Lipid-Lowering Effects of a Novel Polysaccharide Obtained from Fuzhuan Brick Tea In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Purification of Polysaccharides from FBT
2.3. Structural Characterization of FTP3
2.3.1. Polysaccharide, Protein, Uronic Acid, and Total Phenolic Contents
2.3.2. Ultraviolet Spectrum Scan and Fourier-Transform Infrared (FT-IR) Spectrum Analysis
2.3.3. Scanning Electron Microscopy (SEM) Analysis
2.3.4. Molecular Weight (Mw) and Monosaccharide Composition
2.3.5. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
2.3.6. Congo Red Analysis
2.3.7. Atomic Force Microscope (AFM) Analysis
2.4. In Vitro Antioxidant and Glucolipid Ameliorating Activity Assay of FTP3
2.4.1. Antioxidant Activity
2.4.2. Inhibition of α-Glucosidase and α-Amylase Activity
2.4.3. Binding Rates of Cholesterol and Bile Acids
2.4.4. The Survival Rate of HepG2 Cells with FTP3 and OA Treatment
2.4.5. Oil Red O Staining and Lipid Droplet Content
2.4.6. Determination of Biochemical Indicators Related to Lipid Metabolism and Antioxidants in HepG2 Cells
2.4.7. Protein and Gene Expression Related to Lipid Metabolism in HepG2 Cells
2.5. Statistical Analysis
3. Results
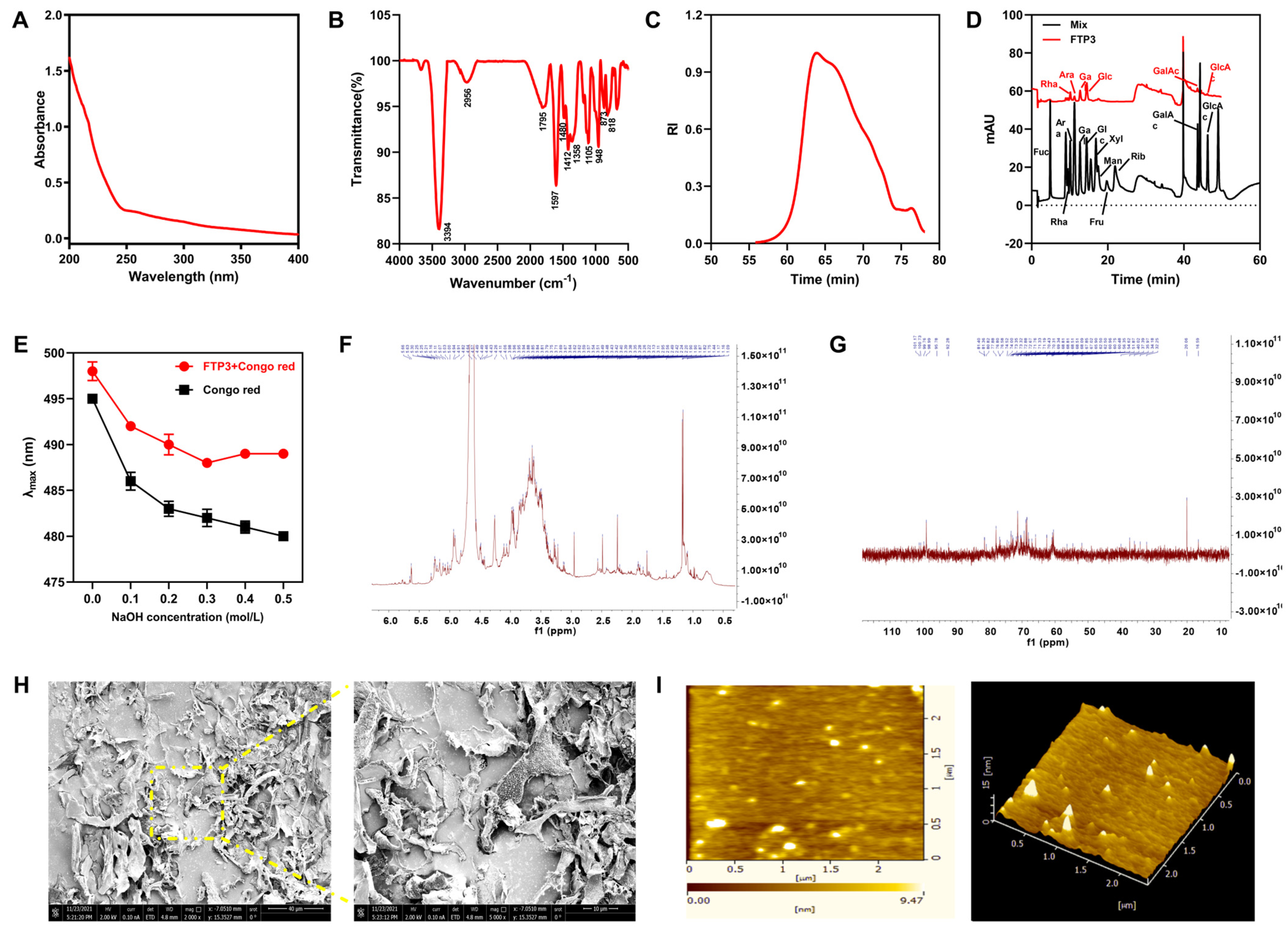
3.1. Structural Characterization of FTP3
3.1.1. Polysaccharide, Protein, Uronic Acid, and Total Phenol Contents
3.1.2. Spectral Analysis
3.1.3. Mw and Monosaccharide Composition
3.1.4. Congo Red Analysis
3.1.5. NMR Analysis
3.1.6. SEM and AFM Analyses
3.2. In Vitro Antioxidant Activity
3.3. In Vitro Inhibition of α-Glucosidase and α-Amylase Activity
3.4. In Vitro Binding to Bile Acids and Cholesterol Activity
3.5. Effect of FTP3 on Lipids Metabolism in HepG2 Cells
3.5.1. The Survival Rate of HepG2 Cells with FTP3 and OA Treatment
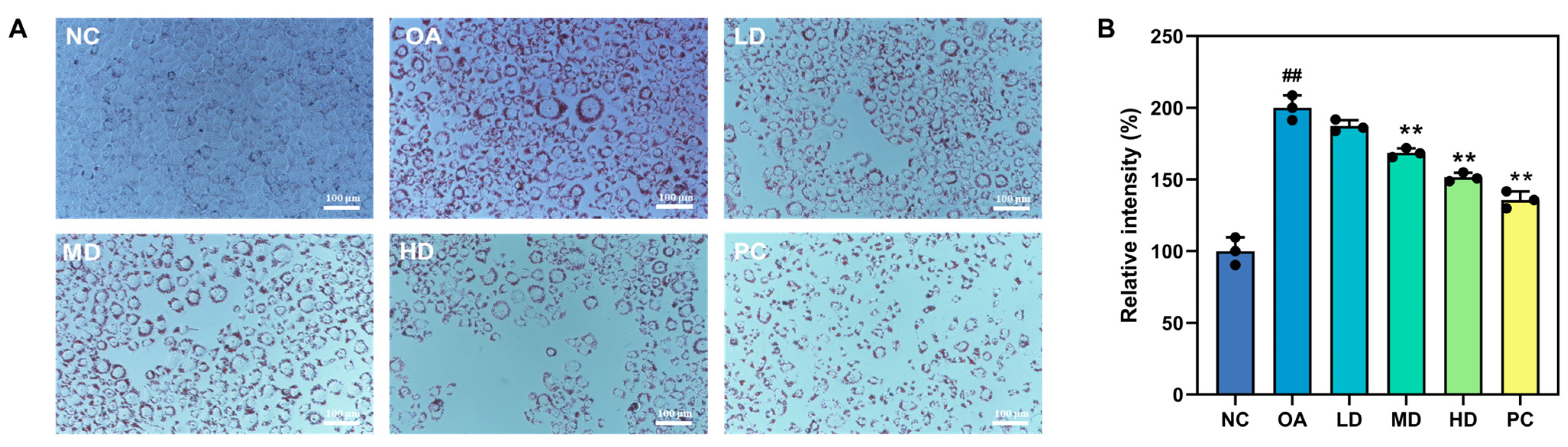
3.5.2. Oil Red O Staining and Lipid Droplet Content Assay
3.5.3. Biochemical Indicators Related to Lipid Metabolism and Antioxidants in HepG2 Cells
3.6. Gene and Protein Expression Associated with Lipid Metabolism
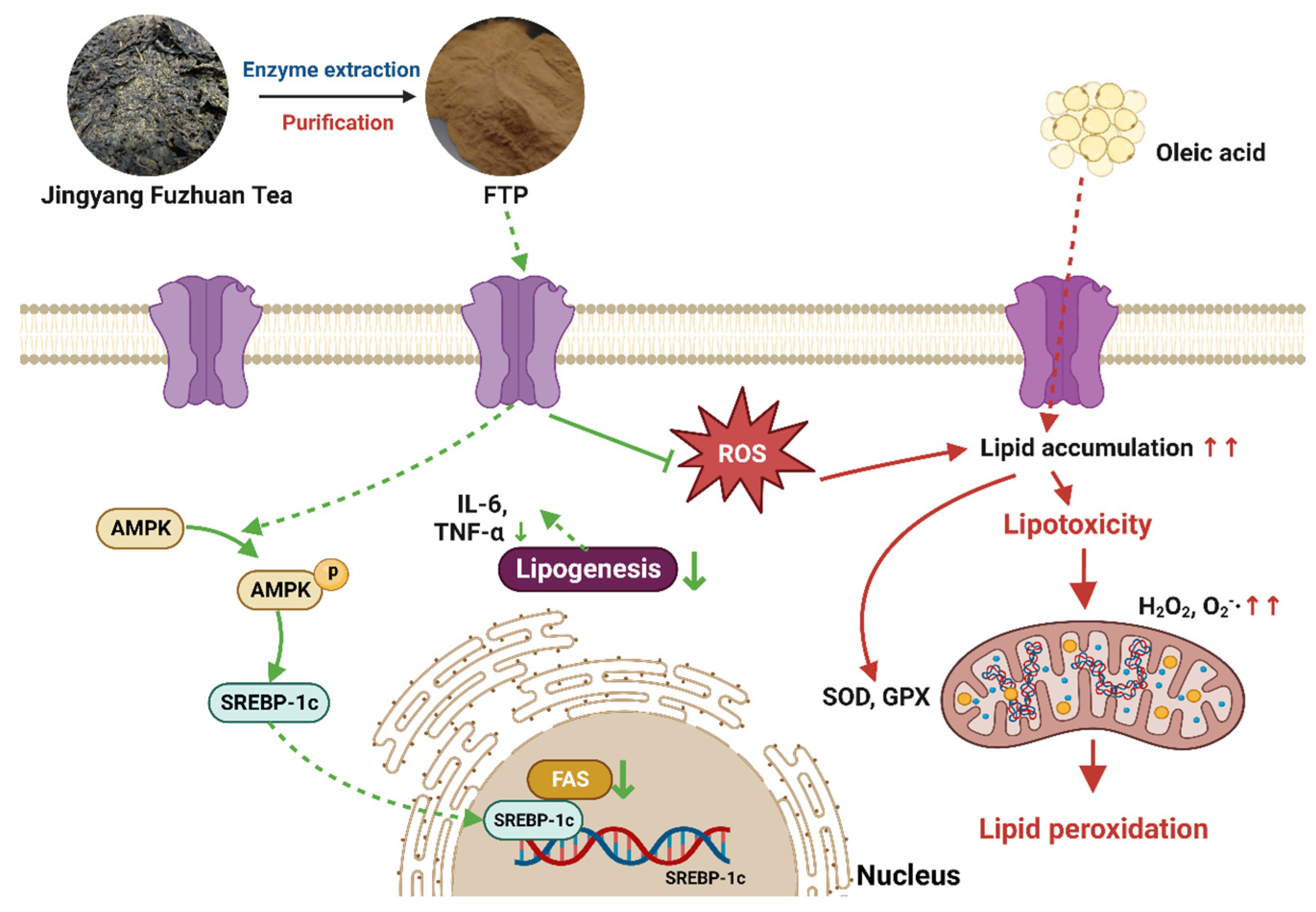
