Antimicrobial Activity of Frankincense (Boswellia sacra) Oil and Smoke against Pathogenic and Airborne Microbes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Frankincense Samples
2.2. Extraction of Frankincense Essential Oil by Steam Distillation
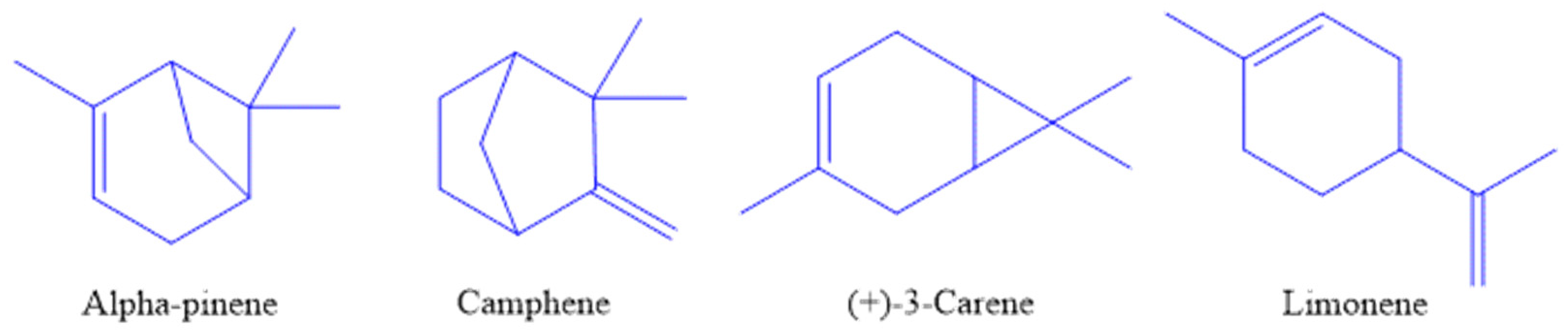
2.3. Chemical Analysis of Essential Oil of Frankincense
2.4. Antimicrobial Activity of Essential Oil of Frankincense
2.4.1. Microorganisms
2.4.2. Screening Antibacterial and Antifungal Activity of Essential Oil of Frankincense
2.4.3. Determination of the Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal/Fungicidal Concentrations (MBCs/MFCs)
2.5. Analysis of Frankincense Smoke Solid Particles
2.6. Screening Antimicrobial Activity of Frankincense Smoke against Airborne Microbes
2.6.1. Collection of Airborne Microorganisms
2.6.2. Exposure of Airborne Microorganisms to Frankincense Smoke
2.7. Statistical Methods
3. Results
3.1. Yield and Chemical Composition of Essential Oils
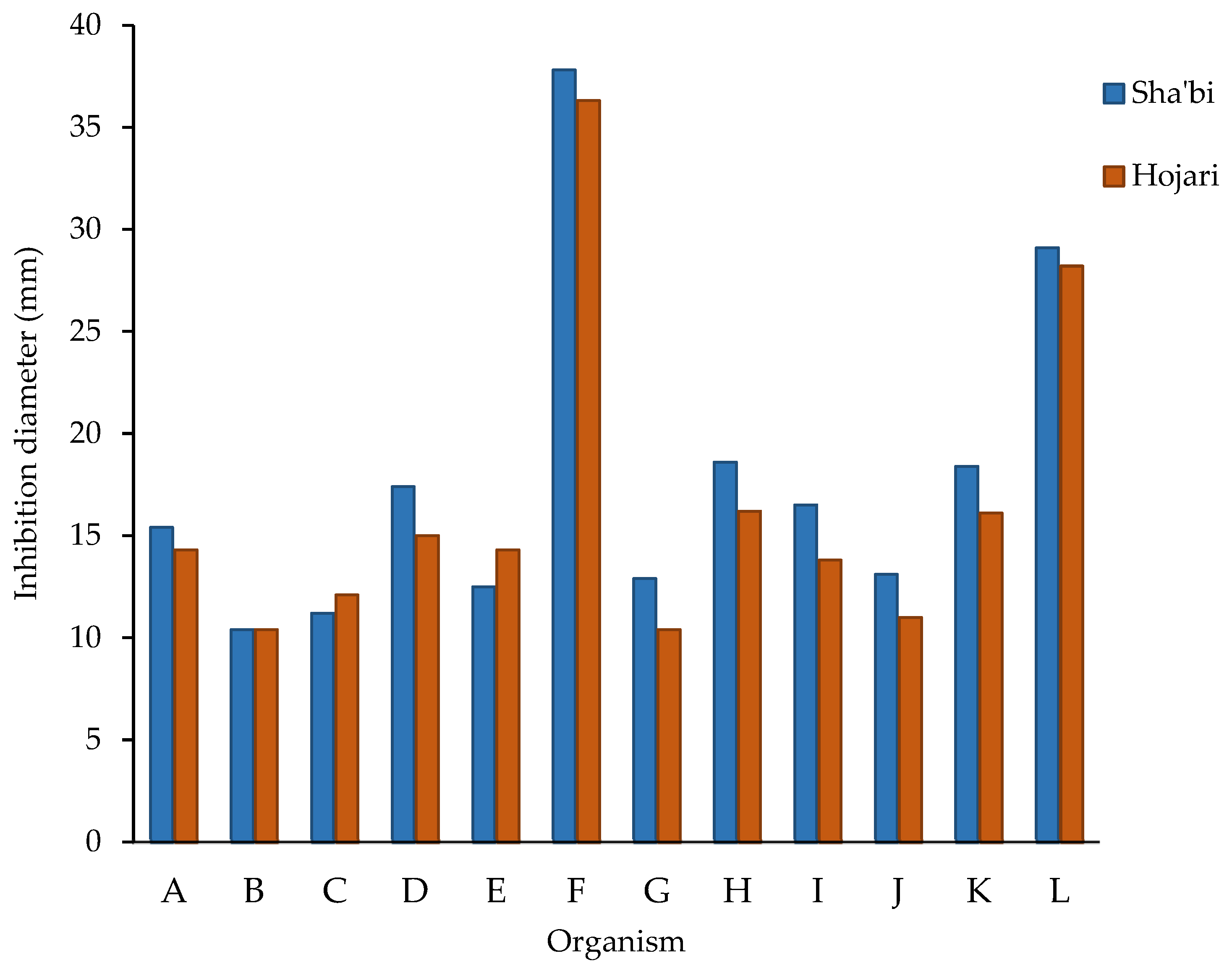
3.2. Antibacterial and Antifungal Activity of Frankincense Oil
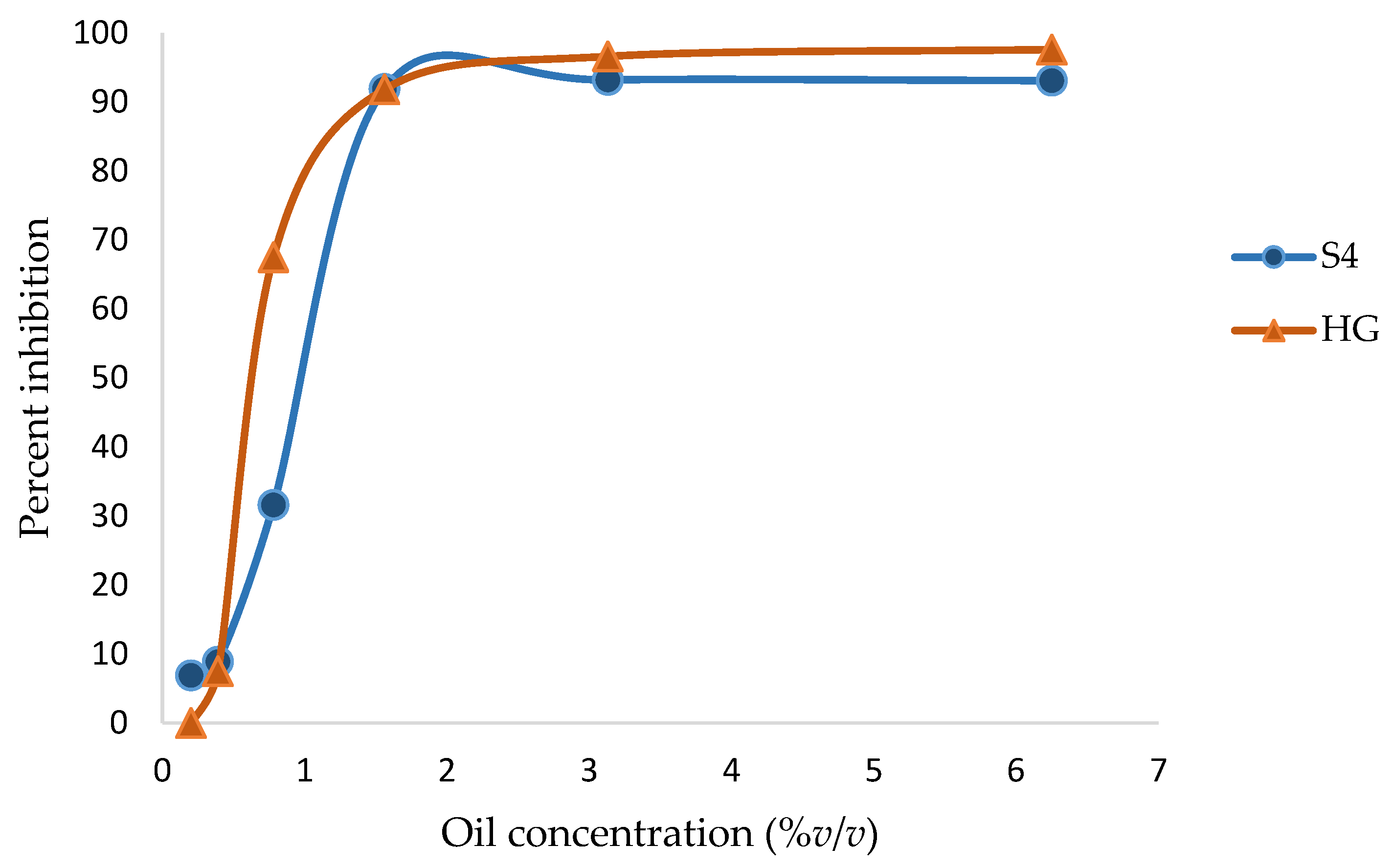
3.3. MICs and MBCs/MFCs of Frankincense Oil
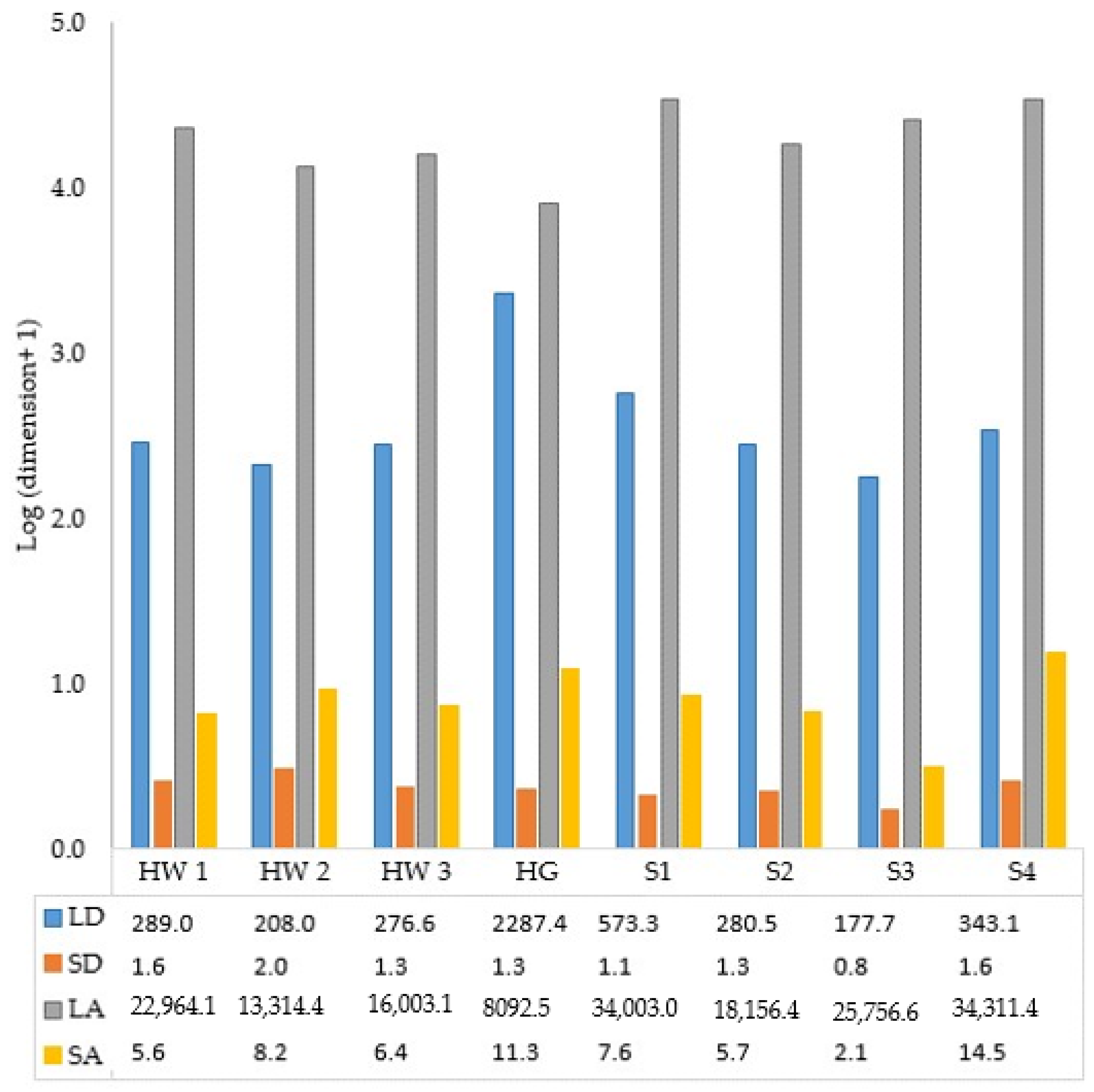
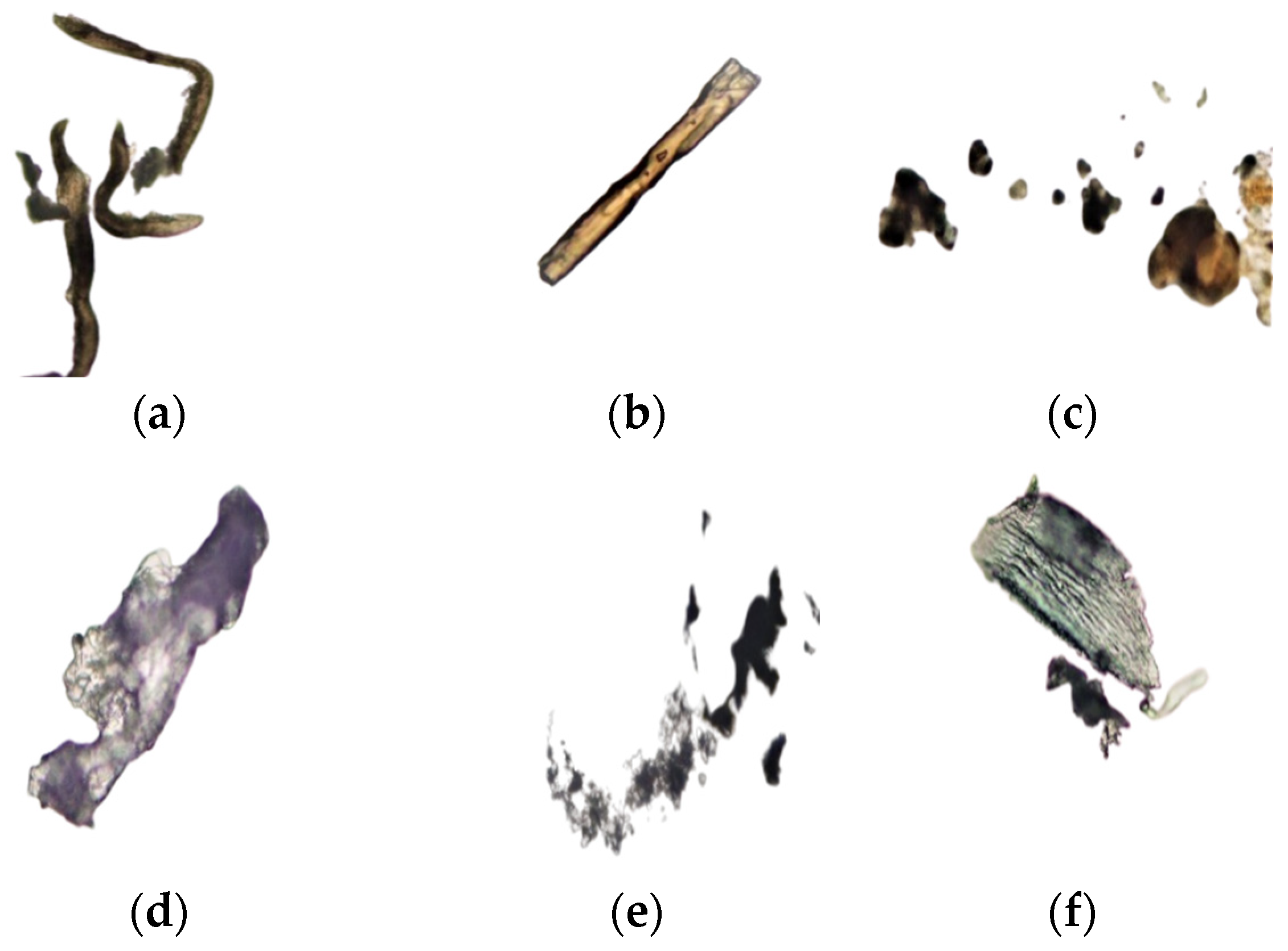
3.4. Dimensions of Frankincense Smoke Solid Particles
3.5. Antimicrobial Activity of Frankincense Smoke
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Tulp, M.; Bohlin, L. Unconventional natural sources for future drug discovery. Drug Discov. Today 2004, 9, 450–458. [Google Scholar] [PubMed]
- Mothana, R.A.A.; Lindequist, U. Antimicrobial activity of some medicinal plants of the island Soqatra. J. Ethnopharmacol. 2005, 96, 177–181. [Google Scholar]
- Abdallah, E.M.; Khalid, A.S.; Ibrahim, N. Antibacterial activity of oleo-gum resins of Commiphora molmol and Boswellia papyrifera against methicillin resistant Staphylococcus aureus (MRSA). Sci. Res. Essay 2009, 4, 351–356. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol 2008, 46, 446–475. [Google Scholar]
