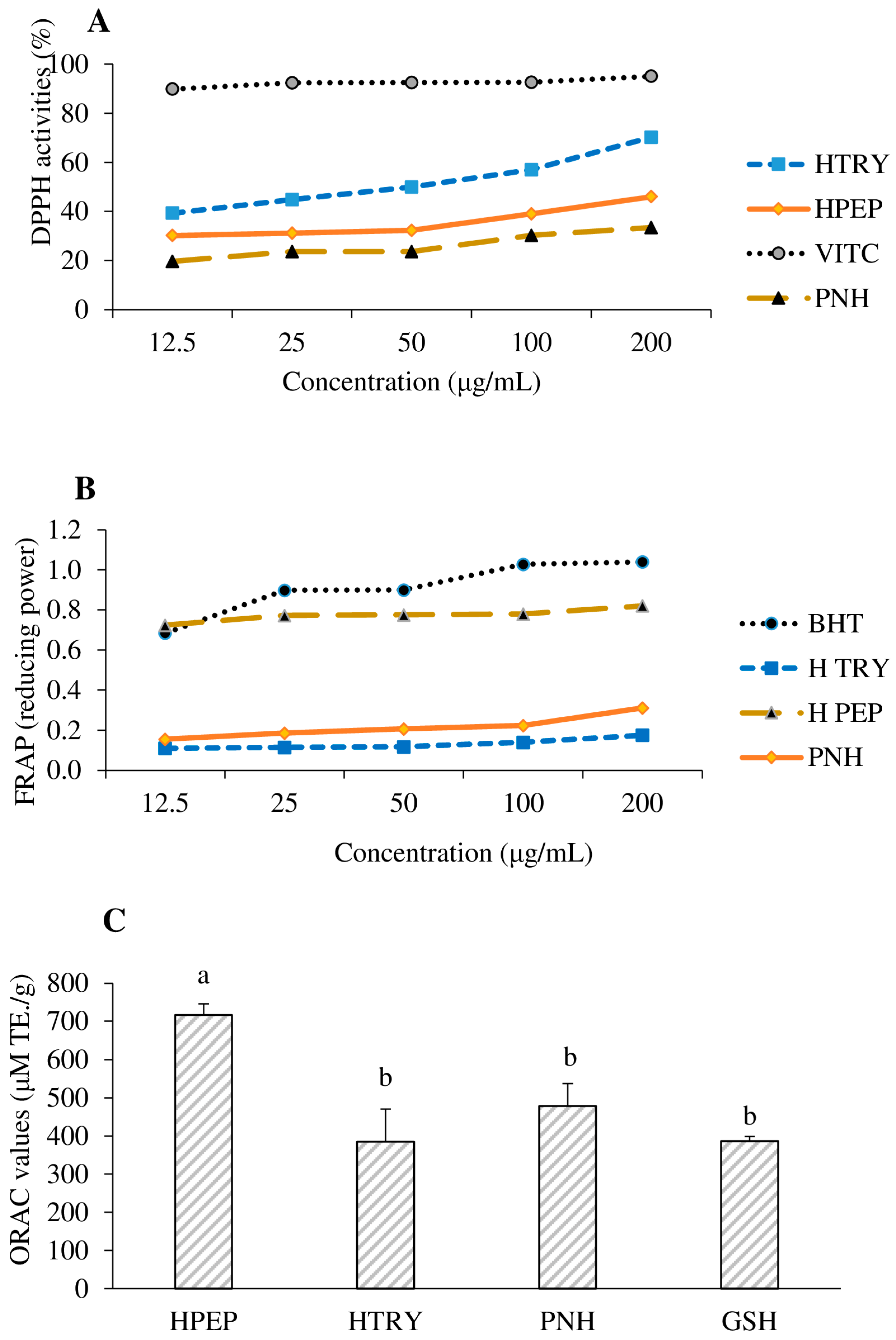
3.3. Antioxidant Activities
3.3.1. Ability to Scavenge 2,2-Diphenyl-1-picrylhydrazyl (DPPH•)
3.3.2. Ferric Reducing Antioxidant Power (FRAP)
3.3.3. Oxygen Radical Absorbance Capacity (ORAC) Properties
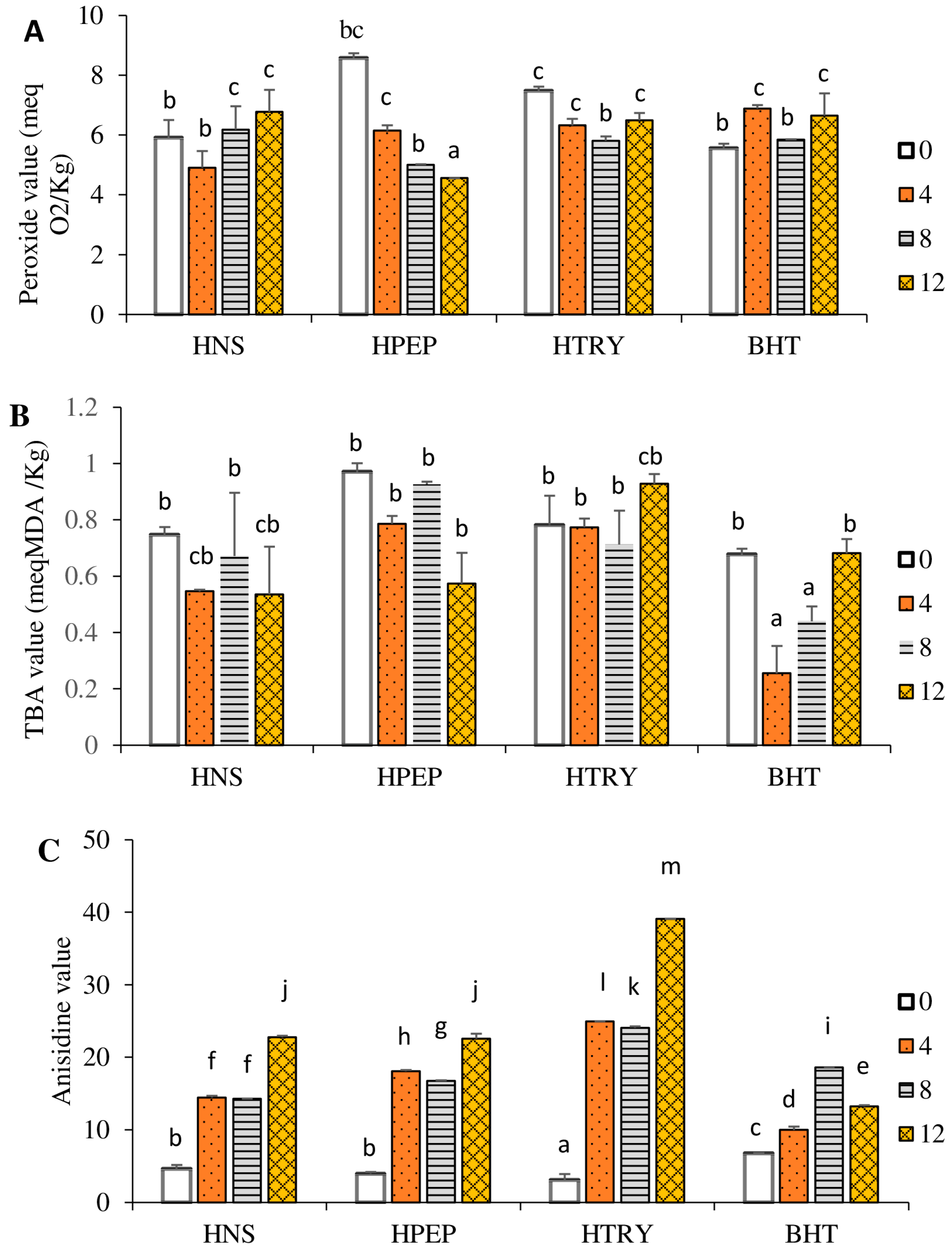
3.4. Quality Chemical Indices
3.4.1. Peroxide Value
3.4.2. Anisidine and Thiobarbituric Acid Values
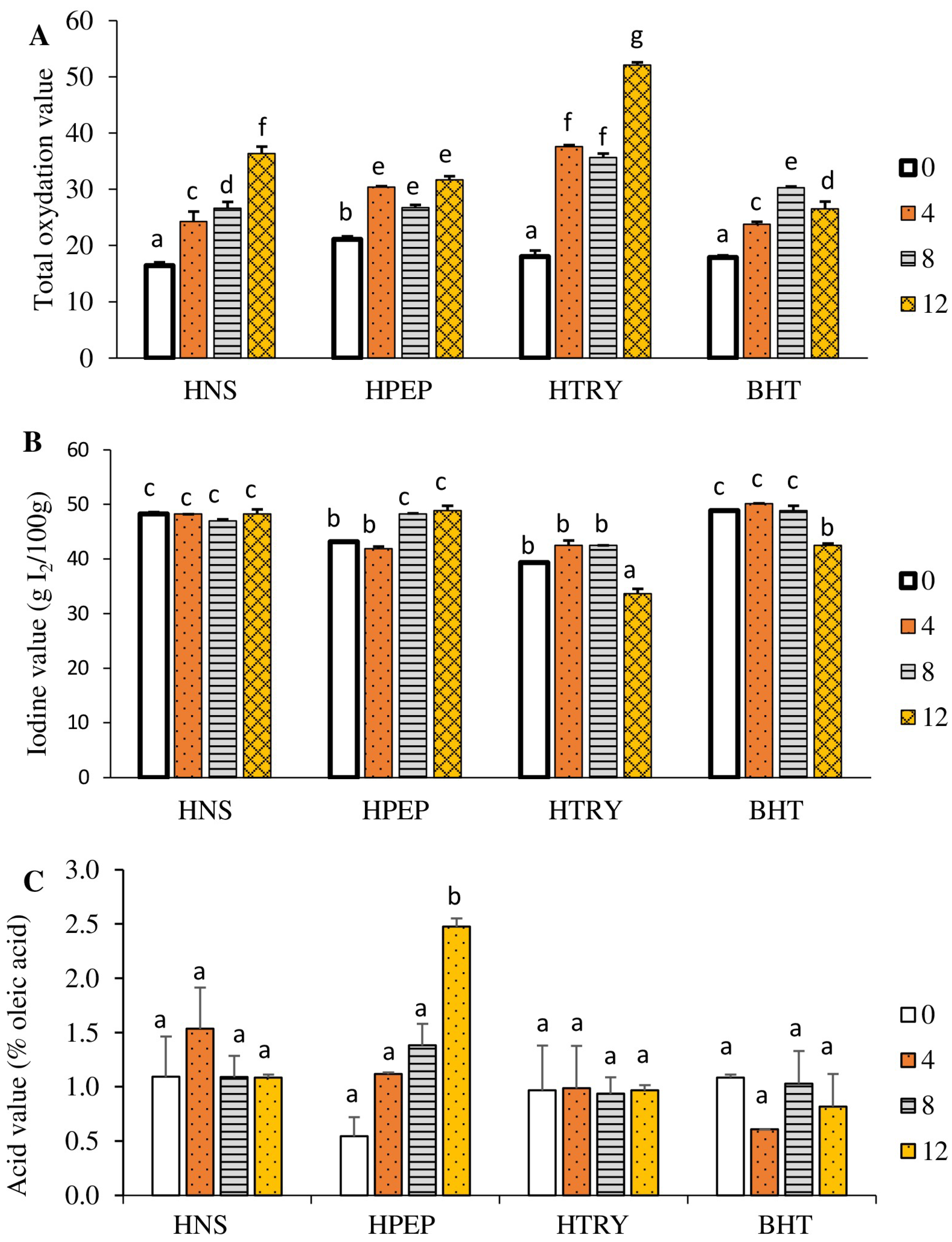
3.4.3. Total Oxidation Value
3.4.4. Iodine Value
3.4.5. Acid Value
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OCDE; FAO. Perspectives Agricoles de l’OCDE et de la FAO 2018–2027. In Oléagineux et Produits Oléagineux; OCDE: Paris, France; FAO: Rome, Italy, 2018; pp. 146–158. [Google Scholar]
- Riaz, M.N. Developing and Producing Protein-Enhanced Snacks and Cereals. In Soy Applications in Food; CRC Press: Boca Raton, FL, USA, 2005; pp. 83–92. ISBN 978-0-429-12564-5. [Google Scholar]
- Zaker, A.; Genitha, T.R.; Hashmi, S.I. Effects of Defatted Soy Flour Incorporation on Physical, Sensorial and Nutritional Properties of Biscuits. J. Food Process. Technol. 2012, 3, 4. [Google Scholar] [CrossRef]
- Ansorena, D.; Ramírez, R.; Lopez de Cerain, A.; Azqueta, A.; Astiasaran, I. Oxidative Stability and Genotoxic Activity of Vegetable Oils Subjected to Accelerated Oxidation and Cooking Conditions. Foods 2023, 12, 2186. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, A.; Arhire, L.I.; Niță, O.; Popa, A.D.; Graur, M.; Mihalache, L. The Relationship between Lifestyle Components and Dietary Patterns. Proc. Nutr. Soc. 2020, 79, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, A.; Yorulmaz, A. The Effect of Rosemary Extract on 3-MCPD and Glycidyl Esters during Frying. Grasas Aceites 2018, 69, e273. [Google Scholar] [CrossRef]
