Assessment of the Efficiency of Technological Processes to Modify Whey Protein Antigenicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Enzyme
2.3. Degree of Hydrolysis
2.4. Indirect Competitive ELISA
2.4.1. Plates Preparation
2.4.2. Polyclonal Antibody
2.4.3. Calibration Curve
2.4.4. Immunochemical Assay
2.4.5. Antigenicity Measure
2.4.6. Validation
Sensitivity
Precision
Accuracy
2.5. Whey Protein Treatments
2.5.1. Control
2.5.2. Heat Treatment
2.5.3. High Hydrostatic Pressure
2.5.4. Enzymatic Hydrolysis
2.5.5. Combined Treatments
Enzymatic Hydrolysis + High Hydrostatic Pressure
Enzymatic Hydrolysis Followed by High Hydrostatic Pressure
Enzymatic Hydrolysis Assisted by High Hydrostatic Pressure
2.6. Statistical Analysis
3. Results
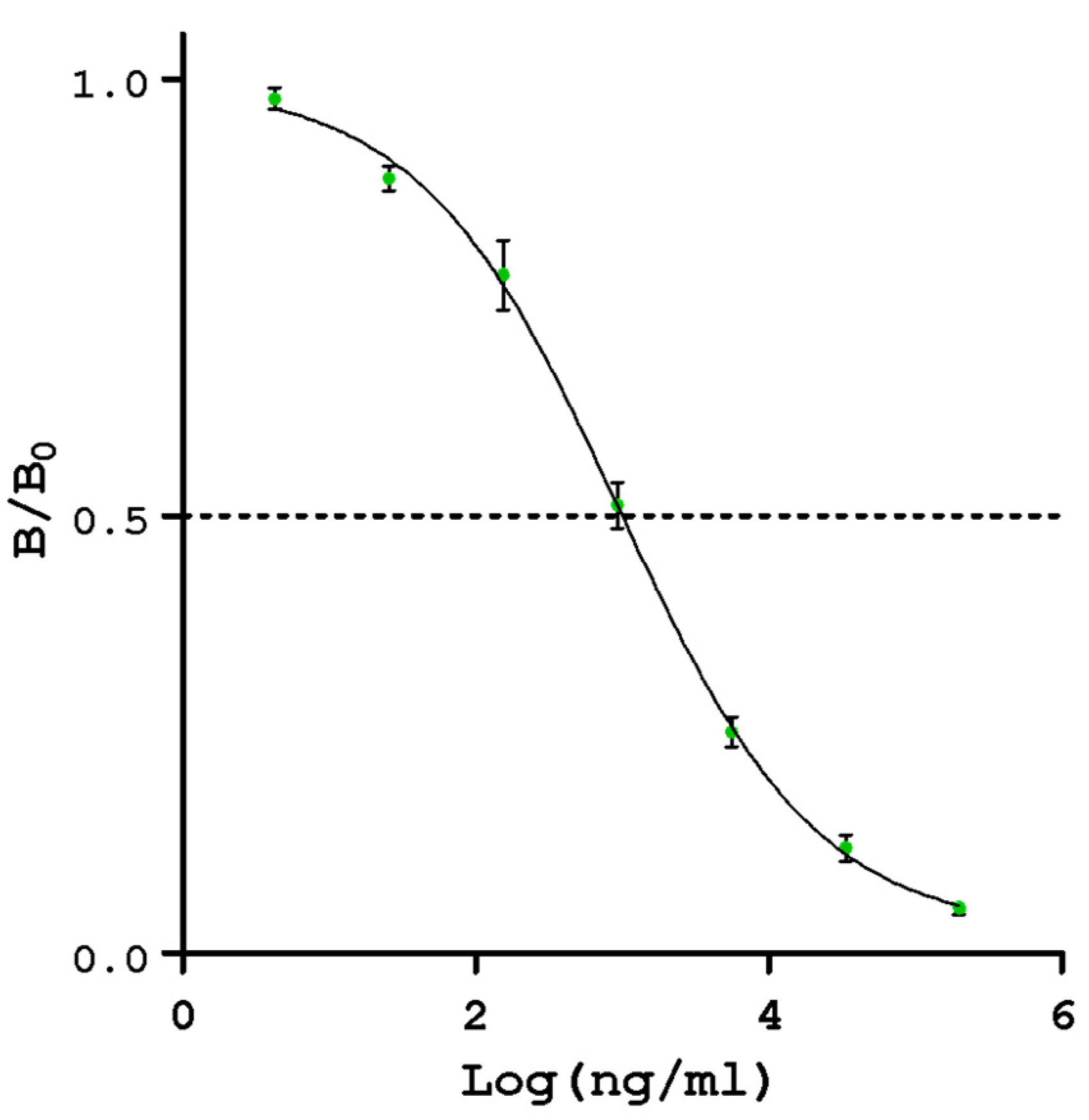
3.1. Evaluation of the Performance of Indirect Competitive ELISA
3.2. ELISA Validation
3.2.1. Sensitivity
3.2.2. Precision
3.2.3. Accuracy
3.3. Effect of the Application of Combined High Hydrostatic Pressure Treatments and Enzymatic Hydrolysis in the Degree of Hydrolysis and in the Antigenicity of BLG in Whey Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elghoudi, A.; Narchi, H. Food allergy in children-the current status and the way forward. World J. Clin. Pediatr. 2022, 11, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Venter, C. Recent Surveys on Food Allergy Prevalence. Nutr. Today 2020, 55, 22–29. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Sancho, A.I.; Hansen, E.B.; Bøgh, K.L. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods 2022, 11, 926. [Google Scholar] [CrossRef]
- Knol, E.F.; de Jong, N.W.; Ulfman, L.H.; Tiemessen, M.M. Management of Cow’s Milk Allergy from an Immunological Perspective: What Are the Options? Nutrients 2019, 11, 2734. [Google Scholar] [CrossRef] [PubMed]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Wróblewska, B.; Karamać, M.; Amarowicz, R.; Szymkiewicz, A.; Troszyńska, A.; Kubicka, E. Immunoreactive properties of peptide fractions of cow whey milk proteins after enzymatic hydrolysis. Int. J. Food Sci. Technol. 2004, 39, 839–850. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S. Bioactive peptides from egg: A review. Nutr. Food Sci. 2015, 45, 190–212. [Google Scholar] [CrossRef]
- Salvatore, S.; Acunzo, M.; Peroni, D.; Pendezza, E.; Di Profio, E.; Fiore, G.; Zuccotti, G.V.; Verduci, E. Hydrolysed Formulas in the MRanagement of Cow’s Milk Allergy: New Insights, Pitfalls and Tips. Nutrients 2022, 13, 2762. [Google Scholar] [CrossRef]
- da Silva, R.R. Enzymatic Synthesis of Protein Hydrolysates From Animal Proteins: Exploring Microbial Peptidases. Front. Microbiol. 2018, 9, 735. [Google Scholar] [CrossRef]