- Manou, I.; Bouillard, L.; Devleechouwer, M.J.; Barel, A.O. Evaluation of the preservative properties of Thymus vulgaris essential oil in topically applied formulations under a challenge test. J. Appl. Microbiol. 1998, 84, 368–376. [Google Scholar]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential application in foods-a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar]
- Khalifa, S.A.M.; Kotb, S.M.; El-Seedi, S.H.; Nahar, L.; Sarker, S.D.; Guo, Z.; Zou, X.; Musharraf, S.G.; Jassbi, A.R.; Du, M.; et al. Frankincense of Boswellia sacra: Traditional and modern applied uses, pharmacological activities, and clinical trials. Ind. Crops Prod. 2023, 203, 117106. [Google Scholar] [CrossRef]
- Culioli, G.; Mathe, C.; Archier, P.; Vieillescazes, C. A lupane triterpene from frankincense (Boswellia sp., Burseraceae). Phytochemistry 2003, 62, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.; Buettner, A.; Kirchhoff, E. The volatile constituents of frankincense-a review. Flavour Fragr. J. 2009, 24, 279–300. [Google Scholar] [CrossRef]
- Di Stefano, V.; Schillaci, D.; Cusimano, M.G.; Rishan, M.; Rashan, L. In vitro antimicrobial activity of frankincense oils from Boswellia sacra grown in different locations of the Dhofar region (Oman). Antibiotics 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Hamm, S.; Bleton, J.; Connan, J.; Tchapla, A. A chemical investigation by headspace SPME and GC-MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry 2005, 66, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Hamm, S.; Lesellier, E.; Bleton, J.; Tchapla, A. Optimization of headspace solid phase microextraction for gas chromatography/mass spectrometry analysis of widely different volatility and polarity terpenoids in olibanum. J. Chromatogr. A 2003, 1018, 73–83. [Google Scholar] [CrossRef]
- Lemenih, M.; Teketay, D. Frankincense and myrrh resources of Ethiopia: II. Medicinal and industrial uses. Ethiop. J. Sci. Sustain. 2003, 26, 161–172. [Google Scholar]