- Womeni, H.M.; Djikeng, F.T.; Tiencheu, B.; Linder, M. Antioxidant Potential of Methanolic Extracts and Powders of Some Cameroonian Spices during Accelerated Storage of Soybean Oil. Adv. Biol. Chem. 2013, 3, 304–313. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Sadawarte, P.D.; Annapure, U.S. Study of the Behavior and Properties of Frying Oil on Repetitive Deep Frying. J. Food. Sci. Technol. 2023, 60, 2549–2556. [Google Scholar] [CrossRef]
- Elaine, E.; Fong, E.L.; Pui, L.P.; Goh, K.M.; Nyam, K.L. The Frying Stability Comparison of Refined Palm Oil, Canola Oil, Corn Oil, Groundnut Oil, and Sunflower Oil during Intermittent Frying of French Fries. J. Food Meas. Charact. 2023, 17, 518–526. [Google Scholar] [CrossRef]
- Wroniak, M.; Raczyk, M.; Kruszewski, B.; Symoniuk, E.; Dach, D. Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants 2021, 10, 1637. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Ahmad, M.; Kousar, M. ul Quality Assessment and Degradative Changes of Deep-Fried Oils in Street Fried Food Chain of Kashmir, India. Food Control. 2022, 141, 109184. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Inhibitory Activities of Soluble and Bound Millet Seed Phenolics on Free Radicals and Reactive Oxygen Species. J. Agric. Food Chem. 2011, 59, 428–436. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef] [PubMed]
- Irwandi, J.; Che Man, Y.B.; Kitts, D.D.; Bakar, J.; Jinap, S. Synergies between Plant Antioxidant Blends in Preventing Peroxidation Reactions in Model and Food Oil Systems. J. Am. Oil Chem. Soc. 2000, 77, 945–951. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; De Souza, D. Detection of Synthetic Antioxidants: What Factors Affect the Efficiency in the Chromatographic Analysis and in the Electrochemical Analysis? Molecules 2022, 27, 7137. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant Properties and Potential Mechanisms of Hydrolyzed Proteins and Peptides from Cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [PubMed]
