The Mechanisms of Plastic Food-Packaging Monomers’ Migration into Food Matrix and the Implications on Human Health
Abstract
1. Introduction
2. Food-Contact Chemicals in Plastic Food-Packaging Types
| Additive Name | Function | Structure | Reference |
|---|---|---|---|
| Plasticizers | Increase the workability and flexibility of final product | Bisphenol A (BPA)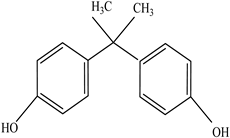 Phthalates 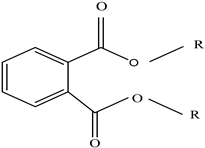 | [58,71] |
| Antioxidants | Scavenge free radicals, reducing the oxidation process that exposure to light causes in polymers | Butylated hydroxytoluene (BHT)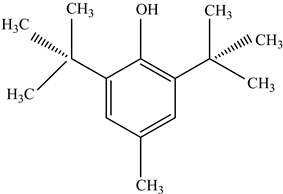 Irganox 1010 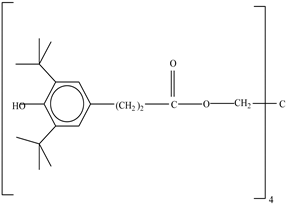 Bisphenol A (BPA) 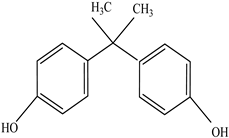 Butylated hydroxyanisole (BHA) 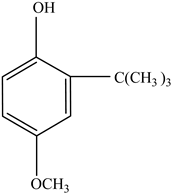 Ionox 100 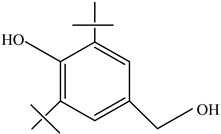 Irganox 1076 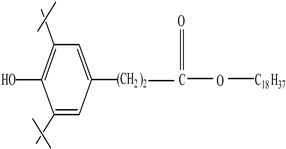 | [72] |
| UV protectants | Stabilize polymers and prevent degradation | UV-326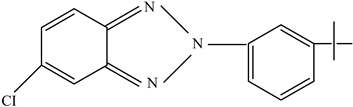 UV-234 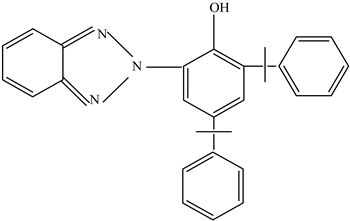 UV-P 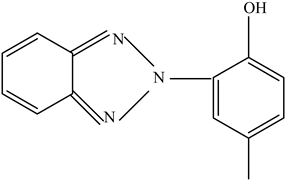 | [73] |
3. Packaging Monomers as Sources of Endocrine-Disrupting Compounds (EDCs) in Foods
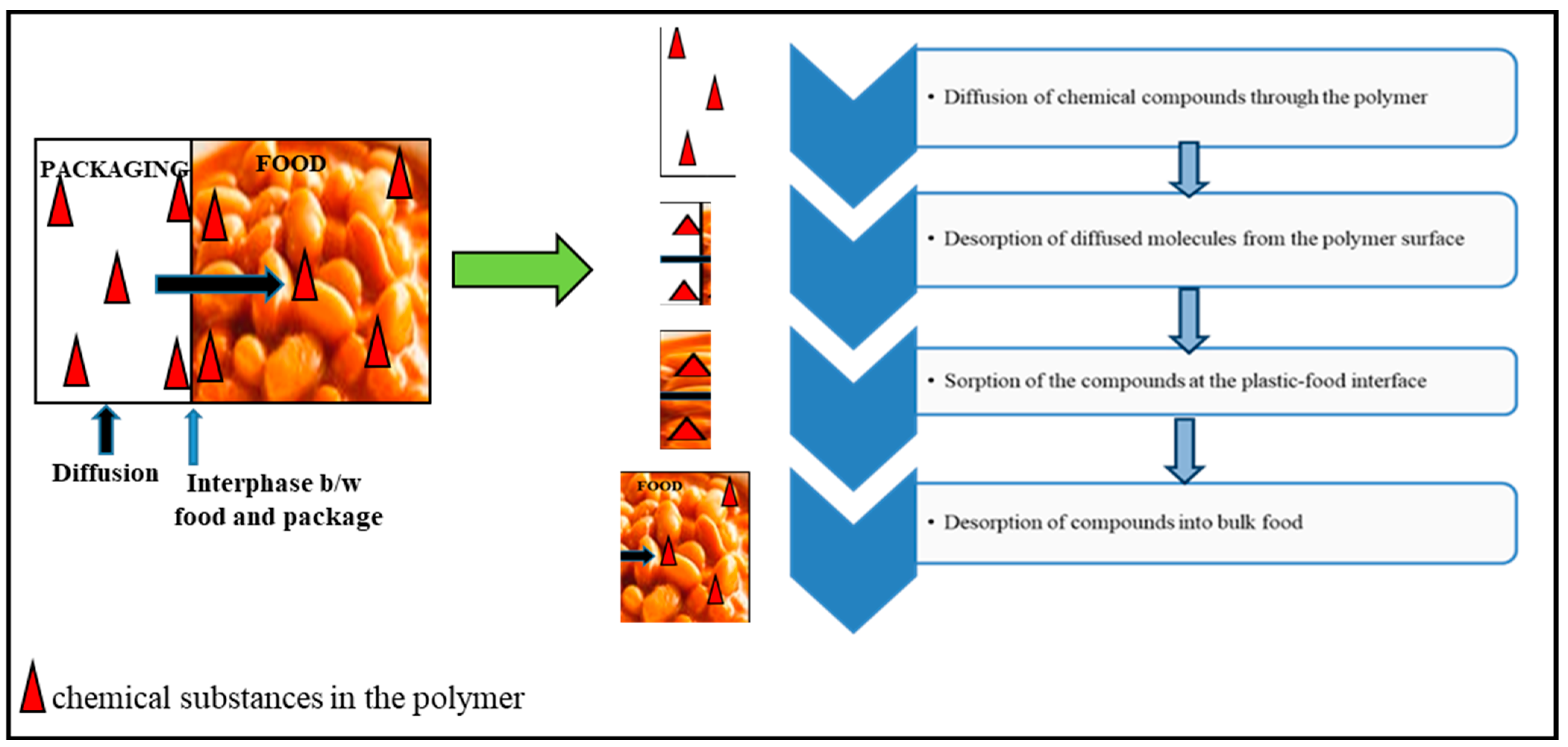
4. Monomer Migration into Food in Food Packaging
4.1. Migration Mechanism Processes in the Migration of Monomers into Food
4.2. Migration Mechanisms Involving Different Chemistries of Monomers
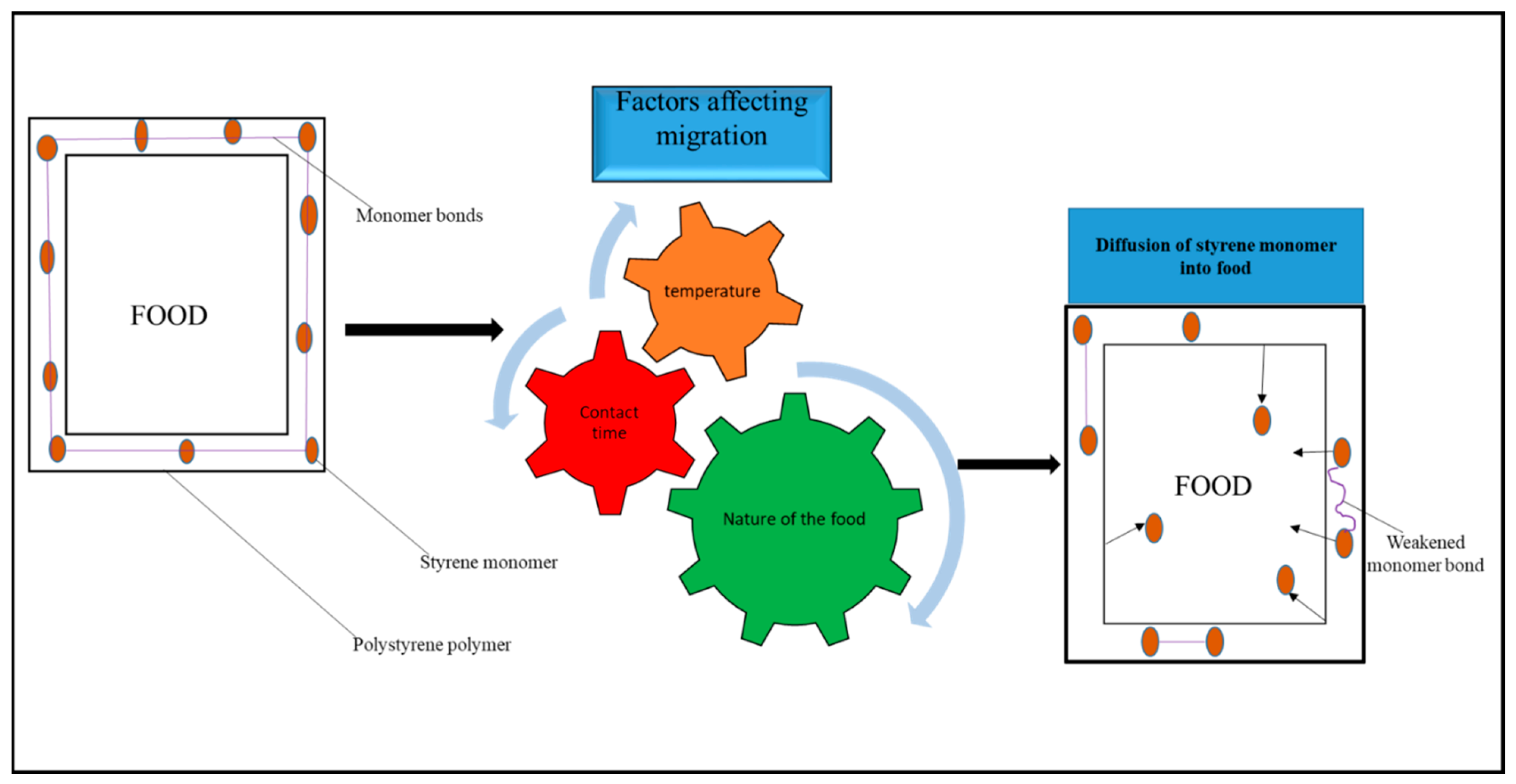
4.3. Factors Influencing the Migration of Food-Packaging Monomers into Food
4.3.1. Nature of Foods
4.3.2. Nature of Contact
4.3.3. Period of Contact
4.3.4. Temperature during Contact
4.3.5. Packaging Material Characteristics
4.3.6. Migrant Characteristics
4.3.7. Migrant Concentration within the Packaging Material
4.3.8. State of Polymer Matrix
4.3.9. Migration Kinetics
5. Interactions between Monomers and Food Nutrients
6. Human Health Risks Due to Monomer Presence in Food
“A container shall be clean and free from any toxic substance, ingredient or any other substance liable to contaminate or spoil the food in the container”.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebnesajjad, S. (Ed.) Plastic Films in Food Packaging Materials, Technology and Applications Plastics Design Library (PDL); William Andrew: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Coles, R.; Mcdowel, D.; Kirwan, M.J. Food Packaging Technology; Blackwell: Oxford, UK, 2003; Volume 3, pp. 9–15. [Google Scholar]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Flint, S.; Yu, P. Enterocins in food preservation. Int. J. Food Microbiol. 2010, 141, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Fortune Business, I. Food Packaging Market Size, Share and COVID-19 Impact Analysis, by Material (Glass, Metal, Paper and Paperboard, Wood and Plastics), by Product Type (Rigid, Semi-Rigid and Flexible) by Application (Fruits and Vegetables, Bakery and Confectionery, Dairy Pr; Fortune Business Insights Pvt. Ltd.: Maharashtra, India, 2021. [Google Scholar]
- Grandview, R. Food Packaging Market Size, Share and Growth Report, 2030; Grand View Research Inc.: San Francisco, CA, USA, 2023. [Google Scholar]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Muncke, J.; Andersson, A.M.; Backhaus, T.; Boucher, J.M.; Carney Almroth, B.; Castillo Castillo, A.; Chevrier, J.; Demeneix, B.A.; Emmanuel, J.A.; Fini, J.B.; et al. Impacts of food contact chemicals on human health: A consensus statement. Environ. Health 2020, 19, 25. [Google Scholar] [CrossRef]
- Mordor, I. Packaging Industry in South Africa Market-Growth, Trends, COVID 19 Impact, and Forecasts (2023–2028); Mordor Intelligence Pvt. Ltd.: Hyderabad, India, 2023; pp. 1–7. [Google Scholar]
- Sadan, Z.; De Kock, L. Plastics: Facts and Futures: Moving beyond Pollution Management towards a Circular Plastics Economy in South Africa. 2020. Available online: https://wwfafrica.awsassets.panda.org/downloads/wwf_plastics_report_final_2nov2020.pdf (accessed on 30 September 2022).