- Tucker, A.O. Frankincense and myrrh. Econ. Bot. 1986, 40, 425–433. [Google Scholar] [CrossRef]
- Coppi, A.; Cecchi, L.; Selvi, F.; Raffaelli, M. The frankincense tree (Boswellia sacra, Burseraceae) from Oman: ITS and ISSR analyses of genetic diversity and implications for conservation. Genet. Resour. Crop Evol. 2010, 57, 1041–1052. [Google Scholar] [CrossRef]
- Farah, M.H. Non-Timber Forest Product (NTFP) Extraction in Arid Environments: Land-Use Change, Frankincense Production and the Sustainability of Boswellia sacra in Dhofar (Oman). Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, 2008. [Google Scholar]
- Schillaci, D.; Arizza, V.; Dayton, T.; Camarda, L.; Di Stefano, V. In vitro anti-biofilm activity of Boswellia spp. oleogum resin essential oils. Lett. Appl. Microbiol. 2008, 47, 433–438. [Google Scholar] [CrossRef]
- Al-Rawas, O.A.; Al-Maniri, A.A.; Al-Riyami, B.M. Home exposure to Arabian incense (bakhour) and asthma symptoms in children: A community survey in two regions in Oman. BMC Pulm. Med. 2009, 9, 23. [Google Scholar] [CrossRef]
- Tran, T.C.; Marriott, P.J. Characterization of incense smoke by solid phase microextraction-comperhensive two-dimensional gas chromatography (GC × GC). Atmos. Environ. 2007, 41, 5756–5768. [Google Scholar] [CrossRef]
- Lin, T.; Krishnaswamy, G.; Chi, D.S. Incense smoke: Clinical, structural and molecular effects on airway disease. Clin. Mol. Allergy CMA 2008, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement Med. Ther. 2021, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Al-Harrasi, A.; Al-Saidi, S. Phytochemical analysis of the essential oil from botanically certified oleogum resin of Boswellia sacra (Omani luban). Molecules 2008, 13, 2181–2189. [Google Scholar] [CrossRef]
- Boutekedjiret, C.; Bentahar, F.; Belabbes, R.; Bessiere, J.M. Extraction of rosemary essential oil by steam distillation and hydrodistillation. Flavour Fragr. J. 2003, 18, 481–484. [Google Scholar] [CrossRef]