- Campos Espinosa, G.Y.; Udenigwe, C.C.; Tsopmo, A. Inhibition of Low-density Lipoprotein Oxidation, Antioxidative and Bile Acid-binding Capacities of Hydrolyzed Proteins from Carbohydrase-treated Oat Bran. J. Food Biochem. 2022, 46, e13675. [Google Scholar] [CrossRef] [PubMed]
- Supawong, S.; Park, J.W.; Thawornchinsombut, S. Effect of Rice Bran Hydrolysates on Physicochemical and Antioxidative Characteristics of Fried Fish Cakes during Repeated Freeze-Thaw Cycles. Food Biosci. 2019, 32, 100471. [Google Scholar] [CrossRef]
- He, S.; Franco, C.; Zhang, W. Fish Protein Hydrolysates: Application in Deep-Fried Food and Food Safety Analysis. J. Food Sci. 2015, 80, E108–E115. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Winkler-Moser, J.K. Antioxidant Activity of Amino Acids in Soybean Oil at Frying Temperature: Structural Effects and Synergism with Tocopherols. Food Chem. 2017, 221, 1168–1177. [Google Scholar] [CrossRef]
- Meshginfar, N.; Sadeghi Mahoonak, A.; Ghorbani, M.; Aalami, M. Effects of Protein Hydrolysate from Sheep Visceral on Oxidative Stability of Soybean Oil and Chicken Sausage. J. Food Process. Preserv. 2017, 41, e12875. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I. Stabilization of Sunflower Oil by Garlic Extract during Accelerated Storage. Food Chem. 2007, 100, 246–254. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Heltiarachchy, N.S. Protein Concentrates from Unstabilized and Stabilized Rice Bran: Preparation and Properties. J. Food Sci. 1995, 60, 1066–1069. [Google Scholar] [CrossRef]
- Goertzen, A.D.; House, J.D.; Nickerson, M.T.; Tanaka, T. The Impact of Enzymatic Hydrolysis Using Three Enzymes on the Nutritional Properties of a Chickpea Protein Isolate. Cereal Chem. 2021, 98, 275–284. [Google Scholar] [CrossRef]
- Walters, M.E.; Udenigwe, C.C.; Tsopmo, A. Structural Characterization and Functional Properties of Proteins from Oat Milling Fractions. J. Am. Oil Chem. Soc. 2018, 95, 991–1000. [Google Scholar] [CrossRef]