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Piergiovanni, S.; Limbo, L. Food Packaging Materials; Springer: London, UK; New York, NY, USA, 2016; ISBN 9783319247304. [Google Scholar]
- Rasul, S.F.; Noori, R.J.; Ali, K.M.; Khdhir, R.B.; Ahmed, S.R.; Qadir, A.M. Roles of different packaging materials on the quality and shelf life of yogurt. Food Sci. Technol. 2022, 42, 1–6. [Google Scholar] [CrossRef]
- The Department of Trade, Industry and Competition (DTIC). South African Plastics Industry. 2023. Available online: www.thedtic.gov.za/sectors-and-services-2/industrial-development/plastics/ (accessed on 4 March 2023).
- Plastics Europe, Plastics—The Facts 2016—An Analysis of European Plastics Production, Demand and Waste Data; Brussels. 2016. Available online: www.plasticseurope.de/informations (accessed on 12 July 2022).
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Eyerer, P. (Ed.) Synthesis (Manufacture, Production) of Plastics. In Polymers–Opportunities and Risks 1: General and Environmental Aspects; Springer: Berlin/Heidelberg, Germany, 2010; Volume 11. [Google Scholar]
- Ai, X.; Wang, D.; Li, X.; Pan, H.; Kong, J.; Yang, H.; Zhang, H.; Dong, L. The properties of chemical cross-linked poly(lactic acid) by bis(tert-butyl dioxy isopropyl) benzene. Polym. Bull. 2019, 76, 575–594. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Gelbke, H.P.; Banton, M.; Block, C.; Dawkins, G.; Eisert, R.; Leibold, E.; Pemberton, M.; Puijk, I.M.; Sakoda, A.; Yasukawa, A. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem. Toxicol. 2019, 124, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Baldev, R. Plastics and their role in food packaging. In Plastics in Food Packaging; Central Food Technological Research Institute: Mysore, India, 2005; pp. 1–32. [Google Scholar]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovation in Food Packaging. A Volume in Food Science and Technology; Academic Press: Cambridge, MA, USA, 2005; pp. 403–428. [Google Scholar]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Denmark, C.; Nilsson, N.H.; Lithner, D.; Lassen, C.; Institute, D.T.; Sweden, C.; Lassen, C.-D.C. Hazardous Substances in Plastic Materials. 2013. Available online: http://www.miljodirektoratet.no/no/Publikasjoner/Publikasjoner/2013/Februar/Hazardous_substances_in_plastic_materials/ (accessed on 12 July 2022).
- Gani, K.M.; Tyagi, V.K.; Kazmi, A.A. Occurrence of phthalates in aquatic environment and their removal during wastewater treatment processes: A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 17267–17284. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Survey and Investigation of Migration of Monomers in Toy Materials and Investigation of Migration of Monomers in Toy Materials; Environmental Protection Agency: Washington, DC, USA, 2019.
- Van Deventer, D.; Mallikarjunan, P. Optimizing an electronic nose for analysis of volatiles from printing inks on assorted plastic films. Innov. Food Sci. Emerg. Technol. 2002, 3, 93–99. [Google Scholar] [CrossRef]
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Kawa, I.A.; Akbar, M.; Fatima, Q.; Mir, S.A.; Jeelani, H.; Manzoor, S.; Rashid, F. Endocrine disrupting chemical Bisphenol A and its potential effects on female health. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 803–811. [Google Scholar] [CrossRef]
- Wójtowicz, A.K.; Sitarz-Głownia, A.M.; Szczęsna, M.; Szychowski, K.A. The Action of Di-(2-Ethylhexyl) Phthalate (DEHP) in Mouse Cerebral Cells Involves an Impairment in Aryl Hydrocarbon Receptor (AhR) Signaling. Neurotox. Res. 2019, 35, 183–195. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Wielgomas, B.; Karwacka, A.; Klimowska, A.; Kałużny, P.; Radwan, P.; Hanke, W. Parameters of ovarian reserve in relation to urinary concentrations of parabens. Environ. Health 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Ajaj, A.; J’bari, S.; Ononogbo, A.; Buonocore, F.; Bear, J.C.; Mayes, A.G.; Morgan, H. An insight into the growing concerns of styrene monomer and poly(Styrene) fragment migration into food and drink simulants from poly(styrene) packaging. Foods 2021, 10, 1136. [Google Scholar] [CrossRef]
- Sax, L. Polyethylene terephthalate May yield endocrine disruptors. Environ. Health Perspect. 2010, 118, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Kim, M.J.; Jung, I.K.; Koo, Y.D.; Ann, H.Y.; Lee, K.J.; Kim, S.H.; Yoon, Y.C.; Cho, B.J.; Park, K.S.; et al. Bisphenol a impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J. Korean Med. Sci. 2012, 27, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Pouech, C.; Kiss, A.; Lafay, F.; Léonard, D.; Wiest, L.; Cren-Olivé, C.; Vulliet, E. Human exposure assessment to a large set of polymer additives through the analysis of urine by solid phase extraction followed by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1423, 111–123. [Google Scholar] [CrossRef] [PubMed]
- HEAL. Food Contact Materials and Chemical Contamination; HEAL: Brussels, Belgium, 2016. [Google Scholar]
- Caner, C. Fundamentals of the Sorption (Scalping) Phenomena in Packaged Foods; Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–12. [Google Scholar]
- Petrović, E.K.; Hamer, L.K. Improving the healthiness of sustainable construction: Example of polyvinyl chloride (PVC). Buildings 2018, 8, 28. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, Z.; Ji, W. Bisphenol A induces apoptosis, oxidative stress and inflammatory response in colon and liver of mice in a mitochondria-dependent manner. Biomed. Pharmacother. 2019, 117, 109182. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, A.; Martínez-Razo, L.D.; MacDonald-Ramos, K.; Morales-Pacheco, M.; Vázquez-Martínez, E.R.; López-López, M.; Rodríguez Dorantes, M.; Cerbón, M. Multisystemic alterations in humans induced by bisphenol A and phthalates: Experimental, epidemiological and clinical studies reveal the need to change health policies. Environ. Pollut. 2021, 271, 116380. [Google Scholar] [CrossRef]
- Neltner, T.G.; Alger, H.M.; O’Reilly, J.T.; Krimsky, S.; Bero, L.A.; Maffini, M.V. Conflicts of interest in approvals of additives to food: Determined to be generally recognized as safe: Out of balance. JAMA Intern. Med. 2013, 173, 2032–2036. [Google Scholar] [CrossRef]
- Trier, X.; Taxvig, C.; Rosenmai, A.K.; Pedersen, G.A. PFAS in Paper and Board for Food Contact, Options for Risk Management of Poly- and Perfluorinated Substances; Noedic Council of Ministers: Copenhagen, Denmark, 2018. [Google Scholar]
- Rosenmai, A.K.; Bengtström, L.; Taxvig, C.; Trier, X.; Petersen, J.H.; Svingen, T.; Binderup, M.; Barbara Medea Alice, v.V.-L.B.M.; Dybdahl, M.; Granby, K. An effect-directed strategy for characterizing emerging chemicals in food contact materials made from paper and board. Food Chem. Toxicol. 2017, 106, 250–259. [Google Scholar] [CrossRef]
- Wagner, M. Know thy unknowns: Why we need to widen our view on endocrine disruptors. J. Epidemiol Community Health 2017, 71, 209–212. [Google Scholar] [CrossRef]
- Wiesinger, H.; Wang, Z.; Hellweg, S. Deep Dive into Plastic Monomers, Additives, and Processing Aids. Environ. Sci. Technol. 2021, 55, 9339–9351. [Google Scholar] [CrossRef]
- Grob, K.; Biedermann, M.; Scherbaum, E.; Roth, M.; Rieger, K. Food contamination with organic materials in perspective: Packaging materials as the largest and least controlled source? A view focusing on the European situation. Crit. Rev. Food Sci. Nutr. 2006, 46, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Azizi, D.; Arif, A.; Blair, D.; Dionne, J.; Filion, Y.; Ouarda, Y.; Pazmino, A.G.; Pulicharla, R.; Rilstone, V.; Tiwari, B.; et al. A comprehensive review on current technologies for removal of endocrine disrupting chemicals from wastewaters. Environ. Res. 2022, 207, 112196. [Google Scholar] [CrossRef]
- Pilevar, Z.; Bahrami, A.; Beikzadeh, S.; Hosseini, H.; Jafari, S.M. Migration of styrene monomer from polystyrene packaging materials into foods: Characterization and safety evaluation. Trends Food Sci. Technol. 2019, 91, 248–261. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J. Analytical methods for the determination of phthalates in food. Curr. Opin. Food Sci. 2018, 22, 122–136. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, H. Phthalates and their impacts on human health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- National Environmental Management: Waste Act 59 of 2008. 2009; pp. 4–6. Available online: https://www.gov.za/documents/national-environmental-management-waste-act (accessed on 19 August 2023).
