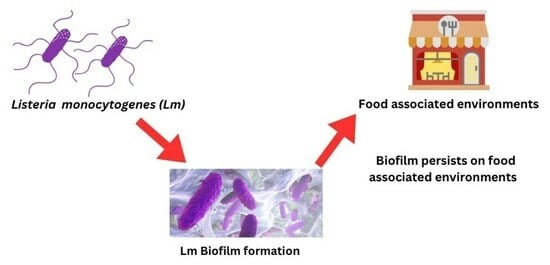
Listeria monocytogenes Biofilms in Food-Associated Environments: A Persistent Enigma
Abstract
:1. Introduction
2. LM Biofilms
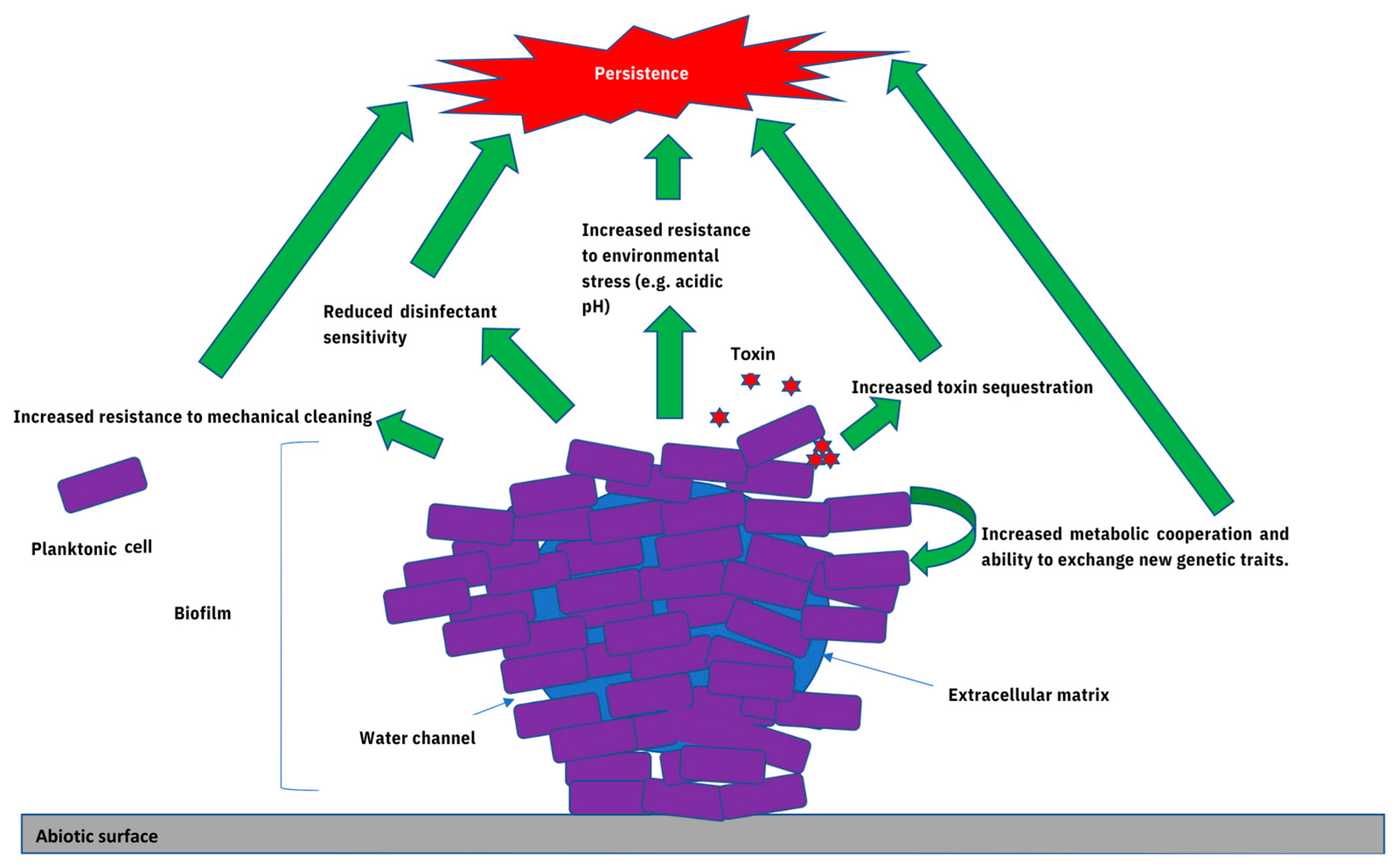
3. The Contribution of Biofilms towards Microbial Persistence within Food-Associated Environments
4. Phenotypic Comparisons of Biofilm Development in Persistent and PNP LM Strains
5. Genetic Mechanisms Involved in Biofilm Development in LM
6. WGS Comparisons of Biofilm Production in Persistent and PNP LM Strains
7. Challenges and Solutions
7.1. Lack of a Standardised Persistence Definition
7.2. Possibility of Repeated Reintroduction
7.3. Assay Limitations
7.4. Experimental Conditions Unrepresentative of Food-Associated Environments
7.5. Lack of Standardised Experimental Protocols
8. Conclusions and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes Biofilms in the Wonderland of Food Industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes Biofilms in the Food Industry: Is the Current Hygiene Program Sufficient to Combat the Persistence of the Pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: A Review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef]
- Unrath, N.; McCabe, E.; Macori, G.; Fanning, S. Application of Whole Genome Sequencing to Aid in Deciphering the Persistence Potential of Listeria monocytogenes in Food Production Environments. Microorganisms 2021, 9, 1856. [Google Scholar] [CrossRef]
- Lyytikäinen, O.; Autio, T.; Maijala, R.; Ruutu, P.; Honkanen-Buzalski, T.; Miettinen, M.; Hatakka, M.; Mikkola, J.; Anttila, V.-J.; Johansson, T.; et al. An Outbreak of Listeria Monocytogenes Serotype 3a Infections from Butter in Finland. J. Infect. Dis. 2000, 181, 1838–1841. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thomas, T.; Govender, N.; Govender, N.P.; McCarthy, K.M.; Erasmus, L.K.; Doyle, T.J.; Allam, M.; Ismail, A.; Ramalwa, N.; et al. Outbreak of Listeriosis in South Africa Associated with Processed Meat. N. Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef]
- McLauchlin, J.; Grant, K.A.; Amar, C.F.L. Human foodborne listeriosis in England and Wales, 1981 to 2015. Epidemiol. Infect. 2020, 148, e54. [Google Scholar] [CrossRef]
- Malley, T.J.V.; Butts, J.; Wiedmann, M. Seek and destroy process: Listeria monocytogenes process controls in the ready-to-eat meat and poultry industry. J. Food Prot. 2015, 78, 436–445. [Google Scholar] [CrossRef]
- EFSA/ECDC. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- de Noordhout, C.M.M.; Devleesschauwer, B.M.; Angulo, F.J.P.; Verbeke, G.P.; Haagsma, J.P.; Kirk, M.P.; Havelaar, A.P.; Speybroeck, N.P. The global burden of listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef] [PubMed]