- Baakdah, M.M.; Tsopmo, A. Identification of Peptides, Metal Binding and Lipid Peroxidation Activities of HPLC Fractions of Hydrolyzed Oat Bran Proteins. J. Food Sci. Technol. 2016, 53, 3593–3601. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical Statistical Model to Estimate the Accuracy of Peptide Identifications Made by MS/MS and Database Search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Jodayree, S.; Smith, J.C.; Tsopmo, A. Use of Carbohydrase to Enhance Protein Extraction Efficiency and Antioxidative Properties of Oat Bran Protein Hydrolysates. Food Res. Int. 2012, 46, 69–75. [Google Scholar] [CrossRef]
- ISO 3976:2006|74A; Anhydrous Milkfat: Determination of Peroxide Value. International Dairy Fédération International IDF Standard: Brussels, Belgium, 1991.
- AOAC. Officials Methods and Recommanded Protices of the American Oil Chemists Society; American Oil Chemists Society: Urbana, IL, USA, 2003. [Google Scholar]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef]
- Association Française de Normalisation. Corps Gras, Graines Oléagineuses, Produits Dérivés: Recueil de Normes Françaises; Recueil de Normes Françaises; AFNOR: Saint-Denis, France, 1988; ISBN 978-2-12-176041-4. [Google Scholar]
- Hamada, J.S. Characterization and Functional Properties of Rice Bran Proteins Modified by Commercial Exoproteases and Endoproteases. J. Food Sci. 2000, 65, 305–310. [Google Scholar] [CrossRef]
- Fabian, C.; Ju, Y.-H. A Review on Rice Bran Protein: Its Properties and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardini, R.; Mullen, A.M.; Bolton, D.; Kerry, J.; O’Neill, E.; Hayes, M. Assessment of the Angiotensin-I-Converting Enzyme (ACE-I) Inhibitory and Antioxidant Activities of Hydrolysates of Bovine Brisket Sarcoplasmic Proteins Produced by Papain and Characterisation of Associated Bioactive Peptidic Fractions. Meat Sci. 2012, 90, 226–235. [Google Scholar] [CrossRef]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Dei Più, L.; Gianotti, A. LC-ESI-QTOF-MS Identification