- National Water Amendment Act 27 of 2014. South African Government. 2014; pp. 4–6. Available online: https://www.gov.za/documents/national-water-amendment-act-0 (accessed on 19 August 2023).
- Schrenk, D. Literature Report on Food Packaging Materials and Their Potential Impact on Human Health. 2014. Available online: http://presspage-production-content.s3.amazonaws.com/uploads/1081/profschrenk-foodpackagingmaterials_final.pdf (accessed on 12 July 2022).
- Geueke, B. Dossier—Non-intentionally added substances (NIAS). Food Packag. Forum 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Yin, S.; Rajarao, R.; Gong, B.; Wang, Y.; Kong, C.; Sahajwalla, V. Thermo-delamination of metallised composite plastic: An innovative approach to generate Aluminium from packaging plastic waste. J. Clean. Prod. 2019, 211, 321–329. [Google Scholar] [CrossRef]
- Seiko Kato, L.; Conte-junior, C.A. Safety of Plastic Food Packaging: The Challenges about Identification and Risk Assessment. Polymers 2021, 13, 33–43. [Google Scholar]
- Muncke, J. Tackling the toxics in plastics packaging. PLoS Biol. 2021, 19, e3000961. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Geueke, B.; Martin, O.; Maffini, M.; Muncke, J. Overview of intentionally used food contact chemicals and their hazards. Environ. Int. 2021, 150, 106225. [Google Scholar] [CrossRef]
- Birgit, G. Dossier—Non-Intentionally Added Substances (NIAS); Food Packaging Forum: Zurich, Switzerland, 2013. [Google Scholar]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.W.; Frelka, N.; Shen, Z.; Chew, A.K.; Banick, J.; Grey, S.; Kim, M.S.; Dumesic, J.A.; Van Lehn, R.C.; Huber, G.W. Recycling of multilayer plastic packaging materials by solvent-targeted recovery and precipitation. Sci. Adv. 2020, 6, eaba7599. [Google Scholar] [CrossRef] [PubMed]
- Fazli, A.; Rodrigue, D. Waste rubber recycling: A review on the evolution and properties of thermoplastic elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef]
- Lohith, K.S.; Mahesh, S.V. Influence of cryogenic treatment on the friction co- efficient of nylon 6 and caprolactam—Graphite composite. Ipsaj Int. J. Mech. Eng. 2014, 1, 10–15. [Google Scholar]
- Alizadeh Sahraei, A.; Mokarizadeh, A.H.; George, D.; Rodrigue, D.; Baniassadi, M.; Foroutan, M. Insights into interphase thickness characterization for graphene/epoxy nanocomposites: A molecular dynamics simulation. Phys. Chem. Chem. Phys. 2019, 21, 19890–19903. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, J.; Bo, T.; Li, H.; Crittenden, J.C. Occurrence and risk assessment of selected phthalates in drinking water from waterworks in China. Environ. Sci. Pollut. Res. 2015, 22, 10690–10698. [Google Scholar] [CrossRef]
- Freire, M.T.D.A.; Santana, I.A.; Reyes, F.G.R. Plasticizers in Brazilian food-packaging materials acquired on the retail market. Food Addit. Contam. 2006, 23, 93–99. [Google Scholar] [CrossRef]
- Moore, M.; Han, I.; Acton, J.; Ogale, A.; Barmore, C.; Dawson, P. Effects of Antioxidants in Polyethylene Film on Fresh Beef Color. J. Food Sci. 2003, 68, 99–104. [Google Scholar] [CrossRef]
- Lin, Q.; Li, B.; Song, H.; Li, X. Determination of 7 Antioxidants, 8 Ultraviolet Absorbents, and 2 Fire Retardants in Plastic food Package by Ultrasonic Extraction and Ultra Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 730–743. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Africa 2023, 6, 117–144. [Google Scholar] [CrossRef]
- Popa, M.; Mitelut, A.; Niculita, P.; Geicu, M.; Ghidurus, M.; Turtoi, M. Biodegradable materials for food packaging applications. J. Environ. Prot. Ecol. 2011, 12, 1825–1834. [Google Scholar]
- Shershneva, E.G. Biodegradable Food Packaging: Benefits and Adverse Effects. IOP Conf. Ser. Earth Environ. Sci. 2022, 988, 022006. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. Biodegradation and physical evaluation of PHB packaging. Polym. Test. 2007, 26, 908–915. [Google Scholar] [CrossRef]
- Scarfato, L.; Di Maio, P.; Incarnato, L. Manufacturing of advanced biodegradable polymeric components. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life. 2022, 31, 100806. [Google Scholar] [CrossRef]
- Zhang, W.; Sani, M.A.; Zhang, Z.; McClements, D.J.; Jafari, S.M. High performance biopolymeric packaging films containing zinc oxide nanoparticles for fresh food preservation: A review. Int. J. Biol. Macromol. 2023, 230, 123188. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Rhim, J.W. Copper-based nanoparticles for biopolymer-based functional films in food packaging applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1933–1952. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO nanoparticles stabilized with gelatin for potential use in food packaging applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef] [PubMed]
- International Panel on Chemical Pollution. Overview Report III: Existing National, Regional, and Global Regulatory Frameworks Addressing Endocrine Disrupting Chemicals (EDCs). United Nations Environment Programme. 2017, pp. 1–59. Available online: https://wedocs.unep.org/handle/20.500.11822/25636 (accessed on 12 July 2022).
- Ferreira, A.P. Endocrine disruptors in sludge wastewater treatment plants: Environmental complications. Acta Sci. 2013, 35, 307–316. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Ibarra-Cabrera, R.; Tecante, A.; Gimeno, M.; Parra, R.; Garcia-Arrazola, R. Removal strategies for endocrine disrupting chemicals using cellulose-based materials as adsorbents: A review. J. Environ. Chem. Eng. 2016, 4, 3122–3142. [Google Scholar] [CrossRef]
- Arambula, S.E.; Patisaul, H.B. Endocrine Disrupting Chemicals and Behavior, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gadupudi, C.K.; Rice, L.; Xiao, L.; Kantamaneni, K. Endocrine Disrupting Compounds Removal Methods from Wastewater in the United Kingdom: A Review. Science 2019, 1, 11. [Google Scholar] [CrossRef]
- Ribeiro, E.; Ladeira, C.; Viegas, S. EDCs mixtures: A stealthy hazard for human health? Toxics 2017, 5, 5. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative stress and BPA toxicity: An antioxidant approach for male and female reproductive dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Abnous, K.; Vahdati Hassani, F.; Hosseinzadeh, H.; Birner-Gruenberger, R.; Mehri, S. Alteration of protein profile in cerebral cortex of rats exposed to bisphenol a: A proteomics study. Neurotoxicology 2020, 78, 1–10. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Roberts, S.C.; Mabrey, N.; Mccaffrey, K.A.; Gear, R.B.; Braun, J.; Belcher, S.M.; Stapleton, H.M. Accumulation and Endocrine Disrupting Effects of the Flame Retardant Mixture Firemaster 550 in Rats: An Exploratory Assessment. J. Biochem. Mol. Toxicol. 2013, 27, 124–136. [Google Scholar] [CrossRef]
- Ciacci, L.; Passarini, F.; Vassura, I. The European PVC cycle: In-use stock and flows. Resour. Conserv. Recycl. 2017, 123, 108–116. [Google Scholar] [CrossRef]
- Biro, F.M.; Greenspan, L.C.; Galvez, M.P.; Pinney, S.M.; Teitelbaum, S.; Windham, G.C.; Deardorff, J.; Herrick, R.L.; Succop, P.A.; Hiatt, R.A.; et al. Onset of breast development in a longitudinal cohort. Pediatrics 2013, 132, 1019–1027. [Google Scholar] [CrossRef]
- Talpade, J.; Shrman, K.; Sharma, R.K.; Gutham, V.; Singh, I.R.; Meena, N.S. Bisphenol a: An endocrine disruptor. J. Entomol. Zool. Stud. 2018, 6, 394–397. Available online: http://www.entomoljournal.com/archives/2018/vol6issue3/PartF/6-2-262-216.pdf (accessed on 12 July 2022).
- Alin, J.; Hakkarainen, M. Migration from polycarbonate packaging to food simulants during microwave heating. Polym. Degrad. Stab. 2012, 97, 1387–1395. [Google Scholar] [CrossRef]
- Maryskova, A.; Rysova, M.; Novotny, M.; Sevcu, V. Polyamide-Laccase Nanofiber Membrane for Degradation of Endocrine-Disrupting Bisphenol A, 17 α- ethinylestradiol, and Triclosan. Polymers 2019, 11, 1560. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Eker, B.; Icoz, A. Packaging Materials and Effects on Quality of Life; Center for Quality, Faculty of Engineering, University of Kragujevac: Kragujevac, Serbia, 2016; pp. 271–278. [Google Scholar]
- Kadri, Z.; Mechnou, I.; Zyade, S. Migration of bisphenol A from epoxy coating to foodstuffs. Mater. Today Proc. 2021, 45, 7584–7587. [Google Scholar] [CrossRef]
- BS EN 1186-1:2002; Materials and Articles in Contact with Foodstuffs–Plastics–Part 1: Guide to the Selection of Conditions and Test Methods for Overall Migration. British Standards Institution: London, UK, 2002; pp. 1–49.
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011. 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:0089:EN:PDF (accessed on 12 July 2022).
- İçöz, A.; Eker, B. Selection of Food Packaging Material, Migration and Its Effects on Food Quality. In Proceedings of the 1st International Conference on Quality of Life, Solo, Indonesia, 14–15 September 2016; pp. 201–210. Available online: http://cqm.rs/2016/cd1/pdf/papers/focus_1/28.pdf (accessed on 18 July 2022).