- Nzeako, B.C.; Al-Kharousi, Z.S.; Al-Mahrooqui, Z. Antimicrobial activities of clove and thyme extracts. Sultan Qaboos Univ. Med. J. 2006, 6, 33–39. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Reimer, L.G.; Stratton, C.W.; Reller, L.B. Minimum inhibitory and bactericidal concentrations of 44 antimicrobial agents against three standard control strains in broth with and without human serum. Antimicrob. Agents Chemother. 1981, 19, 1050–1055. [Google Scholar] [CrossRef]
- Hili, P.; Evans, C.S.; Venes, R.G. Antimicrobial action of essential oils: The effects of dimethylsulphoxide on the activity of cinnamon oil. Lett. Appl. Microbiol. 1997, 24, 269–275. [Google Scholar] [CrossRef]
- Reponen, T.; Grinshpun, S.A.; Conwell, K.L.; Wiest, J.; Anderson, M. Aerodynamic versus physical size of spores: Measurement and implication for respiratory deposition. Grana 2001, 40, 119–125. [Google Scholar] [CrossRef]
- Mothershaw, A.S.; Al-Raisi, A.N. Air as a source of bacterial contamination in a slaughterhouse prior to implementation of hygienic control systems. Int. J. Postharvest Technol. Innov. 2008, 1, 337–347. [Google Scholar] [CrossRef]
- Al-Kharousi, Z.S.; Nzeako, B.; Mothershaw, A.S. Initial observation on the interaction of frankincense oil with selected plastics. Int. J. Food Prop. 2014, 17, 1182–1184. [Google Scholar] [CrossRef]
- Mikhaeil, B.R.; Maatooq, G.T.; Badria, F.A.; Amer, M.M.A. Chemistry and Immunomodulatory activity of frankincense oil. Z. Naturforschung C 2003, 58, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ribechini, E.; Raffaelli, M.; Colombini, M.P. Botanical and chemical characterization of frankincense resin from Dhofar. In A Port in Arabia between Rome and the Indian Ocean, (3rd C.BC–5thC.AD), Khor Rori Report 2, 3rd ed.; Avanzini, A., Ed.; L’Erma di Breitschneider: Roma, Italy, 2008; pp. 681–686. [Google Scholar]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E. Extraction of Santalum album and Boswellia carteriii Birdw. volatile oil by supercritical carbon dioxide: Influence of some process parameters. Flavour Fragr. J. 2006, 21, 718–724. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.; Dekebo, A.; Dagne, E. Essential oils of some Boswellia spp., myrrh and opopanax. Flavour Fragr. J. 2003, 18, 153–156. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Kamatou, G.P.P.; Viljoen, A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010, 76, 686–691. [Google Scholar] [CrossRef]
- Chao, S.; Young, G.; Oberg, C.; Nakaoka, K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Fragr. J. 2008, 23, 444–449. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Weckesser, S.; Engel, K.; Simon-Haarhaus, B.; Wittmer, A.; Pelz, K.; Schempp, C.M. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine 2007, 14, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Rahman, A.; Choi, U.K.; Youn, S.J.; Kang, S.C. Inhibitory parameters of the essential oil and various extracts of Metasequoia glyptostroboides Miki ex Hu to reduce food spoilage and food-borne pathogens. Food Chem. 2007, 105, 1061–1066. [Google Scholar] [CrossRef]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998, 26, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; Moss, M.O. Food Microbiology, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2000; p. 10. [Google Scholar]
- Venette, J.R.; Harrison, M.D. Factors affecting infection of potato tubers by Alternaria solani in Colorado. Am. Potato J. 1973, 50, 283–292. [Google Scholar] [CrossRef]
- Udomsilp, J.; Piyo, A.; Khang-Khun, P.; Thobunluepop, P. Antifungal properties of essential oils from Thai medicinal plants against rice pathogenic fungi. Asian J. Food Agro-Ind. 2009, 2, 24–30. [Google Scholar]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Jetter, J.J.; Guo, Z.; McBrian, J.A.; Flynn, M.R. Characterization of emissions from burning incense. Sci. Total Environ. 2002, 295, 51–67. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Hussain, H.; Hussain, J.; Al-Rawahi, A.; Hakkim, F.L.; Khan, H.Y.; Ur Rehman, N.; Ali, L.; Al-Harrasi, R.; Al-Hadrami, S.; et al. Two pyrolysate products from Omani frankincense smoke: First evidence of thermal aromatization of boswellic acids. J. Anal. Appl. Pyrolysis 2014, 110, 430–434. [Google Scholar] [CrossRef]