- Kathariou, S. Listeria monocytogenes Virulence and Pathogenicity, a Food Safety Perspective. J. Food Prot. 2002, 65, 1811–1829. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, W.B.; Cutter, C.N. An Ecological Perspective of Listeria monocytogenes Biofilms in Food Processing Facilities. Crit. Rev. Food Sci. Nutr. 2013, 53, 801–817. [Google Scholar] [CrossRef]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef] [PubMed]
- Tompkin, R.B. Control of Listeria monocytogenes in the Food-Processing Environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Natalia, W.-K.; Ewa, W.-Z.; Krzysztof, S.; Agnieszka, K.; Zuzanna, B.; Justyna, B.-K.; Zuzanna, K.; Eugenia, G.-K. Comparison of Selected Phenotypic Features of Persistent and Sporadic Strains of Listeria monocytogenes Sampled from Fish Processing Plants. Foods 2022, 11, 1492. [Google Scholar] [CrossRef]
- Kastbjerg, V.G.; Gram, L. Model systems allowing quantification of sensitivity to disinfectants and comparison of disinfectant susceptibility of persistent and presumed nonpersistent Listeria monocytogenes. J. Appl. Microbiol. 2009, 106, 1667–1681. [Google Scholar] [CrossRef]
- Orsi, R.H.; Borowsky, M.L.; Lauer, P.; Young, S.K.; Nusbaum, C.; Galagan, J.E.; Birren, B.W.; Ivy, R.A.; Sun, Q.; Graves, L.M.; et al. Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMC Genom. 2008, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Ivanek, R.; GrÖHn, Y.T.; Tauer, L.W.; Wiedmann, M. The Cost and Benefit of Listeria Monocytogenes Food Safety Measures. Crit. Rev. Food Sci. Nutr. 2005, 44, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Batz, M.B.; Morris, J.J.G. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Prot. 2012, 75, 1292–1302. [Google Scholar] [CrossRef]
- Forauer, E.; Wu, S.T.; Etter, A.J. Listeria monocytogenes in the retail deli environment: A review. Food Control 2021, 119, 107443. [Google Scholar] [CrossRef]
- Holah, J.T.; Taylor, J.H.; Dawson, D.J.; Hall, K.E. Biocide use in the food industry and the disinfectant resistance of persistent strains of Listeria monocytogenes and Escherichia coli. Symp. Ser. (Soc. Appl. Microbiol.) 2002, 92, 111S–120S. [Google Scholar] [CrossRef]
- Wulff, G.; Gram, L.; Ahrens, P.; Vogel, B.F. One Group of Genetically Similar Listeria monocytogenes Strains Frequently Dominates and Persists in Several Fish Slaughter- and Smokehouses. Appl. Environ. Microbiol. 2006, 72, 4313–4322. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. Adherence of Listeria monocytogenes strains to stainless steel coupons. J. Appl. Microbiol. 1999, 86, 576–582. [Google Scholar] [CrossRef]
- Lunden, J.M.; Miettinen, M.K.; Autio, T.J.; Korkeala, H.J. Persistent Listeria monocytogenes Strains Show Enhanced Adherence to Food Contact Surface after Short Contact Times. J. Food Prot. 2000, 63, 1204–1207. [Google Scholar] [CrossRef]
- Borucki, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in Biofilm Formation among Strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef]
- Monds, R.D.; O’Toole, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2008, 17, 73–87. [Google Scholar] [CrossRef]