- Castle, L.; Defra, C.S.L. Chemical migration into food: An overview. In Chemical Migration and Food Contact Materials; Barnes, K., Sinclair, C., Watson, D., Eds.; Woodhead Publishing: Sawston, UK; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–481. ISBN 9772081415. [Google Scholar]
- Makowska, K.; Staniszewska, M.; Bodziach, K.; Calka, J.; Gonkowski, S. Concentrations of bisphenol a (BPA) in fresh pork loin meat under standard stock-farming conditions and after oral exposure—A preliminary study. Chemosphere 2022, 295, 133816. [Google Scholar] [CrossRef] [PubMed]
- Kubwabo, C.; Kosarac, I.; Stewart, B.; Gauthier, B.R.; Lalonde, K.; Lalonde, P.J. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit. Contam. 2009, 26, 928–937. [Google Scholar] [CrossRef]
- Munguia-Lopez, E.M.; Soto-Valdez, H. Effect of heat processing and storage time on migration of bisphenol A (BPA) and bisphenol A-diglycidyl ether (BADGE) to aqueous food simulant from Mexican can coatings. J. Agric. Food Chem. 2001, 49, 3666–3671. [Google Scholar] [CrossRef]
- Marć, M.; Formela, K.; Klein, M.; Namieśnik, J.; Zabiegała, B. The emissions of monoaromatic hydrocarbons from small polymeric toys placed in chocolate food products. Sci. Total Environ. 2015, 530, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.S.; Huyghebaert, A. Polystyrene cups and containers: Styrene migration. Food Addit. Contam. 1998, 15, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Guazzotti, V.; Hendrich, V.; Gruner, A.; Fiedler, D.; Störmer, A.; Welle, F. Migration of Styrene in Yogurt and Dairy Products Packaged in Polystyrene: Results from Market Samples. Foods 2022, 11, 2120. [Google Scholar] [CrossRef] [PubMed]
- Lickly, T.D.; Lehr, K.M.; Welsh, G.C. Migration of styrene from polystyrene foam food-contact articles. Food Chem. Toxicol. 1995, 33, 475–481. [Google Scholar] [CrossRef]
- El-Ziney, M.G.; Tawfik, M.S. Migration Levels of Monostyrene from Polystyrene Containers to Dairy Products. MOJ Food Process. Technol. 2016, 3, 267–271. [Google Scholar] [CrossRef][Green Version]
- Khaksar, M.; Ghazi-Khansari, M. Determination of migration monomer styrene from GPPS (general purpose polystyrene) and HIPS (high impact polystyrene) cups to hot drinks. Toxicol. Mech. Methods 2009, 19, 257–261. [Google Scholar] [CrossRef]
- Bradley, E.; Coulier, L. Report FD 07/01: An Investigation into the Reaction and Breakdown Products from Starting Substances Used to Produce Food Contact Plastics. 2007. Available online: http://www.foodpackagingforum.org/wp-content/uploads/2014/06/Bradley-and-Coulier-2007.pdf (accessed on 12 July 2022).
- Begley, T.H.; Gay, M.L.; Hollifield, H.C. Determination of migrants in and migration from nylon food packaging. Food Addit. Contam. 1995, 12, 671–676. [Google Scholar] [CrossRef]
- Bomfim, M.V.J.; Zamith, H.P.S.; Abrantes, S.M.P. Migration of ε-caprolactam residues in packaging intended for contact with fatty foods. Food Control 2011, 22, 681–684. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1282/2011 of 28 November 2011 Amending and Correcting Commission Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:328:0022:0029:En:PDF (accessed on 12 July 2022).
- Huang, H.B.; Pan, W.H.; Chang, J.W.; Chiang, H.C.; Guo, Y.L.; Jaakkola, J.J.K.; Huang, P.C. Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environ. Res. 2017, 153, 63–72. [Google Scholar] [CrossRef]
- Younker, J.M.; Beste, A.; Iii, A.C.B. Computational study of bond dissociation enthalpies for lignin model compounds: B -5 Arylcoumaran. Chem. Phys. Lett. 2012, 545, 100–106. [Google Scholar] [CrossRef]
- Huang, J.B.; Zeng, G.S.; Li, X.S.; Cheng, X.C.; Tong, H. Theoretical studies on bond dissociation enthalpies for model compounds of typical plastic polymers. IOP Conf. Ser. Earth Environ. Sci. 2018, 167, 012029. [Google Scholar] [CrossRef]
- Majder-Łopatka, M.; Węsierski, T.; Ankowski, A.; Ratajczak, K.; Duralski, D.; Piechota-Polanczyk, A.; Polanczyk, A. Thermal analysis of plastics used in the food industry. Materials 2022, 15, 248. [Google Scholar] [CrossRef] [PubMed]
- Begley, T.H.; Biles, J.E.; Cunningham, C.; Piringer, O. Migration of a UV stabilizer from polyethylene terephthalate (PET) into food simulants. Food Addit. Contam. 2004, 21, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Naziruddin, M.A.; Nurulhuda, K.; Sulaiman, R.; Sanny, M. Assessment of residual styrene monomer migration into yoghurt packed in High Impact Polystyrene pots using a modelling approach. Food Control 2023, 148, 109612. [Google Scholar] [CrossRef]
- Mercea, P. Physicochemical Processes Involved in Migration of Bisphenol A from Polycarbonate. J. Appl. Polym. Sci. 2009, 112, 579–593. [Google Scholar] [CrossRef]
- Oduneye, T. Migration of Styrene from Polystyrene Food Containers into Food Simulants: A Meta-Analysis; Master of Applied Science; Dalhousie University: Halifax, NS, Canada, 2020; Volume 91, pp. 1–108. [Google Scholar]
- Muncke, J. Endocrine disrupting chemicals and other substances of concern in food contact materials: An updated review of exposure, effect and risk assessment. J. Steroid Biochem. Mol. Biol. 2011, 127, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, V.I.; Akrida-Demertzi, K.; Demertzis, P.G. A study on the migration of organic pollutants from recycled paperboard packaging materials to solid food matrices. Food Chem. 2007, 101, 1759–1768. [Google Scholar] [CrossRef]
- Goodson, A.; Robin, H.; Summerfield, W.; Cooper, I. Migration of bisphenol A from can coatings—Effects of damage, storage conditions and heating. Food Addit. Contam. 2004, 21, 1015–1026. [Google Scholar] [CrossRef]
- Paraskevopoulou, D.; Achilias, D.S.; Paraskevopoulou, A. Migration of styrene from plastic packaging based on polystyrene into food simulants. Polym. Int. 2012, 61, 141–148. [Google Scholar] [CrossRef]
- Anderson, W.A.C.; Castle, L. Benzophenone in cartonboard packaging materials and the factors that influence its migration into food. Food Addit. Contam. 2003, 20, 607–618. [Google Scholar] [CrossRef]
- Castle, L.; Mayo, A.; Crews, C.; Gilbert, J. Migration of poly(ethylene terephthalate) (PET) oligomers from PET plastics into food during microwave and conventional cooking and into bottled beverages. J. Food Prot. 1989, 52, 337–342. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Bosnea, L. Migration of Substances from Food Packaging Materials to Foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Lickly, T.D.; Breder, C.V.; Rainey, M.L. A Model for Estimating the Daily Dietary Intake of a Substance from Food-Contact Articles: Styrene from Polystyrene Food Contact Polymers. Regul. Toxicol. Pharmacol. 1995, 21, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Seo, Y.M.; Kim, M.G. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere 2010, 79, 949–952. [Google Scholar] [CrossRef]
- Poças, M.; Oliveira, J.C.; Pereira, J.R.; Brandsch, R.; Hogg, T. Modelling migration from paper into a food simulant. Food Control 2011, 22, 303–312. [Google Scholar] [CrossRef]
- Boccaci Mariani, M.; Chiacchierini, E.; Gesumundo, C. Potential migration of Diisopropyl naphthalenes from recycled paperboard packaging into dry foods. Food Addit. Contam. 1999, 16, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Garban, Z.; Garban, G.; Ghibu, G.D.; Baltă, C.; Avacovici, A.E.; Mitroi, E.M.; Miclău, L. Xenobiochemistry at the interface of packaging materials-food. Note I. Packaging materials and specific interactions in vivo. J. Agroaliment. Process. Technol. 2010, 16, 265–275. [Google Scholar]
- Acerini, C.L.; Hughes, I.A. Endocrine disrupting chemicals: A new and emerging public health problem? Arch. Dis. Child. 2006, 91, 633–638. [Google Scholar] [CrossRef]
- Groh, K.J.; Muncke, J. In Vitro Toxicity Testing of Food Contact Materials: State-of-the-Art and Future Challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1123–1150. [Google Scholar] [CrossRef]
- Zelko, I.N.; Taylor, B.S.; Das, T.P.; Watson, W.H.; Sithu, I.D.; Wahlang, B.; Malovichko, M.V.; Cave, M.C.; Srivastava, S. Effect of vinyl chloride exposure on cardiometabolic toxicity. Environ. Toxicol. 2022, 37, 245–255. [Google Scholar] [CrossRef]
- Jones, B.A.; Watson, N.V. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Horm. Behav. 2012, 61, 605–610. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Han, L.; Zhang, C.; Wang, Y.; Tu, K.; Peng, J.; Wang, J.; Pan, L. Effects of caprolactam content on curdlan-based food packaging film and detection by infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118942. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: Findings from a dietary intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Genthe, B.; Steyn, M. Health Risk Assessment Protocol for Endocrine Disrupting Chemicals; CSIR, Natural Resources and the Environment: Stellenbosch, South Africa, 2008. [Google Scholar]
- Stroheker, T.; Cabaton, N.; Nourdin, G.; Régnier, J.F.; Lhuguenot, J.C.; Chagnon, M.C. Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology 2005, 208, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.; Frederiksen, H.; Feldt-Rasmussen, U.; Skakkebæk, N.E.; Hegedüs, L.; Hilsted, L.; Juul, A.; Main, K.M. Childhood exposure to phthalates: Associations with thyroid function, insulin-like growth factor I, and growth. Environ. Health Perspect. 2010, 118, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Teitelbaum, S.L.; McGovern, K.; Windham, G.C.; Pinney, S.M.; Galvez, M.; Calafat, A.M.; Kushi, L.H.; Biro, F.M. Phthalate exposure and pubertal development in a longitudinal study of US girls. Hum. Reprod. 2014, 29, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Dufour, P.; Pirard, C.; Seghaye, M.; Charlier, C. Association between organohalogenated pollutants in cord blood and thyroid function in newborns and mothers from Belgian population. Environ. Pollut. 2018, 238, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; Vandevoort, C.A.; et al. Bisphenol A and reproductive health: Update of Experimental and Human Evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Kim, J.; Chevrier, J. Exposure to parabens and prevalence of obesity and metabolic syndrome: An analysis of the Canadian Health Measures Survey. Sci. Total Environ. 2020, 713, 135116. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kambayashi, Y.; Tsujiguchi, H.; Hara, A.; Hori, D.; Thi Thu Nguyen, T.; Suzuki, F.; Hamagishi, T.; Yamada, Y.; Nakamura, H.; et al. Relationship between the Use of Parabens and Allergic Diseases in Japanese Adults—A Cross-Sectional Study. J. Multidiscip. Sci. J. 2018, 1, 148–158. [Google Scholar] [CrossRef]
- Simoneau, C.; Raffael, B.; Garbin, S.; Hoekstra, E.; Mieth, A.; Lopes, J.A.; Reina, V. JRC Science for Policy Report: Non-Harmonised Food Contact Materials in the EU: Regulatory and Market Situation Baseline Study Final Report. 2016. Available online: https://doi.org/10.2788/234276 (accessed on 12 July 2022).