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.X.; Guo, X.X.; Zeng, Z.; Wang, Y.; Qiu, J. Puerarin improves hepatic glucose and lipid homeostasis in vitro and in vivo by regulating the AMPK pathway. Food Funct. 2021, 12, 2726–2740. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, X.F.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Xu, A.Q.; Wang, Y.L.; Wen, J.Y.; Liu, P.; Liu, Z.Y.; Li, Z.J. Fungal community associated with fermentation and storage of Fuzhuan brick-tea. Int. J. Food Microbiol. 2011, 146, 14–22. [Google Scholar] [CrossRef]
- Chen, G.J.; Xie, M.H.; Wan, P.; Chen, D.; Ye, H.; Chen, L.G.; Zeng, X.X.; Liu, Z.H. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Xiao, J.B.; Huo, J.L.; Jiang, H.X.; Yang, F. Chemical compositions and bioactivities of crude polysaccharides from tea leaves beyond their useful date. Int. J. Biol. Macromol. 2011, 49, 1143–1151. [Google Scholar] [CrossRef]
- Chen, G.J.; Xie, M.H.; Wan, P.; Chen, D.; Dai, Z.Q.; Ye, H.; Hu, B.; Zeng, X.X.; Liu, Z.H. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Chen, G.J.; Wang, M.J.; Xie, M.H.; Wan, P.; Chen, D.; Hu, B.; Ye, H.; Zeng, X.X.; Liu, Z.H. Evaluation of chemical property, cytotoxicity and antioxidant activity in vitro and in vivo of polysaccharides from Fuzhuan brick teas. Int. J. Biol. Macromol. 2018, 116, 120–127. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, D.J.; Jiang, H.; Sun, H.Y.; Zhang, C.; Zhao, H.; Li, X.S.; Yan, F.; Chen, C.; Xu, Z.M. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography-mass spectrometry and olfactometry and sensory analysis. Food Chem. 2018, 274, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Z.H.; Huang, J.A.; Luo, G.A.; Liang, Q.L.; Wang, D.; Ye, X.Y.; Wu, C.B.; Wang, L.L.; Hu, J.H. Anti-obesity and hypolipidemic effects of Fuzhuan brick tea water extract in high-fat diet-induced obese rats. J. Sci. Food Agr. 2013, 93, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.H.; Ryan, E.P.; Huang, J.N.; Liu, Z.H.; Weir, T.L.; Snook, R.L.; Ryan, T.P. Fermented Camellia sinensis, Fu Zhuan Tea, regulates hyperlipidemia and transcription factors involved in lipid catabolism. Food Res. Int. 2011, 44, 2999–3005. [Google Scholar] [CrossRef]
- Zhu, J.X.; Yu, C.; Zhou, H.; Wei, X.L.; Wang, Y.F. Comparative evaluation for phytochemical composition and regulation of blood glucose, hepatic oxidative stress and insulin resistance in mice and HepG2 models of four typical Chinese dark teas. J. Sci. Food Agr. 2021, 101, 6563–6577. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhong, K.; Bai, J.R.; Wu, Y.P.; Zhang, J.Q.; Gao, H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (MF800948) and its hypolipidemic activity in a zebrafish model. LWT–Food Sci. Technol. 2020, 117, 108629. [Google Scholar] [CrossRef]