of Novel Antioxidant Peptides Obtained by Enzymatic and Microbial Hydrolysis of Vegetable Proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Chen, N.; Chen, J.; Yao, B.; Li, Z. QSAR Study on Antioxidant Tripeptides and the Antioxidant Activity of the Designed Tripeptides in Free Radical Systems. Molecules 2018, 23, 1407. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Asi, M.R.; Chatha, S.A.S. Antioxidant Potential of Extracts from Different Agro Wastes: Stabilization of Corn Oil. Grasas Aceites 2008, 59, 205–217. [Google Scholar] [CrossRef]
- Codex Alimentarius. Commission Codex-Stan 210-1999: Codex Standard for Named Vegetable Oils. In Fats, Oils and Related Products; FAO; WHO: Rome, Italy, 2001; Volume 8, ISBN 92-5-104682-4. [Google Scholar]
- Debnath, S.; Rastogi, N.K.; Gopala Krishna, A.G.; Lokesh, B.R. Effect of Frying Cycles on Physical, Chemical and Heat Transfer Quality of Rice Bran Oil during Deep-Fat Frying of Poori: An Indian Traditional Fried Food. Food Bioprod. Process. 2012, 90, 249–256. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the Ability of Antioxidants to Counteract Lipid Oxidation: Existing Methods, New Trends and Challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Ying, Z.; Lei, Y.; YuanGang, Z.; XiaoQiang, C.; Fuji, W.; Fang, L. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants during Accelerated Storage. Food Chem. 2010, 118, 656–662. [Google Scholar]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W.; Wu, J. Antioxidative and Functional Properties of Protein Hydrolysate from Defatted Skipjack (Katsuwonous Pelamis) Roe. Food Chem. 2012, 135, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Shabanpour, B.; Pourashouri, P.; Hajfathalian, M.; Jacobsen, C. Extraction of Unsaturated Fatty Acid-Rich Oil from Common Carp (Cyprinus Carpio) Roe and Production of Defatted Roe Hydrolysates with Functional, Antioxidant, and Antibacterial Properties: Common Carp Roe Oil and Functional, Antioxidant, and Antibacterial Hydrolysate. J. Sci. Food Agric. 