- Neltner, T.G.; Kulkarni, N.R.; Alger, H.M.; Maffini, M.V.; Bongard, E.D.; Fortin, N.D.; Olson, E.D. Navigating the U.S. Food Additive Regulatory Program. Compr. Rev. Food Sci. Food Saf. 2011, 10, 342–368. [Google Scholar] [CrossRef]
- South Africa. Department of Health, Foodstuffs, Cosmetics and Disinfectants Act, 1972 (Act No. 54 of 1972). 2018. Available online: www.gpwonline.co.za (accessed on 12 July 2022).
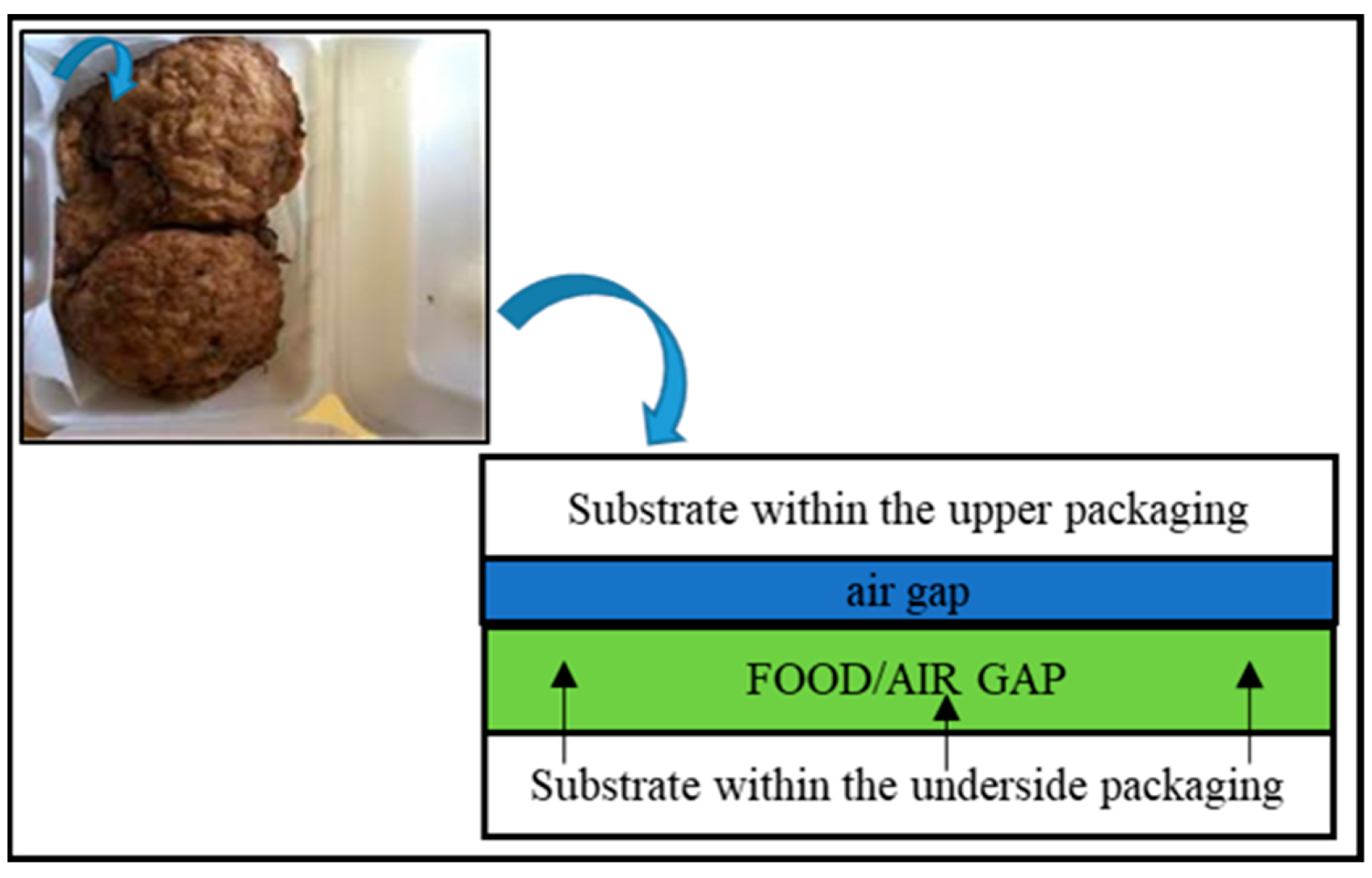
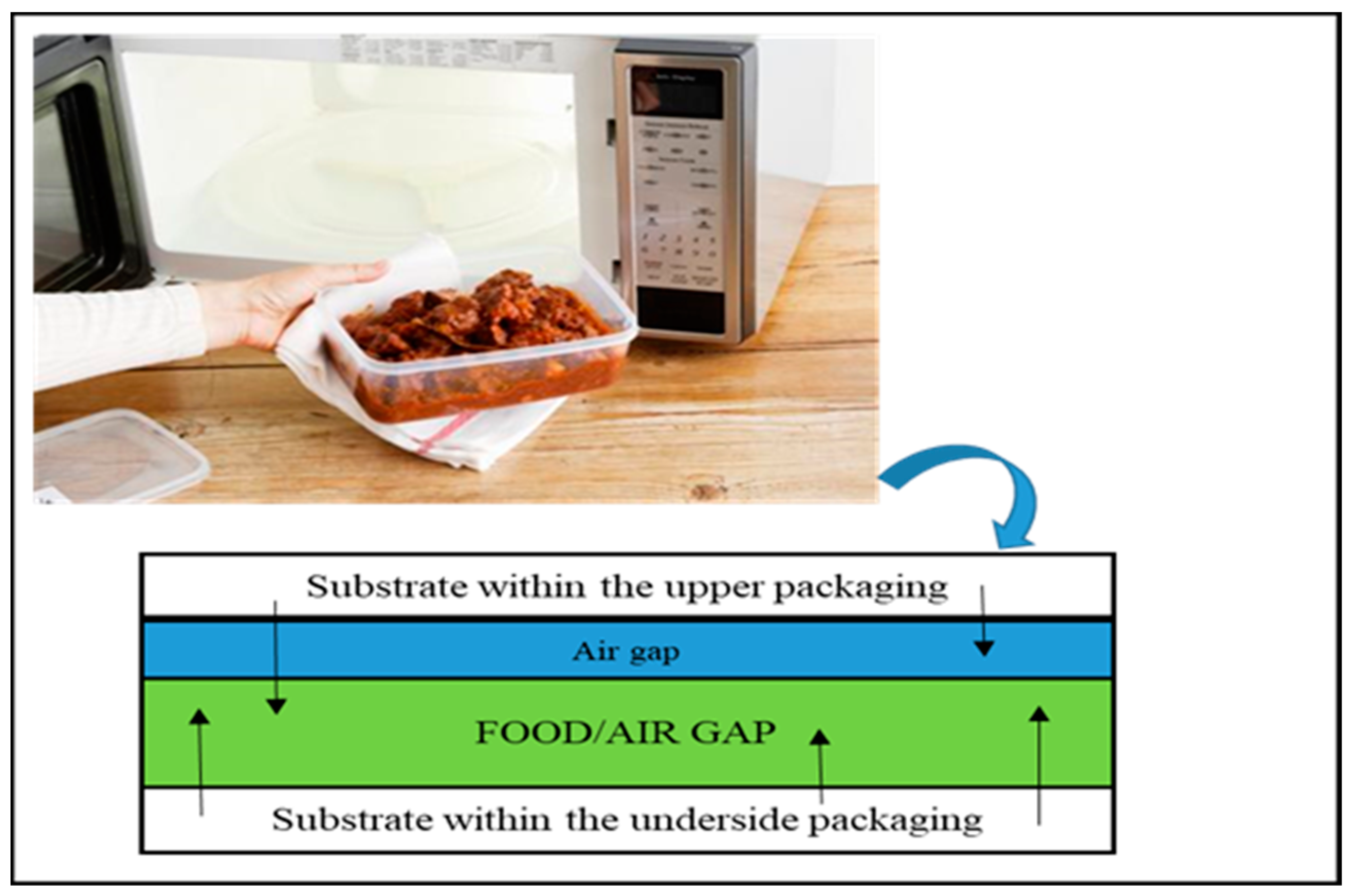
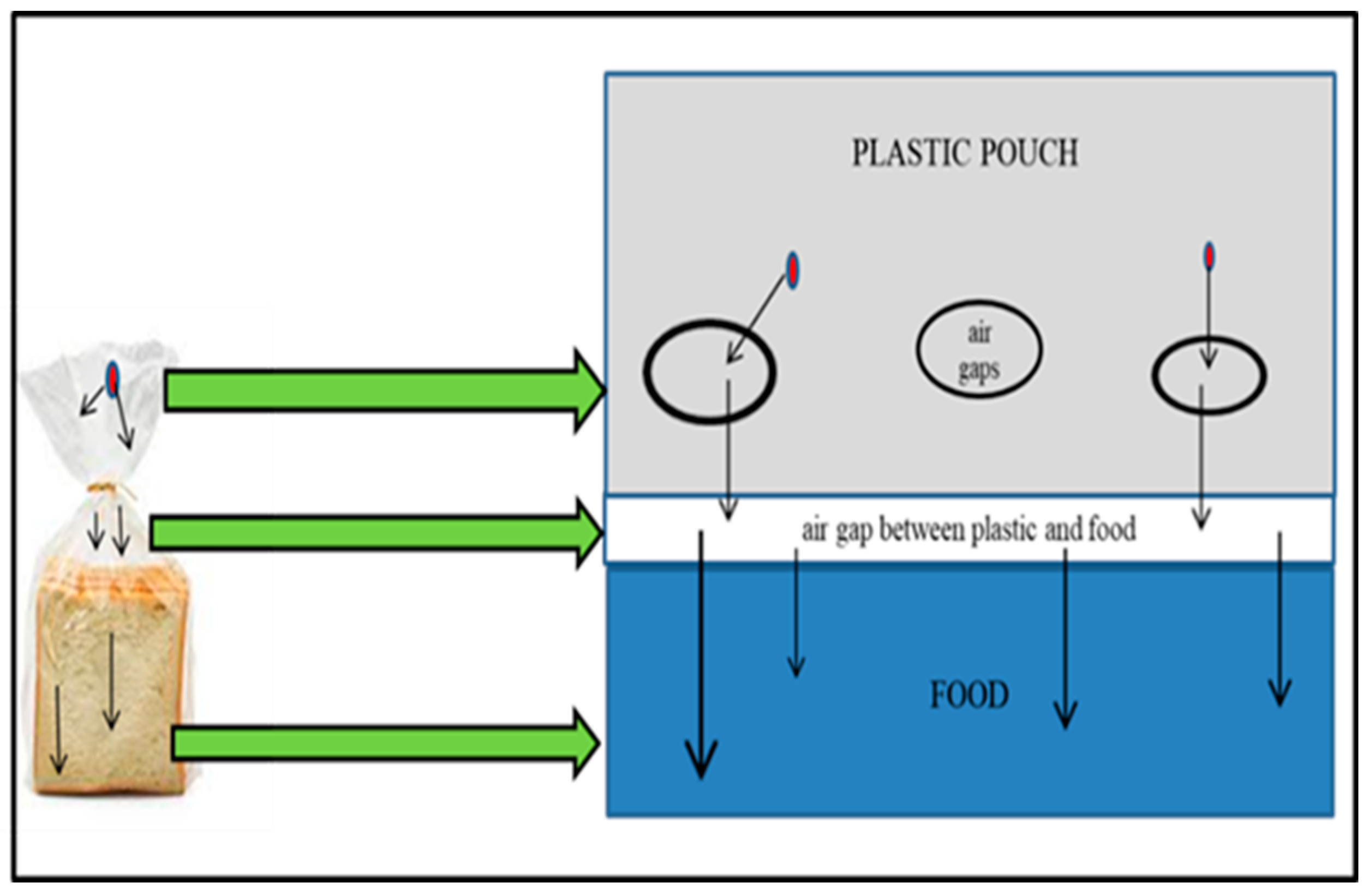
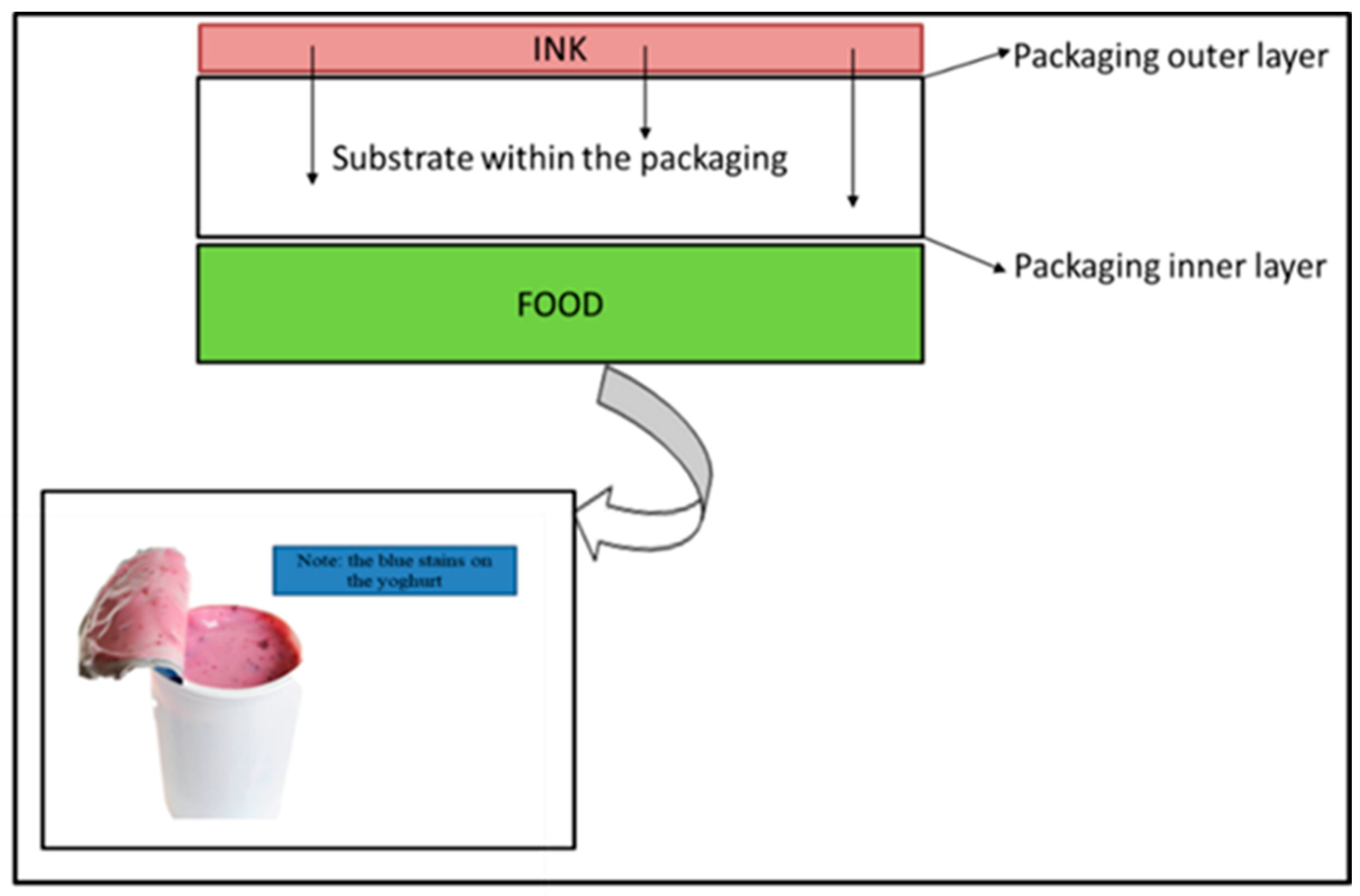
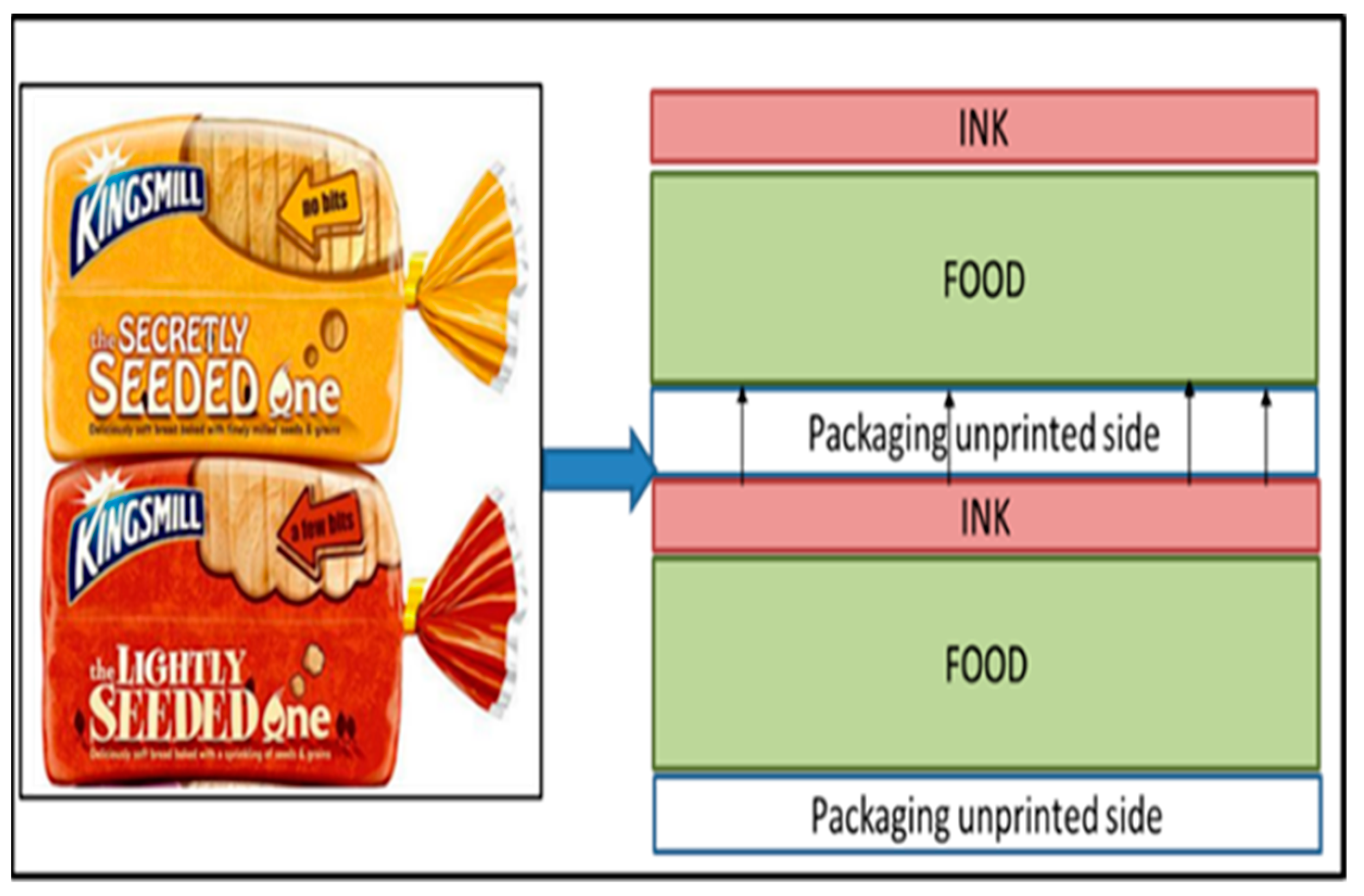
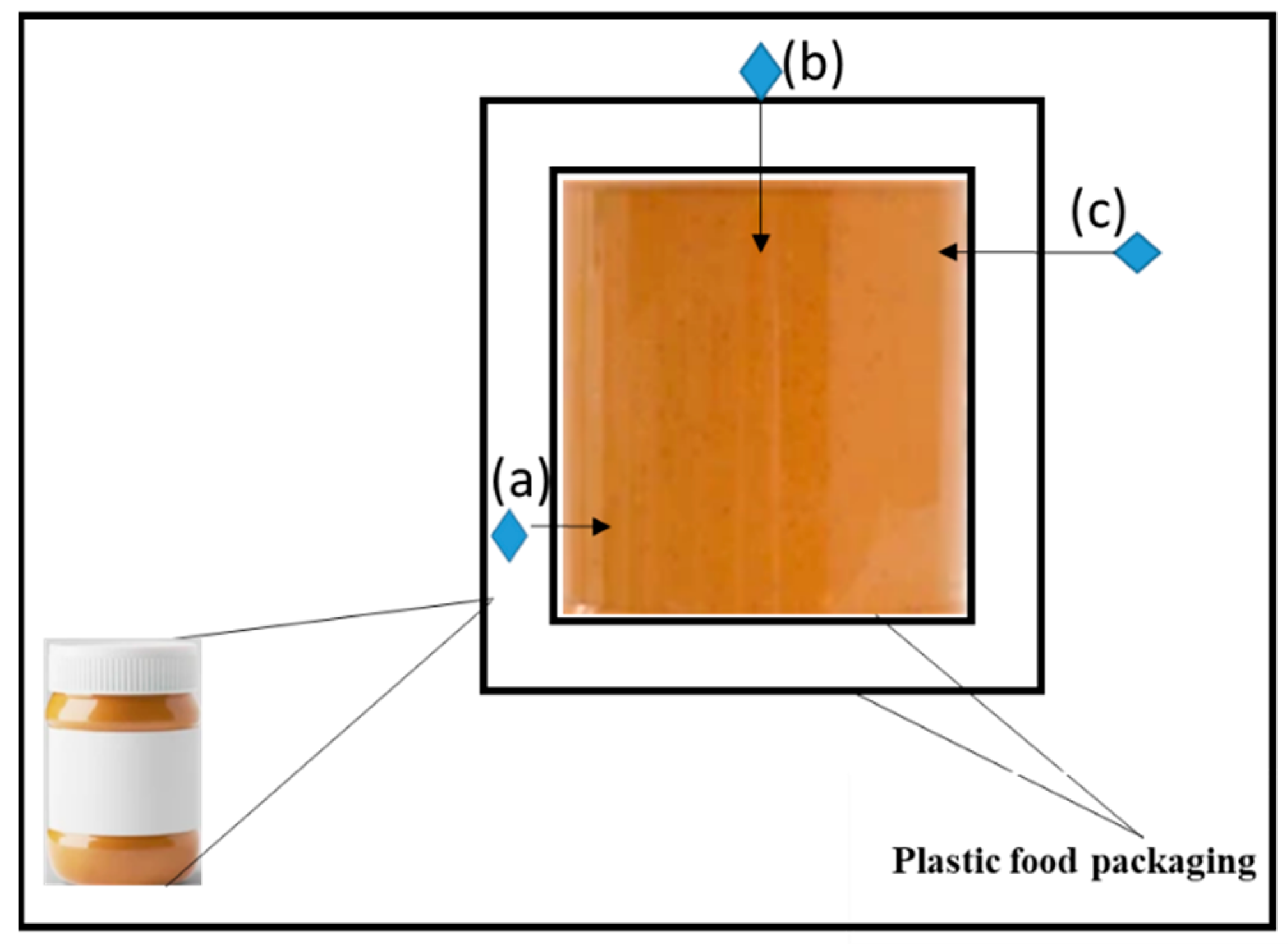

| Type of Packaging | Applications (Types of Foods) | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Plastics | Fast foods Solid products, such as pasta, rice, biscuits, bread, and sugar Liquid products, such as concentrate juices, oils, and methylated spirits |
|
| [27] |
| Packaging Material | Synthetic Materials Present | Food-Contact Chemicals | References | |
|---|---|---|---|---|
| Intentionally Added Substances (IASs) | Nonintentionally Added Substances (NIASs) | |||
| Plastic packaging material | Aluminium Coatings Adhesives Printing inks | Monomers Oligomers Additives Pigments Metals | Impurities By-products of reactions Breakdown products Recycling-product contaminants Starting-material impurities Unwanted side products | [59,60,61,62] |
| Plastic Type | Recycling Code and Symbol | Monomer Name | Monomer Structure | References |
|---|---|---|---|---|
| Polyethylene terephthalate (PET) |  | Ethylene terephthalate | 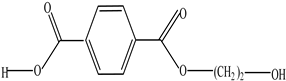 | [65,66] |
| High-density polyethylene (HDPE) |  | Ethylene |  | [65] |
| Polyvinyl chloride (PVC) |  | Vinyl chloride | 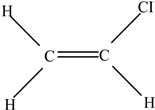 | [65] |
| Low-density polyethylene (LDPE) |  | Ethylene | 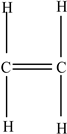 | [67] |
| Polypropylene (PP) |  | Propylene | 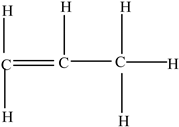 | [65,67] |
| Polystyrene (PS) |  | Styrene | 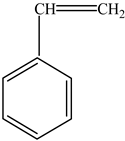 | [65] |
| Other |  | Bisphenol A for PC Caprolactam for Nylon-6 Bisphenol A diglycidyl ether for epoxy resins | 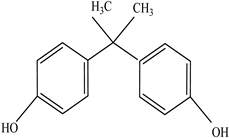  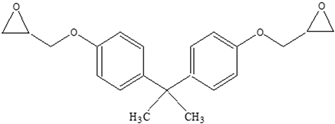 | [68,69] |
| Biopolymer | Monomers | References |
|---|---|---|
| Polylactic acid (PLA) | Lactic acid | [74,76] |
| Polylactide aliphatic copolymer (CPLA) | Lactide + aliphatic polyesters | [74,76] |
| Polyglycolide (PGA) | Glycolic acid | [74] |
| Polybutylene succinate (PBS) | Glycols + aliphatic polyesters | [74] |
| PBAT | 1,4 butanediol + terephthalic acid + adipic acid | [74] |
| Packaging Material | Structure | EDCs Identified in Such Compounds | Uses of Packaging | Ref. |
|---|---|---|---|---|
| Polypropylene (PP) | 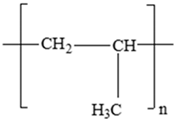 | Antioxidants (vinyl and polymer with a methyl group) Plasticizers (phthalates) | Margarine tubs, microwaveable meal trays, lunch boxes, plastic bottle caps, and sweets and snack wrappers | [90,91,92] |