- Beigi, M.; Jahanbin, K. A water-soluble polysaccharide from the roots of Eremurus spectabilis M. B. subsp. spectabilis: Extraction, purification and structural features. Int. J. Biol. Macromol. 2019, 128, 648–654. [Google Scholar]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Wang, M.R.; Yao, W.B.; Yang, W.J.; Chen, F.X. Effects of simulated saliva-gastrointestinal digestion on the physicochemical properties and bioactivities of Siraitia grosvenorii polysaccharides. Food Sci. Technol. 2022, 57, 4495–4506. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Wang, M.R.; Yao, W.B.; Yang, W.J.; Chen, F.X. In vitro digestion and fecal fermentation of Siraitia grosvenorii polysaccharide and its impact on human gut microbiota. Food Funct. 2022, 13, 9443–9458. [Google Scholar] [CrossRef]
- Zhang, T.T.; Ye, J.F.; Xue, C.H.; Wang, Y.M.; Liao, W.Z.; Mao, L.M.; Yuan, M.M.; Lian, S. Structural characteristics and bioactive properties of a novel polysaccharide from Flammulina velutipes. Carbohydr. Polym. 2018, 197, 147–156. [Google Scholar] [CrossRef]
- Xu, S.Y.; Liu, J.P.; Huang, X.; Du, L.P.; Shi, F.L.; Dong, R.; Huang, X.T.; Zheng, K.; Liu, Y.; Cheong, K.L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. Food Sci. Technol. 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Zhou, X.; Sun, P.; Li, B.; Zhang, X. Preliminary characterization and antioxidant and hypoglycemic activities in vivo of polysaccharides from Huidouba. Food Funct. 2018, 9, 6337–6348. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.H.; Wu, J.L.; Xu, W.Q.; Wang, X.Q.; Lu, X. Improvement of simultaneous determination of neutral monosaccharides and uronic acids by gas chromatography. Food Chem. 2017, 220, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Huang, G.L.; Chen, G.Y. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Hou, C.Y.; Yan, Y.Z.; Shi, M.M.; Liu, Y.Q. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Dong, M.S.; Jiang, Y.H.; Wang, C.; Yang, Q.; Jiang, X.L.; Zhu, C.L. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis. Mar. Drugs 2020, 18, 539. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Zhao, J.; Wang, C.; Wei, M.; Dang, T.; Deng, Y.; Sun, J.; Song, S.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of the degradation products from Porphyra haitanensis polysaccharides. Process Biochem. 2018, 74, 185–193. [Google Scholar] [CrossRef]