| Organism | Oil | Oil Concentration (% v/v; µg/mL between Brackets) | |||||
|---|---|---|---|---|---|---|---|
| 6.25 (62,500) | 3.13 (31,300) | 1.56 (15,600) | 0.78 (7800) | 0.39 (3900) | 0.20 (2000) | ||
| S. aureus | S1 | 88.0 ± 1.9 | 65.8 ± 19.0 | 28.0 ± 28.7 | 13.4 ± 32.6 | 10.2 ± 31.0 | 11.7 ± 30.8 |
| HW3 | 87.7 ± 0.9 | 59.1 ± 10.6 | 34.7 ± 8.8 | 24.8 ± 10.2 | 23.7 ± 11.1 | 22.8 ± 13.5 | |
| E. coli | S1 | 90.8 ± 1.1 | 90.5 ± 3.5 | 92.6 ± 0.5 | 49.4 ± 1.7 | 18.4 ± 3.0 | 6.0 ± 5.7 |
| HW1 | 91.8 ± 0.8 | 92.7 ± 0.4 | 91.5 ± 2.1 | 32.0 ± 6.4 | 16.3 ± 6.7 | 13.5 ± 4.6 | |
| P. aeruginosa | S3 | 83.4 ± 2.7 | 5.3 ± 6.0 | 2.4 ± 4.4 | −0.8 ± 5.6 | −3.1 ± 4.0 | −1.3 ± 4.3 |
| HW1 | 89.9 ± 1.1 | 9.1 ± 7.5 | 7.5 ± 5.9 | 5.2 ± 6.3 | 2.5 ± 6.3 | 3.4 ± 6.1 | |
| Bacillus spp. | S1 | 83.6 ± 0.5 | 5.9 ± 3.7 | 7.6 ± 11.5 | 11.2 ± 7.2 | 9.6 ± 4.7 | 7.8 ± 5.7 |
| HW3 | 81.2 ± 6.0 | 34.4 ± 11.2 | 34.0 ± 11.5 | 35.7 ± 9.9 | 33.6 ± 7.1 | 38.4 ± 5.8 | |
| C. albicans | S1 | 95.3 ± 0.3 | 95.4 ± 0.2 | 17.9 ± 8.8 | 6.3 ± 4.9 | 1.2 ± 6.7 | 3.7 ± 4.3 |
| HW2 | 96.2 ± 0.6 | 38.8 ± 23.8 | 30.3 ± 6.2 | 17.4 ± 8.8 | 9.9 ± 5.5 | 9.6 ± 3.0 | |
| S. cerevisiae | S1 | 91.6 ± 1.7 | 94.3 ± 0.3 | 92.5 ± 1.9 | 31.7 ± 14.6 | 11.7 ± 3.6 | 9.4 ± 5.0 |
| HW3 | 93.9 ± 0.7 | 94.8 ± 0.3 | 18.0 ± 22.0 | 51.0 ± 3.6 | 18.7 ± 12.1 | 22.2 ± 47.2 | |
| A. flavous | S4 | 89.3 ± 1.1 | 83.6 ± 2.0 | 65.5 ± 6.3 | 46.6 ± 11.3 | 37.3 ± 11.1 | 11.7 ± 7.8 |
| HW2 | 83.7 ± 3.5 | 81.1 ± 6.4 | 72.3 ± 9.6 | 66.1 ± 7.7 | 37.2 ± 23.0 | 38.0 ± 25.0 | |
| A. ochraceus | S3 | 83.0 ± 1.3 | 65.9 ± 15.5 | 69.3 ± 1.2 | 61.0 ± 1.5 | 39.4 ± 8.1 | −8.5 ± 29.9 |
| HW3 | 60.1 ± 16.0 | 61.8 ± 9.6 | 49.0 ± 15.7 | 23.8 ± 19.7 | −0.7 ± 16.4 | 20.7 ± 14.7 | |