| Polyvinyl chloride (PVC) | 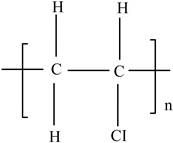 | Heat stabilizers (Pb, Zn, and Sn compounds) Dioxins Plasticizers (phthalates) Bisphenol A (BPA) | Meat trays, bottles containing liquid foods (oils, vinegars, and beverage foods), flexible films for wrapping solid foods (fresh fruits, cheese, meat, and vegetables), coatings in metal cans, and lunch boxes | [93,94] |
| Polyethylene (HDPE and LDPE) | 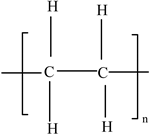 | Plasticizers (phthalates) Antioxidants Ethylene and olefins (butene, hexene, and octene) | Freezer bags; milk cartons; yoghurt, fruit juice, and soup pots; caps for plastic bottles; Tupperware; plastic grocery bags; and shrink wrap | [25] |
| Polystyrene (PS) | 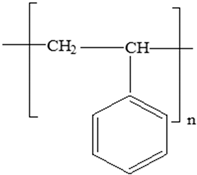 | Plasticizers (phthalates) Styrene | Disposable coffee cups; plastic food boxes; containers for yoghurt, ice cream, fruit juice, and cheese; egg cartons; and biscuit trays | [24,25,51] |
| Polyethylene terephthalate (PET) | 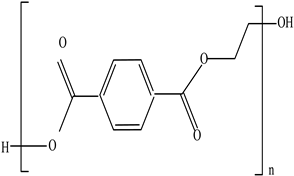 | BPA Phthalates Dioxins Colourants Fillers Plasticizers | Water, soft drink, and alcohol beverage bottles as well as edible oil and fruit/vegetable punnets | [95,96] |
| Polycarbonate (PC) | 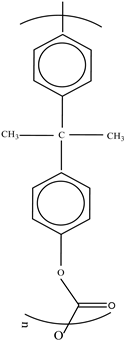 | BPA Phenol Volatile aromatic and aliphatic hydrocarbons Chlorinated hydrocarbons | Recyclable beverage containers, ovenable frozen-food trays, and convenience meals | [97] |
| Polyamides (PAs) |  | BPA 17α ethinyl estradiol Triclosan | Vacuum packaging of frozen foods, bacon, cheese, and fresh and processed meats | [98] |
| Country | Food Description | Residual Styrene Monomer Levels (µg/g) | Reference |
|---|---|---|---|
| Italy | Stirred yogurt, 3.2% fat | 266 ± 1 | [111] |
| Germany | Stirred yogurt, 3.5% fat | 275 ± 2–351 ± 23 | |
| Germany | Set yogurt, 3.5% fat | 278 ± 12–308 ± 6 | |
| Germany | Stirred sour cream with 10% fat | 260 ± 8–292 ± 20 |
| Different Methods’ Chemical Bond Average Values (kJ mol−1) | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plastic type | Bond types | ||||||||
| C-C bonds | C-CH3 bonds | C-C aromatic bonds | C–Cl bonds | ||||||
| M06-2X/6 | B3P86/6- 31 G (d,p) | M062X/6 −31 G (d) | B3P86/6- 31 G (d,p) | M062X/6 31 G (d) | B3P86/6 −31 G (d,p) | M062X/6-31 G (d) | B3P86/6 −31 G (d,p) | ||
| PE | 364.3 | 350.9 | - | - | - | - | - | - | [121,122] |
| 0.003 | 0.003 | ||||||||
| PP | 357.1 | 329.5 | 361.9 | 342.6 | - | - | - | - | |
| 0.003 | 0.003 | 0.003 | 0.003 | ||||||
| PS | 331.5 | 291.7 | - | - | 424.1 | 395.9 | - | - | |
| 0.003 | 0.003 | 0.003 | 0.003 | ||||||
| PVC | 373.8 | 345.8 | - | - | - | - | 355.6 | 343.7 | |
| 0.003 | 0.003 | 0.003 | 0.003 | ||||||
| Nutrients | Monomer | Reaction/Interaction |
|---|---|---|
Carbohydrates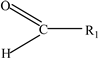 | Styrene | 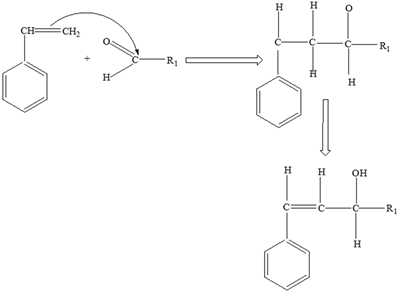 |
Bisphenol A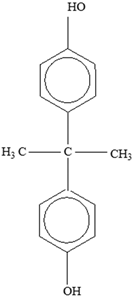 | 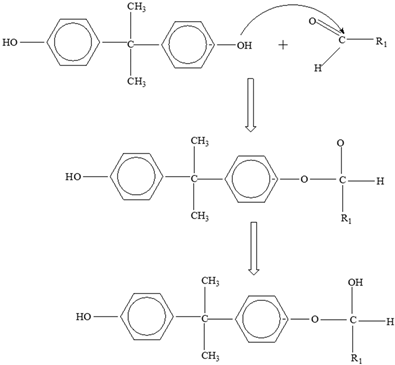 | |
Proteins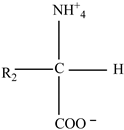 | Styrene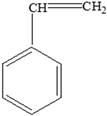 | 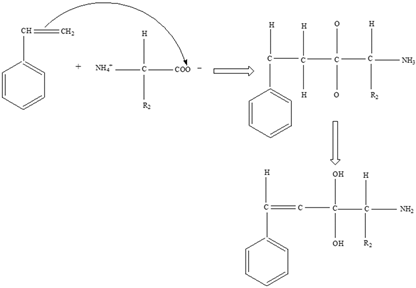 |
Bisphenol A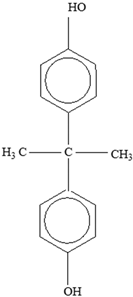 | 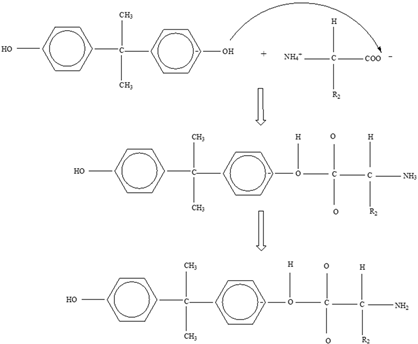 | |