- Marsh, E.J.; Luo, H.; Wang, H. A three-tiered approach to differentiate Listeria monocytogenes biofilm-forming abilities. FEMS Microbiol. Lett. 2003, 228, 203–210. [Google Scholar] [CrossRef]
- Rieu, A.; Briandet, R.; Habimana, O.; Garmyn, D.; Guzzo, J.; Piveteau, P. Listeria monocytogenes EGD-e Biofilms: No Mushrooms but a Network of Knitted Chains. Appl. Environ. Microbiol. 2008, 74, 4491–4497. [Google Scholar] [CrossRef]
- Milanov, D.; Ašanin, R.; Vidić, R.; Katić, V.; Plavša, N. Examination of the capabilities of attachment and biofilm formation of different Listeria monocytogenes STRAINS. Biotechnol. Anim. Husb. 2009, 5–6, 1255–1265. [Google Scholar]
- Blackman, I.C.; Frank, J.F. Growth of Listeria monocytogenes as a biofilm on various food-processing surfaces. J. Food Prot. 1996, 59, 827–831. [Google Scholar] [CrossRef]
- Chavant, P.; Martinie, B.; Thierry, M.; Marie-Noëlle, B.-F.; Michel, H. Listeria monocytogenes LO28: Surface Physicochemical Properties and Ability To Form Biofilms at Different Temperatures and Growth Phases. Appl. Environ. Microbiol. 2002, 68, 728–737. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Di Ciccio, P.; Zanardi, E.; Ghidini, S.; Ianieri, A. A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix. Microorganisms 2016, 4, 22. [Google Scholar] [CrossRef]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm Formation and Control in Food Processing Facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef]
- Kumar, C.G.; Anand, S.K. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Latorre, A.A.; Van Kessel, J.A.S.; Karns, J.S.; Zurakowski, M.J.; Pradhan, A.K.; Boor, K.J.; Adolph, E.; Sukhnanand, S.; Schukken, Y.H. Increased In Vitro Adherence and On-Farm Persistence of Predominant and Persistent Listeria monocytogenes Strains in the Milking System. Appl. Environ. Microbiol. 2011, 77, 3676–3684. [Google Scholar] [CrossRef]
- Russo, P.; Hadjilouka, A.; Beneduce, L.; Capozzi, V.; Paramithiotis, S.; Drosinos, E.H.; Spano, G. Effect of different conditions on Listeria monocytogenes biofilm formation and removal. Czech J. Food Sci. 2018, 36, 208–214. [Google Scholar] [CrossRef]
- Duffy, G.; Sheridan, J.J. The effect of temperature, pH and medium in a surface adhesion immunofluorescent technique for detection of Listeria monocytogenes. J. Appl. Microbiol. 1997, 83, 95–101. [Google Scholar] [CrossRef]
- Briandet, R.; Leriche, V.; Carpentier, B.; Bellon-Fontaine, M.-N. Effects of the Growth Procedure on the Surface Hydrophobicity of Listeria monocytogenes Cells and Their Adhesion to Stainless Steel. J. Food Prot. 1999, 62, 994–998. [Google Scholar] [CrossRef]
- Lee, B.-H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hébraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm Formation of Listeria monocytogenes Strains Under Food Processing Environments and Pan-Genome-Wide Association Study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef]