- da Silva, R.R. Comment on mRNA-Sequencing analysis reveals transcriptional changes in root of maize seedlings treated with two increasing concentrations of a new biostimulant. J. Agric. Food Chem. 2018, 66, 2061–2062. [Google Scholar] [CrossRef]
- Landim, A.P.M.; Tiburski, J.H.; Mellinger, C.G.; Juliano, P.; Rosenthal, A. Potential Application of High Hydrostatic Pressure on the Production of Hydrolyzed Proteins with Antioxidant and Antihypertensive Properties and Low Allergenicity: A Review. Foods 2023, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Sihag, M.; Kaushik, R. High Pressure Processing and Its Impact on Milk Proteins: A Review. J. Dairy Sci. Technol. 2013, 2, 2319–3409. [Google Scholar]
- Somkuti, J.; Smeller, L. High pressure effects on allergen food proteins. Biophys. Chem. 2013, 183, 19–29. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Chicón, R.; Belloque, J.; López-Fandiño, R.; Berin, M.C. In Vivo Methods for Testing Allergenicity Show That High Hydrostatic Pressure Hydrolysates of β-Lactoglobulin Are Immunologically Inert. J. Dairy Sci. 2012, 95, 541–548. [Google Scholar] [CrossRef]
- Ambrosi, V.; Polenta, G.; Gonzalez, C.; Ferrari, G.; Maresca, P. High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innov. Food Sci. Emerg. Technol. 2016, 38, 294–301. [Google Scholar] [CrossRef]
- Boukil, A.; Perreault, V.; Chamberland, J.; Mezdour, S.; Pouliot, Y.; Doyen, A. High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis Affect Mealworm Allergenic Proteins. Molecules 2020, 25, 2685. [Google Scholar] [CrossRef] [PubMed]
- Broersen, K. Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin. Foods 2020, 9, 874. [Google Scholar] [CrossRef]
- Gottschalk, M.; Nilsson, H.; Roos, H.; Halle, B. Protein self-association in solution: The bovine beta-lactoglobulin dimer and octamer. Protein Sci. 2003, 12, 2404–2411. [Google Scholar] [CrossRef]
- AOAC International. Nitrogen (total) in milk—Kjeldahl methods, method no. 991.20.1994 (1996). In Official Methods of Analysis of AOAC International, 19th ed.; Latimer, G.W., Jr., Ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: New York, NY, USA, 1986; pp. 110–169. [Google Scholar]
- Church, F.C.; Porter, D.H.; Catignani, G.L.; Swaisgood, H.E. An o-phthalaldehyde spectrophotometric assay for proteinases. AnalBiochem 1985, 146, 343–348. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Polenta, G.; Godefroy-Benrejeb, S.; Delahaut, P.; Dorcas, W.; Abbot, M. Development of a Competitive ELISA for the detection of Pecan (Carya illinoinensis (Wangenh.)K. Koch) traces in food. Food Anal. Methods 2010, 3, 375–381. [Google Scholar] [CrossRef]
- Schneider, N.; Weigel, I.; Werkmeister, K.; Pischetsrieder, M. Development and validation of an enzyme-linked Immunosorbent assay (ELISA) for quantification of lysozyme in cheese. J. Agric. Food Chem. 2010, 58, 76–81. [Google Scholar] [CrossRef]