| A. niger | S1 | 92.5 ± 2.6 | 88.4 ± 6.0 | 90.0 ± 3.1 | 83.2 ± 8.5 | 26.7 ± 22.5 | −34.6 ± 34.8 |
| HW2 | 90.6 ± 2.7 | 90.0 ± 8.7 | 79.7 ± 36.9 | 27.4 ± 30.6 | −9.0 ± 9.7 | −35.9 ± 18.6 | |
| P. citrinum | S4 | 93.5 ± 2.7 | 77.2 ± 8.7 | 47.4 ± 36.8 | 59.1 ± 30.6 | 12.2 ± 9.7 | −19.7 ± 18.6 |
| HW3 | 75.0 ± 2.1 | 63.1 ±6.0 | 50.0 ± 17.7 | 14.2 ± 5.3 | 17.7 ±8.0 | −11.3 ± 9.7 | |
| A. alternata | S4 | 84.9 ± 7.3 | 72.6 ± 10.2 | 56.7 ± 14.5 | 56.1 ± 22.1 | 32.5 ± 32.3 | 2.2 ± 6.3 |
| HW2 | 82.3 ± 9.8 | 78.7 ± 6.5 | 44.3 ± 12.3 | 50.7 ± 8.3 | 0.9 ± 41.3 | −59.4 ± 37.5 | |
| F. solani | S4 | 93.1 ± 0.7 | 93.2 ± 0.2 | 91.9 ± 1.2 | 31.6 ± 3.9 | 8.9 ± 2.9 | 6.9 ± 0.7 |
| HG | 97.6 ± 0.9 | 96.6 ± 0.3 | 91.8 ± 1.3 | 67.5 ± 12.8 | 7.5 ± 18.3 | 0.0 ± 1.1 | |
| Microorganism | Oil Sample | MIC | MBC/MFC | |
|---|---|---|---|---|
| Visual | OD620 | |||
| S. aureus | S1 | 6.25 (62,500) | >6.25 (>62,500) | 12.50 (125,000) |
| HW3 | 6.25 (62,500) | >6.25 (>62,500) | 12.50 (125,000) | |
| E. coli | S1 | 1.56 (15,600) | 1.56 (15,600) | 1.56 (15,600) |
| HW1 | 1.56 (15,600) | 1.56 (15,600) | 3.13 (31,300) | |
| P. aeruginosa | S3 | 6.25 (62,500) | >6.25 (>62,500) | 12.50 (125,000) |
| HW1 | 6.25 (62,500) | 6.25 (62,500) | 12.50 (125,000) | |
| Bacillus spp. | S1 | 6.25 (62,500) | >6.25 (>62,500) | 12.50 (125,000) |
| HW3 | 6.25 (62,500) | >6.25 (>62,500) | 12.50 (125,000) | |
| C. albicans | S1 | 3.13 (31,300) | 3.13 (31,300) | 3.13 (31,300) |
| HW2 | 6.25 (62,500) | 6.25 (62,500) | 6.25 (62,500) | |
| S. cerevisiae | S1 | 1.56 (15,600) | 1.56 (15,600) | 1.56 (15,600) |
| HW3 | 3.13 (31,300) | 3.13 (31,300) | 3.13 (31,300) | |
| A. flavous | S4 | 12.50 (125,000) | >6.25 (>62,500) | 50.00 (500,000) |
| HW2 | 12.50 (125,000) | >6.25 (>62,500) | 50.00 (500,000) | |
| A. ochraceus | S3 | 12.50 (125,000) | >6.25 (>62,500) | 50.00 (500,000) |
| HW3 | 12.50 (125,000) | >6.25 (>62,500) | >50.00 (>500,000) | |
| A. niger | S1 | 6.25 (62,500) | 1.56 (15,600) | 50.00 (500,000) |
| HW2 | 6.25 (62,500) | 3.13 (31,300) | 50.00 (500,000) | |