- He, Y.L.; Ye, M.; Jing, L.Y.; Du, Z.Z.; Surahio, M.; Xu, H.M.; Li, J. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohydr. Polym. 2015, 117, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Fu, X.; Cao, C.L.; Li, C.; Chen, C.; Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 121, 407–414. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Cao, C.L.; Huang, Q.; Zhang, B.; Li, C.; Fu, X. Physicochemical characterization and in vitro hypoglycemic activities of polysaccharides from Sargassum pallidum by microwave-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 109, 357–368. [Google Scholar] [CrossRef]
- Wang, D.Y.; Li, C.X.; Fan, W.C.; Yi, T.; Wei, A.C.; Ma, Y.X. Hypoglycemic and hypolipidemic effects of a polysaccharide from Fructus Corni in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2019, 133, 420–427. [Google Scholar] [CrossRef]
- Zhao, J.H.; Liang, K.Y.; Zhong, H.; Liu, S.Z.; Sun, P.L.; He, R.J. A cold-water polysaccharide-protein complex from Grifola frondosa exhibited antiproliferative activity via mitochondrial apoptotic and Fas/FasL pathways in HepG2 cells. Int. J. Biol. Macromol. 2022, 218, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.T.; Cho, Y.Y.; Hsieh, S.C.; Chiang, A.N. Chinese olive extract ameliorates hepatic lipid accumulation in vitro and in vivo by regulating lipid metabolism. Sci. Rep. 2018, 8, 1057. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, S.; Song, M.X. Polygonatum sibiricum polysaccharide inhibits high glucose-induced oxidative stress, inflammatory response, and apoptosis in RPE cells. J. Recept. Sig. Transd. 2022, 42, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, S.J.; Yang, C.; Du, R.Y.; Zhao, J.Q.; Li, J.J.; Xu, Y.S.; Qin, Y.Y.; Gao, Y.; Huang, W.X. Ginsenoside Rg1 Ameliorates Palmitic Acid-Induced Hepatic Steatosis and Inflammation in HepG2 Cells via the AMPK/NF-κB Pathway. Int. J. Endocrinol. 2019, 2019, 7514802. [Google Scholar] [CrossRef]