Fats | Styrene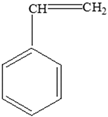 | 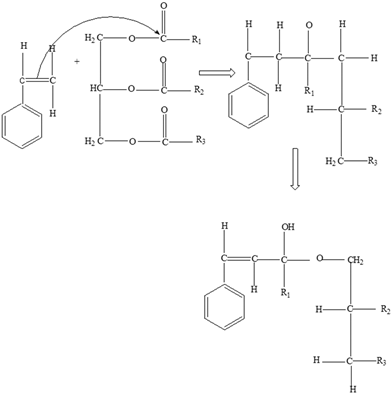 |
Bisphenol A | 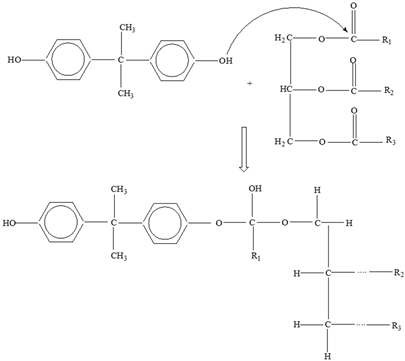 |
| Monomer | Health Effects | References |
|---|---|---|
| Styrene | -Toxic effect on the liver, chromosomal abnormalities, carcinogen, mucous membrane irritation, eye irritation, gastrointestinal effects, CNS dysfunction (reaction time and memory), effects on some kidney enzyme functions and on the blood, stimulates cell replication, cell proliferation, and cytogenetic damage promotion. | [36,140] |
| Vinyl chloride | -Liver, kidney, and lung toxicity; effects on liver, kidney, lung, spleen, nervous system and blood; cancer; causes steatohepatitis; affects glucose homeostasis; and enhances alcoholic liver disease. | [141] |
| Bisphenol A | Breast, ovarian, uterine, prostate, and testicular cancer. | [142] |
| Caprolactam | Cause neurasthenia syndrome and damages the central nervous system. | [143] |
| EDCs Present in Packaging Materials | Monomer Structures in the Food | EDC Health Effects | Sources |
|---|---|---|---|
| Plasticizers (phthalates) | DMP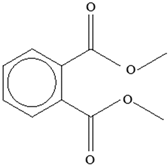 BBP 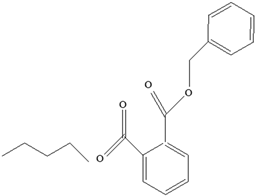 DBP  DEP 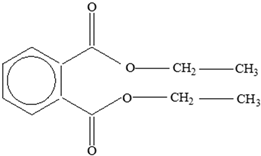 DEHP 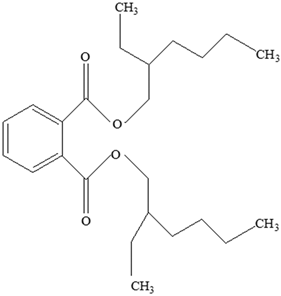 | Has antiandrogenic effects when it interacts with the androgen receptor. Interacts with the aryl hydrocarbon (AhR) and PPAR receptors. Affects thyroid signalling. Reproductive disorders, including low sperm count. Reduced anogenital distance in males. Increased risk of preterm birth. Elevated oestrogen levels in pregnant women. Birth defects. Thyroid axis dysfunction in men. Asthma. Hypospadias. Cryptorchidism. Neurobehaviour problems. | [34,141,143,144] |
| Perfluoroalkyl substances (PFASs) | Perfluoroalkyl carboxylic acids (PFCAs)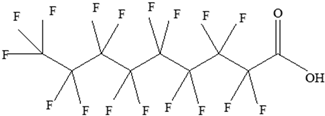 Perfluoroalkyl sulfonic acids (PFSAs)  | Decreased thyroid hormones. Has an effect on both pregnant women and children’s thyroid hormone levels. Increases hyperactivity. Developmental and immune toxicity. Cancer. Weight gain. Kidney and testicular cancer. Liver degeneration. Changes in nervous system development. Suppressed immune response. Decreased foetal and birth weights. | [145,146,147,148] |
| Dioxins | Polychlorinated biphenyls (PCB)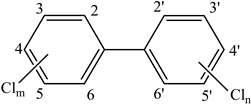 | Increased metabolism. Suppressed concentrations of thyroxine. Reduction in blood insulin and glucose levels. Increase in serum gastrin. Infertility and foetal loss. Decreased spermatogenesis. Decreased circulating androgens. Endometriosis. Inhibition of growth factor and vitamin A expression. Ovarian dysfunction. | [149] |
| Styrene | Styrene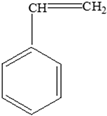 | Reproductive toxicity. Developmental toxicity. Impaired immune response to concanavalin and reduced cell-mediated immunity. Neurotoxicity, which includes the suppression of the activity of the central nervous system, including slow reaction time and altered performance on neurobehavioural tests of memory and learning. Respiratory effects, including mucous membrane irritation. Gastrointestinal effects. Effects on the liver, kidney, and eye. Nasal irritation. Lung tumours. | [36] |