- Kadam, S.R.; den Besten, H.M.W.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef]
- Nilsson, R.E.; Ross, T.; Bowman, J.P. Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int. J. Food Microbiol. 2011, 150, 14–24. [Google Scholar] [CrossRef]
- Smoot, L.M.; Pierson, M.D. Influence of Environmental Stress on the Kinetics and Strength of Attachment of Listeria monocytogenes Scott A to Buna-N Rubber and Stainless Steel. J. Food Prot. 1998, 61, 1286–1292. [Google Scholar] [CrossRef]
- Holah, J.T.; Thorpe, R.H. Cleanability in relation to bacterial retention on unused and abraded domestic sink materials. J. Appl. Bacteriol. 1990, 69, 599–608. [Google Scholar] [CrossRef]
- Mustapha, A.; Liewen, M.B. Destruction of Listeria monocytogenes by sodium hypochlorite and quaternary ammonium sanitizers. J. Food Prot. 1989, 52, 306–311. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio)control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, E.; Naghoni, A.; Rahvar, H.; Kialha, M.; Tabaraie, B. Evaluating the Effect of Copper Nanoparticles in Inhibiting Pseudomonas aeruginosa and Listeria monocytogenes Biofilm Formation. Jundishapur J. Microbiol. 2015, 8, e17430. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Patuzzi, I.; Rigo, L.; Conficoni, D.; Gallocchio, F.; Cibin, V.; Catellani, P.; Segato, S.; Ricci, A. Silver As Antibacterial toward Listeria monocytogenes. Front. Microbiol. 2016, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Smoot, L.M.; Pierson, M.D. Effect of Environmental Stress on the Ability of Listeria monocytogenes Scott A To Attach to Food Contact Surfaces. J. Food Prot. 1998, 61, 1293–1298. [Google Scholar] [CrossRef]
- Bonsaglia, E.C.R.; Silva, N.C.C.; Fernades Júnior, A.; Araújo Júnior, J.P.; Tsunemi, M.H.; Rall, V.L.M. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control 2014, 35, 386–391. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter Plate Assay for Assessment of Listeria monocytogenes Biofilm Formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Svabic-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Takahashi, H.; Suda, T.; Tanaka, Y.; Kimura, B. Cellular hydrophobicity of Listeria monocytogenes involves initial attachment and biofilm formation on the surface of polyvinyl chloride. Lett. Appl. Microbiol. 2010, 50, 618–625. [Google Scholar] [CrossRef]
- Bereksi, N.; Gavini, F.; Bénézech, T.; Faille, C. Growth, morphology and surface properties of Listeria monocytogenes Scott A and LO28 under saline and acid environments. J. Appl. Microbiol. 2002, 92, 556–565. [Google Scholar] [CrossRef]
- Mafu, A.A.; Roy, D.; Goulet, J.; Savoie, L. Characterization of physicochemical forces involved in adhesion of Listeria monocytogenes to surfaces. Appl. Environ. Microbiol. 1991, 57, 1969–1973. [Google Scholar] [CrossRef]