- Peng, C.; Han, B.; Wang, B.S.; Zhao, G.Y.; Yuan, F.; Zhao, Y.Y.; Liang, L.; Liu, D.G.; Zhai, Z.H.; Shen, Y.P. Therapy of Prostate Cancer by Nanoyam Polysaccharide. Int. J. Polym. Sci. 2019, 2019, 9543526. [Google Scholar] [CrossRef]
- Bonetti, G.; Tedeschi, P.; Meca, G.; Bertelli, D.; Manes, J.; Brandolini, V.; Maietti, A. In vitro bioaccessibility, transepithelial transport and antioxidant activity of Urtica dioica L. phenolic compounds in nettle based food products. Food Funct. 2016, 7, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Wang, R.T.; Han, A.Y.; Deng, Z.F.; Qi, Z.Y.; Long, H.; Wang, J.T.; Yao, W.B.; et al. Advances in the Role and Mechanisms of Essential Oils and Plant Extracts as Natural Preservatives to Extend the Postharvest Shelf Life of Edible Mushrooms. Foods 2023, 12, 801. [Google Scholar] [CrossRef]
- Sun, Y.F.; Yang, X.B.; Lu, X.S.; Wang, D.Y.; Zhao, Y. Protective effects of Keemun black tea polysaccharides on acute carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food Chem. Toxicol. 2013, 58, 184–192. [Google Scholar] [CrossRef]
- Chen, G.T.; Yuan, B.; Wang, H.X.; Qi, G.H.; Cheng, S.J. Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int. J. Biol. Macromol. 2019, 139, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Zhou, H.; Zhang, J.Y.; Li, F.L.; Wei, K.; Wei, X.L.; Wang, Y.F. Valorization of Polysaccharides Obtained from Dark Tea: Preparation, Physicochemical, Antioxidant, and Hypoglycemic Properties. Foods 2021, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hu, D.; Cui, Z.Y.; Li, H.X.; Man, C.X.; Jiang, Y.J. Lipid-Lowering Effects of Inonotus obliquus Polysaccharide In Vivo and In Vitro. Foods 2021, 10, 3085. [Google Scholar] [CrossRef] [PubMed]
- Copikova, J.; Taubner, T.; Tuma, J.; Synytsya, A.; Duskova, D.; Marounek, M. Cholesterol and fat lowering with hydrophobic polysaccharide derivatives. Carbohydr. Polym. 2015, 116, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.S.; Lin, J.K.; Kuo, Y.Y.; Chen, Y.W.; Chen, P.C. Chronic palmitic acid-induced lipotoxicity correlates with defective trafficking of ATP sensitive potassium channels in pancreatic beta cells. J. Nutr. Biochem. 2018, 59, 37–48. [Google Scholar] [CrossRef]
- Tian, W.; Chen, L.; Zhang, L.; Wang, B.; Li, X.B.; Fan, K.R.; Ai, C.H.; Xia, X.; Li, S.D.; Li, Y. Effects of ginsenoside Rg1 on glucose metabolism and liver injury in streptozotocin-induced type 2 diabetic rats. Genet. Mol. Res. 2017, 16, 9463. [Google Scholar] [CrossRef]