- Ecker, C.; Ertl, A.; Cichna-Markl, M. Development and Validation of Two Competitive ELISAs for the Detection of Potentially Allergenic Lupine (Lupinus Species) in Food. Food Anal. Methods 2013, 6, 248–257. [Google Scholar] [CrossRef]
- Huber, L. Validation of Analytical Methods; Agilent Technologies: Waldbronn, Germany, 2010. [Google Scholar]
- Segura-Gil, I.; Blázquez-Soro, A.; Galán-Malo, P.; Mata, L.; Tobajas, A.P.; Sánchez, L.; Pérez, M.D. Development of sandwich and competitive ELISA formats to determine β-conglycinin: Evaluation of their performance to detect soy in processed food. Food Control 2019, 103, 78–85. [Google Scholar] [CrossRef]
- Abbott, M.; Hayward, S.; Ross, W.; Godefroy, S.; Ulberth, F.; Van Hengel, A.; Roberts, J.; Akiyama, H.; Popping, B.; Yeung, J.; et al. Validation Procedures for Quantitative Food Allergen ELISA Methods: Community Guidance and Best Practices. J. AOAC Int. 2010, 93, 442–450. [Google Scholar] [CrossRef]
- Cordle, C. Allergen quality assurance for hypoallergenic formula. In Detecting Allergens in Food, 1st ed.; Koppelman, S.J., Hefle, S.L., Eds.; Woodhead Publishing Limited: Sawston, UK, 2006; pp. 293–314. [Google Scholar] [CrossRef]
- Svenning, C.; Brynhildsvold, J.; Molland, T.; Langsrud, T.; Vegarud, G.E. Antigenic response of whey proteins and genetic variants of b-lactoglobulin—The effect of proteolysis and processing. Int. Dairy J. 2000, 10, 699–711. [Google Scholar] [CrossRef]
- Villa, C. Food Allergens: Detection and Immunogenic Pro; Erties as Affected by Processing and In Vitro Digestibility. Ph.D. Thesis, University of Porteo, Porto, Portugal, 2020. [Google Scholar]
- Cucu, T.; Devreese, B.; Kerkaert, B.; Rogge, M.; Vercruysse, L.; De Meulenaer, B. ELISA based detection of soybean proteins: A comparative study using antibodies against modified and native proteins. Food Anal. Methods 2012, 5, 1121–1130. [Google Scholar] [CrossRef]
- Karamonova, L.; Fukal, L.; Kodicek, M.; Rauch, P.; Mills, E.N.C.; Morgan, M.R.A. Immunoprobes for thermally-induced alterations in whey protein structure and their application to the analysis of thermally-treated milks. Food Agric. Immunol. 2003, 15, 77–91. [Google Scholar] [CrossRef]
- Kleber, N.; Krause, I.; Illgner, S.; Hinrichs, J. The antigenic response of beta-lactoglobulin is modulated by thermally induced aggregation. Eur. Food Res. Technol. 2004, 219, 105–110. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.-Y. Potential Utility of High-Pressure Processing to Address the Risk of Food Allergen Concerns. Compr. Rev. Food Sci. Food Saf. 2014, 13, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Kleber, N.; Maier, S.; Hinrichs, J. Antigenic response of bovine b-lactoglobulin influenced by ultra-high pressure treatment and temperature. Inn. Food Sci Emer. Tech. 2007, 8, 39–45. [Google Scholar] [CrossRef]