- Caly, D.; Takilt, D.; Lebret, V.; Tresse, O. Sodium chloride affects Listeria monocytogenes adhesion to polystyrene and stainless steel by regulating flagella expression. Lett. Appl. Microbiol. 2009, 49, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Briandet, R.; Meylheuc, T.; Maher, C.; Bellon-Fontaine, M.N. Listeria monocytogenes Scott A: Cell Surface Charge, Hydrophobicity, and Electron Donor and Acceptor Characteristics under Different Environmental Growth Conditions. Appl. Environ. Microbiol. 1999, 65, 5328–5333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, X.; Zhang, Q.; Feng, F.; Yin, X.; Shang, J.; Qu, H.; Luo, Q. Carbon Catabolite Control is Important for Listeria monocytogenes Biofilm Formation in Response to Nutrient Availability. Curr. Microbiol. 2012, 65, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Combrouse, T.; Sadovskaya, I.; Faille, C.; Kol, O.; Guérardel, Y.; Midelet-Bourdin, G. Quantification of the extracellular matrix of the Listeria monocytogenes biofilms of different phylogenic lineages with optimization of culture conditions. J. Appl. Microbiol. 2013, 114, 1120–1131. [Google Scholar] [CrossRef]
- Hood, S.K.; Zottola, E.A. Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Int. J. Food Microbiol. 1997, 37, 145–153. [Google Scholar] [CrossRef]
- Mai, T.L.; Conner, D.E. Effect of temperature and growth media on the attachment of Listeria monocytogenes to stainless steel. Int. J. Food Microbiol. 2007, 120, 282–286. [Google Scholar] [CrossRef]
- Ronner, A.B.; Wong, A.C.L. Biofilm Development and Sanitizer Inactivation of Listeria monocytogenes and Salmonella typhimurium on Stainless Steel and Buna-n Rubber. J. Food Prot. 1993, 56, 750–758. [Google Scholar] [CrossRef]
- Moltz, A.G.; Martin, S.E. Formation of Biofilms by Listeria monocytogenes under Various Growth Conditions. J. Food Prot. 2005, 68, 92–97. [Google Scholar] [CrossRef]
- Folsom, J.P.; Siragusa, G.R.; Frank, J.F. Formation of Biofilm at Different Nutrient Levels by Various Genotypes of Listeria monocytogenes. J. Food Prot. 2006, 69, 826–834. [Google Scholar] [CrossRef]
- Sashara, K.C.; Zottola, E.A. Biofilm formation by Listeria monocytogenes utilizes a primary colonizing microorganism in flowing systems. J. Food Prot. 1993, 56, 1022–1028. [Google Scholar] [CrossRef]
- Carpentier, B.; Chassaing, D. Interactions in biofilms between Listeria monocytogenes and resident microorganisms from food industry premises. Int. J. Food Microbiol. 2004, 97, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Ripolles-Avila, C.; Hascoët, A.S.; Guerrero-Navarro, A.E.; Rodríguez-Jerez, J.J. Establishment of incubation conditions to optimize the in vitro formation of mature Listeria monocytogenes biofilms on food-contact surfaces. Food Control 2018, 92, 240–248. [Google Scholar] [CrossRef]
- Davey, M.E.; O’Toole, G.A. Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef]