- Kianbakht, S.; Khalighi-Sigaroodi, F.; Dabaghian, F.H. Improved Glycemic Control in Patients with Advanced Type 2 Diabetes Mellitus Taking Urtica dioica Leaf Extract: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Clin. Lab. 2013, 59, 1071–1076. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, W.J.; Yin, J.J.; Feng, L.J.; Li, Y.B.; Yao, X.R.; Zhou, B.J. Alisol A 24-Acetate Prevents Hepatic Steatosis and Metabolic Disorders in HepG2 Cells. Cell. Physiol. Biochem. 2016, 40, 453–464. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Yan, L.J. Chronic Inhibition of Mitochondrial Dihydrolipoamide Dehydrogenase (DLDH) as an Approach to Managing Diabetic Oxidative Stress. Antioxidants 2019, 8, 32. [Google Scholar] [CrossRef]
- Lee, H.I.; Yun, K.W.; Seo, K.I.; Kim, M.J.; Lee, M.K. Scopoletin prevents alcohol-induced hepatic lipid accumulation by modulating the AMPK-SREBP pathway in diet-induced obese mice. Metabolism. 2014, 63, 593–601. [Google Scholar] [CrossRef]
- Pietrzyk, N.; Zakłos-Szyda, M.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus L. fruit phenolic compounds protect against FFA-induced steatosis of HepG2 cells via AMPK pathway. J. Funct. Foods 2021, 80, 104437. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhao, T.; Feng, W.W.; Mao, G.H.; Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; Wang, X.T.; Yang, L.Q.; et al. Schisandra polysaccharide increased glucose consumption by up-regulating the expression of GLUT-4. Int. J. Biol. Macromol. 2016, 87, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Komura, D.L.; Carbonero, E.R.; Gracher, A.H.; Baggio, C.H.; Freitas, C.S.; Marcon, R.; Santos, A.R.; Gorin, P.A.; Iacomini, M. Structure of Agaricus spp. fucogalactans and their anti-inflammatory and antinociceptive properties. Bioresour. Technol. 2010, 101, 6192–6199. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Martera, G.; Goñi, I.; Villanueva-Suárez, M.-J.; Redondo-Cuenca, A. Chemical structure and molecular weight influence the in vitro fermentability of polysaccharide extracts from the edible seaweeds Himathalia elongata and Gigartina pistillata. Food Hydrocoll. 2018, 83, 348–354. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.P.; Zhang, J.M.; Xie, Y.Z.; Cai, W.; Tan, J.B. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 down-regulated hepatic glucose regulatory enzymes in diabetic mice. J. Ethnopharmacol. 2017, 196, 47–57. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Li, G.L.; Yao, W.B.; Yang, W.J. The Gut-Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology. Int. J. Mol. 2023, 24, 4089. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.E.; Wang, N.; Liu, X.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; et al. Chemical Composition and Antioxidant Activities of Polysaccharides from Yingshan Cloud Mist Tea. Oxid. Med. Cell. Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, J.; Liu, Y.L.; Gong, L.X.; Sun, B.G. Rice bran proteins and their hydrolysates modulate cholesterol metabolism in mice on hypercholesterolemic diets. Food Funct. 2016, 7, 2747–2753. [Google Scholar] [CrossRef]




| Gene | Sequence (5′–3′) |
|---|---|
| SREBP-1c-F | GCGGAGCCATGGATTGCAC |
| SREBP-1c-R | CTCTTCCTTGATACCAGGCCC |
| FAS-F | TACATCGACTGCATCAGGCA |
| FAS-R | GATACTTTCCCGTCGCATAC |
| IL-6-F | ACTCACCTCTTCAGAACGAATTG |
| IL-6-R | CCATCTTTGGAAGGTTCAGGTTG |
| TNF-α-F | GAGGCCAAGCCCTGGTATG |
| TNF-α-R | CGGGCCGATTGATCTCAGC |
| β-actin-F | GCCGACAGGATGCAGAAGG |
| β-actin-R | TGGAAGGTGGACAGCGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Cheng, S.; Liu, M.; Li, N.; Wang, J.; Yao, W.; Chen, F.; Xie, J.; Gong, P. Lipid-Lowering Effects of a Novel Polysaccharide Obtained from Fuzhuan Brick Tea In Vitro. Foods 2023, 12, 3428. https://doi.org/10.3390/foods12183428
Yang W, Cheng S, Liu M, Li N, Wang J, Yao W, Chen F, Xie J, Gong P. Lipid-Lowering Effects of a Novel Polysaccharide Obtained from Fuzhuan Brick Tea In Vitro. Foods. 2023; 12(18):3428. https://doi.org/10.3390/foods12183428
Chicago/Turabian StyleYang, Wenjuan, Shirui Cheng, Meng Liu, Nan Li, Jing Wang, Wenbo Yao, Fuxin Chen, Jianwu Xie, and Pin Gong. 2023. "Lipid-Lowering Effects of a Novel Polysaccharide Obtained from Fuzhuan Brick Tea In Vitro" Foods 12, no. 18: 3428. https://doi.org/10.3390/foods12183428
APA StyleYang, W., Cheng, S., Liu, M., Li, N., Wang, J., Yao, W., Chen, F., Xie, J., & Gong, P. (2023). Lipid-Lowering Effects of a Novel Polysaccharide Obtained from Fuzhuan Brick Tea In Vitro. Foods, 12(18), 3428. https://doi.org/10.3390/foods12183428