- Belloque, R.; Chicon, R.; López-Fandino, R. Unfolding and refolding of betalactoglobulinsubjected to high hydrostatic pressure at different pH values and temperatures and its influence on proteolysis. J. Agric. Food Chem. 2007, 55, 5282–5288. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, H.; Sun, J.; Cheng, J.; Luo, X.; Wang, Z.; Yang, M.; Tao, D.B.; Yue, X.; Zheng, Y. Effects of enzymatic treatments on the hydrolysis and antigenicity reduction of natural cow milk. Food Sci. Nutr. 2021, 9, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.B.; Candreva, A.M.; Rizzo, G.; Ramos, M.V.; Oliveira, J.S.; Oliveira, H.D.; Ary, M.B.; Docena, G.; Freitas, C.D.T. Allergenicity reduction of cow’s milk proteins using latex peptidases. Food Chem. 2019, 284, 245–253. [Google Scholar] [CrossRef]
- Asselin, J.; Hebert, J.; Amiot, J. Effects of in vitro proteolysis on the allergenicity of major whey proteins. J. Food Sci. 1989, 54, 1037–1039. [Google Scholar] [CrossRef]
- Lieske, B.; Konrad, G. Interrelation between pH and availability of a-lactalbumin and b-lactoglobulin for proteolysis by papain. Int. Dairy J. 1996, 6, 359–370. [Google Scholar] [CrossRef]
- Bonomi, F.; Fiocchi, A.; Frøkiaer, H.; Gaiaschi, A.; Iametti, S.; Poiesi, C.; Rasmussen, P.; Restani, P.; Rovere, P. Reduction of immunoreactivity of bovine beta-lactoglobulin upon combined physical and proteolytic treatment. J. Dairy Res. 2003, 70, 51–59. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Recio, I.; López-Fandiño, R. Influence of high hydrostatic pressure on the proteolysis pattern of beta-lactoglobulin A treated with trypsin. J. Dairy Res. 2006, 73, 121–128. [Google Scholar] [CrossRef]
- Chicón, R.; López-Fandiño, R.; Quirós, A.; Belloque, J. Changes of chymotrypsin hydrolysis of beta-lactoblobulin A induced by high hydrostatic pressure. J Agric. Food Chem. 2006, 54, 2333–2341. [Google Scholar] [CrossRef]
- Peñas, E.; Snel, H.; Floris, R.; Préstamo, G.; Gomez, R. High pressure can reduce the antigenicity of bovine whey protein hydrolysates. Int. Dairy J. 2006, 16, 969–975. [Google Scholar] [CrossRef]
- Zeece, M.; Huppertz, T.; Kelly, A. Effect of high-pressure treatment on in-vitro digestibility of beta-lactoglobulin. Innov. Food Sci. Emerg. Technol. 2008, 9, 62–69. [Google Scholar] [CrossRef]
- Espinosa-Pesqueira, D.; Hernández-Herrero, M.M.; Roig-Sagués, A.X. High Hydrostatic Pressure as a Tool to Reduce Formation of Biogenic Amines in Artisanal Spanish Cheeses. Foods 2018, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Gomez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef] [PubMed]
| Treatment † | HHP • | H ⋄ | TIE ⋆ | Note ‡ | |
|---|---|---|---|---|---|
| 1: | C | - | - | - | Untreated WPC, control |