| Bisphenol A | BPA | Oestrogenic properties. Interacts with a variety of nuclear receptors, including ERR, orphan receptor, oestrogen receptor, glucocorticoid receptor, human oestrogen-related receptor, PPARy, androgen receptor, and gamma receptor. Disrupts the thyroid axis. Causes metabolic disorders, which result in hyperactivity, neurodevelopment disorders, and type 2 diabetes. Causes infertility. Gut permeability. Breast and prostate cancers. It directly impairs oxidative homeostasis and indirectly impairs redox homeostasis by increasing oxidative mediators and reducing antioxidant enzymes. Increases hydrogen peroxide and lipid peroxidation. Alters organogenesis of kidneys, brain, and testes in foetus. Anxiety in childhood. Cardiovascular function disorders. Increases hydrogen peroxide and lipid peroxidation. In menopausal women, it can bind to ER (oestrogen receptor), triggering noxious cellular responses, such as binding to and stimulating oestrogen receptors (ERs) as well as disrupting action of other steroid hormones and DNA methylation. Disrupts normal action of androgens and alters thyroid hormone synthesis. | [150] |
| Parabens | Methylparaben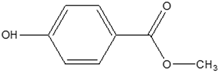 Butylparaben, isobutylparaben  | Exerts oestrogenic and antiandrogenic activities. Results in fecundity. Affects postnatal growth of boys. Increased weight. Cardiovascular diseases. More abnormal sperm. Lower testosterone levels. Cancer. Weakens enzyme activity that metabolizes endogenic hormones. Mimics oestrogens. | [35,151] |
| Heavy metals | Cadmium | Cadmium, lead, mercury, and aluminium specifically linked to oestrogenic and breast-cancer-related effects. Mercury compounds also disrupt the thyroid gland function, the hypothalamic–pituitary–adrenal axis, and thyroid hormone function. Lead inhibits cellular enzymes and binding of sulfhydryl groups. It also affects membrane stability of red blood cells, inducing functional disturbances in peripheral nerves and development of the skeleton. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzeza, C.; Ngole-Jeme, V.; Msagati, T.A.M. The Mechanisms of Plastic Food-Packaging Monomers’ Migration into Food Matrix and the Implications on Human Health. Foods 2023, 12, 3364. https://doi.org/10.3390/foods12183364
Muzeza C, Ngole-Jeme V, Msagati TAM. The Mechanisms of Plastic Food-Packaging Monomers’ Migration into Food Matrix and the Implications on Human Health. Foods. 2023; 12(18):3364. https://doi.org/10.3390/foods12183364
Chicago/Turabian StyleMuzeza, Celia, Veronica Ngole-Jeme, and Titus Alfred Makudali Msagati. 2023. "The Mechanisms of Plastic Food-Packaging Monomers’ Migration into Food Matrix and the Implications on Human Health" Foods 12, no. 18: 3364. https://doi.org/10.3390/foods12183364
APA StyleMuzeza, C., Ngole-Jeme, V., & Msagati, T. A. M. (2023). The Mechanisms of Plastic Food-Packaging Monomers’ Migration into Food Matrix and the Implications on Human Health. Foods, 12(18), 3364. https://doi.org/10.3390/foods12183364