| P. citrinum | S4 | 12.50 (125,000) | 6.25 (62,500) | 50.00 (500,000) |
| HW3 | 12.50 (125,000) | >6.25 (>62,500) | 50.00 (500,000) | |
| A. alternata | S4 | 6.25 (62,500) | >6.25 (>62,500) | >50.00 (>500,000) |
| HW2 | 6.25 (62,500) | >6.25 (>62,500) | >50.00 (>500,000) | |
| F. solani | S4 | 1.56 (15,600) | 1.56 (15,600) | 3.13 (31,300) |
| HG | 1.56 (15,600) | 1.56 (15,600) | 3.13 (31,300) | |
| Microorganism | Mean Plate Colony Counts ± SD | |||
|---|---|---|---|---|
| Control Bacteria | Hojari White | Hojari Green | ||
| Controls | Tests | Controls | Tests | |
| S. aureus (NCTC 6571) | 29.3 ± 16.6 | 10 ± 1 | 380.7 ± 155.5 | ND |
| E. coli (NCTC 10418) | 40.7 ± 14.5 | 11.0 ± 4.6 | 320.7 ± 152.0 | ND |
| Air isolates | ||||
| B14 (Gram + bacteria) | 203.3 ± 106.4 | ND | - | - |
| B17 (Gram + bacteria) | 160.7 ± 12.9 | ND | 431.67 ± 133.6 | ND |
| B18 (Gram + bacteria) | 225.0 ± 61.3 | ND | - | - |
| M7 (yeast) | 280.3 ± 89.5 | ND | - | - |
| Growth diameter (mm) | ||||
| M1 (mold) | - | - | 27 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kharousi, Z.S.; Mothershaw, A.S.; Nzeako, B. Antimicrobial Activity of Frankincense (Boswellia sacra) Oil and Smoke against Pathogenic and Airborne Microbes. Foods 2023, 12, 3442. https://doi.org/10.3390/foods12183442
Al-Kharousi ZS, Mothershaw AS, Nzeako B. Antimicrobial Activity of Frankincense (Boswellia sacra) Oil and Smoke against Pathogenic and Airborne Microbes. Foods. 2023; 12(18):3442. https://doi.org/10.3390/foods12183442
Chicago/Turabian StyleAl-Kharousi, Zahra S., Ann S. Mothershaw, and Basil Nzeako. 2023. "Antimicrobial Activity of Frankincense (Boswellia sacra) Oil and Smoke against Pathogenic and Airborne Microbes" Foods 12, no. 18: 3442. https://doi.org/10.3390/foods12183442
APA StyleAl-Kharousi, Z. S., Mothershaw, A. S., & Nzeako, B. (2023). Antimicrobial Activity of Frankincense (Boswellia sacra) Oil and Smoke against Pathogenic and Airborne Microbes. Foods, 12(18), 3442. https://doi.org/10.3390/foods12183442