| 2: | T-In | - | - | 100 °C—10 min | WPC + thermal inactivation of the enzyme, TIE control |
| 3: | HHP | 300 MPa—5 min—45 °C | - | - | WPC + HHP |
| 4: | H-In | - | 45 °C—15 min + 25 °C—10 min | 100 °C—10 min | Enzymatic hydrolysis |
| 5: | H-In-HHP | 300 MPa—5 min—45 °C | 45 °C—15 min | 100 °C—10 min | Enzymatic hydrolysis followed by TIE + HHP treatment |
| 6: | Hext-In | - | 45 °C—30 min + 25 °C—10 min | 100 °C—10 min | Extended enzymatic hydrolysis |
| 7: | H+HHP-In | 300 MPa—5 min—45 °C | 45 °C—15 min | 100 °C—10 min | Enzymatic hydrolysis treatment combined with HHP |
| 8: | HHPaH-In | 300 MPa—5 min—45 °C | 45 °C—5 min ∎ | 100 °C—10 min | HHP-assisted enzymatic hydrolysis treatment |
| 4PL Regression Parameters | |
|---|---|
| A | 0.995 |
| B | 0.025 |
| C | 942 |
| D | 0.644 |
| R2 | 0.9959 |
| Concentration Range (ng whey protein/mL) | 4–200,000 |
| Parameter | Value |
|---|---|
| Limit of detection (ng whey protein/mL) (n = 24) | 6.6 ± 0.02 |
| Limit of quantification (ng whey protein/mL) (n = 24) | 94.3 ± 0.05 |
| Intra-assay variability (n = 24), CV (%) | 8.0–11.9 |
| Inter-assay variability (n = 27), CV (%) | 7.53–12.5 |
| Recovery (7 ng/mL, n = 5) (%) | 70 ± 8.8 |
| Recovery (95 ng/mL, n = 5) (%) | 135 ± 10.6 |
| Recovery (925 ng/mL, n = 5) (%) | 98 ± 12.5 |
| Treatment † | %DH ‡ | %BLG • | |
|---|---|---|---|
| 1: | C | 0.00 ± 0.44 a | 100.0 ± 4.8 B |
| 2: | T-In | −0.21 ± 0.11 a | 129.7 ± 7.2 A |
| 3: | HHP | −0.55 ± 0.43 a | 122.8 ± 0.7 A |
| 4: | H-In | 4.05 ± 0.31 b | 86.6 ± 1.7 C |
| 5: | H-In-HHP | 4.18 ± 0.50 b | 82.4 ± 8.6 C |
| 6: | Hext-In | 7.69 ± 0.45 c | 72.9 ± 5.1 D |
| 7: | H+HHP-In | 8.20 ± 0.45 d | 43.2 ± 1.3 E |
| 8: | (HHPaH)-In | 9.00 ± 0.40 d | 20.7 ± 1.0 F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosi, V.A.; Guidi, S.M.; Primrose, D.M.; Gonzalez, C.B.; Polenta, G.A. Assessment of the Efficiency of Technological Processes to Modify Whey Protein Antigenicity. Foods 2023, 12, 3361. https://doi.org/10.3390/foods12183361
Ambrosi VA, Guidi SM, Primrose DM, Gonzalez CB, Polenta GA. Assessment of the Efficiency of Technological Processes to Modify Whey Protein Antigenicity. Foods. 2023; 12(18):3361. https://doi.org/10.3390/foods12183361
Chicago/Turabian StyleAmbrosi, Vanina Andrea, Silvina Mabel Guidi, Debora Marina Primrose, Claudia Beatriz Gonzalez, and Gustavo Alberto Polenta. 2023. "Assessment of the Efficiency of Technological Processes to Modify Whey Protein Antigenicity" Foods 12, no. 18: 3361. https://doi.org/10.3390/foods12183361
APA StyleAmbrosi, V. A., Guidi, S. M., Primrose, D. M., Gonzalez, C. B., & Polenta, G. A. (2023). Assessment of the Efficiency of Technological Processes to Modify Whey Protein Antigenicity. Foods, 12(18), 3361. https://doi.org/10.3390/foods12183361