2018, 98, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, J.; Mei, T.; Xu, M.; Jia, C.; Duan, C.; Dai, H.; Liu, X.; Pi, F. Spectroscopic Studies on Thermal Degradation and Quantitative Prediction on Acid Value of Edible Oil during Frying by Raman Spectroscopy. Spectrochim. Acta Part A 2023, 293, 122477. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pougoue Ngueukam, A.A.; Klang, M.J.; Zokou, R.; Teboukeu Boungo, G.; Djikeng Tonfack, F.; Azeez, B.K.; Womeni, H.M.; Tsopmo, A. Peptidomics Analysis of Soy Protein Hydrolysates—Antioxidant Properties and Mechanism of their Inhibition of the Oxidation of Palm Olein during Frying Cycles. Foods 2023, 12, 3498. https://doi.org/10.3390/foods12183498
Pougoue Ngueukam AA, Klang MJ, Zokou R, Teboukeu Boungo G, Djikeng Tonfack F, Azeez BK, Womeni HM, Tsopmo A. Peptidomics Analysis of Soy Protein Hydrolysates—Antioxidant Properties and Mechanism of their Inhibition of the Oxidation of Palm Olein during Frying Cycles. Foods. 2023; 12(18):3498. https://doi.org/10.3390/foods12183498
Chicago/Turabian StylePougoue Ngueukam, Annick Arcelle, Mathilde Julie Klang, Ronice Zokou, Gires Teboukeu Boungo, Fabrice Djikeng Tonfack, Barakat Koyinsola Azeez, Hilaire Macaire Womeni, and Apollinaire Tsopmo. 2023. "Peptidomics Analysis of Soy Protein Hydrolysates—Antioxidant Properties and Mechanism of their Inhibition of the Oxidation of Palm Olein during Frying Cycles" Foods 12, no. 18: 3498. https://doi.org/10.3390/foods12183498
APA StylePougoue Ngueukam, A. A., Klang, M. J., Zokou, R., Teboukeu Boungo, G., Djikeng Tonfack, F., Azeez, B. K., Womeni, H. M., & Tsopmo, A. (2023). Peptidomics Analysis of Soy Protein Hydrolysates—Antioxidant Properties and Mechanism of their Inhibition of the Oxidation of Palm Olein during Frying Cycles. Foods, 12(18), 3498. https://doi.org/10.3390/foods12183498