- Vestby, L.K.; Møretrø, T.; Langsrud, S.; Heir, E.; Nesse, L.L. Biofilm forming abilities of Salmonella are correlated with persistence in fish meal- and feed factories. BMC Vet. Res. 2009, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Dantas, S.T.A.; Camargo, C.H.; Tiba-Casas, M.R.; Vivian, R.C.; Pinto, J.P.A.N.; Pantoja, J.C.F.; Hernandes, R.T.; Fernandes Júnior, A.; Rall, V.L.M. Environmental persistence and virulence of Salmonella spp. Isolated from a poultry slaughterhouse. Food Res. Int. 2020, 129, 108835. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Luedtke, B.E.; Bosilevac, J.M.; Schmidt, J.W.; Kalchayanand, N.; Arthur, T.M. Escherichia coli O157:H7 Strains Isolated from High-Event Period Beef Contamination Have Strong Biofilm-Forming Ability and Low Sanitizer Susceptibility, Which Are Associated with High pO157 Plasmid Copy Number. J. Food Prot. 2016, 79, 1875–1883. [Google Scholar] [CrossRef]
- Jaakkonen, A.; Kivistö, R.; Aarnio, M.; Kalekivi, J.; Hakkinen, M. Persistent contamination of raw milk by Campylobacter jejuni ST-883. PLoS ONE 2020, 15, e0231810. [Google Scholar] [CrossRef]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C.J. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- White, A.P.; Gibson, D.L.; Grassl, G.A.; Kay, W.W.; Finlay, B.B.; Vallance, B.A.; Surette, M.G. Aggregation via the red, dry, and rough morphotype is not a virulence adaptation in Salmonella enterica serovar Typhimurium. Infect. Immun. 2008, 76, 1048–1058. [Google Scholar] [CrossRef]
- White, A.P.; Gibson, D.L.; Kim, W.; Kay, W.W.; Surette, M.G. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J. Bacteriol. 2006, 188, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Salmonella and Campylobacter biofilm formation: A comparative assessment from farm to fork. J. Sci. Food Agric. 2018, 98, 4014–4032. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tran, F.; Youssef, M.K.; Gill, C.O. Determination of Sources of Escherichia coli on Beef by Multiple-Locus Variable-Number Tandem Repeat Analysis. J. Food Prot. 2015, 78, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in Food Processing Environments: Challenges and Opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Van Houdt, R.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef]
- Larsen, M.H.; Dalmasso, M.; Ingmer, H.; Langsrud, S.; Malakauskas, M.; Mader, A.; Møretrø, T.; Smole Možina, S.; Rychli, K.; Wagner, M.; et al. Persistence of foodborne pathogens and their control in primary and secondary food production chains. Food Control 2014, 44, 92–109. [Google Scholar] [CrossRef]
- Lunden, J.M.; Autio, T.J.; Korkeala, H.J. Transfer of Persistent Listeria monocytogenes Contamination between Food-Processing Plants Associated with a Dicing Machine. J. Food Prot. 2002, 65, 1129–1133. [Google Scholar] [CrossRef]
- Poimenidou, S.V.; Chrysadakou, M.; Tzakoniati, A.; Bikouli, V.C.; Nychas, G.-J.; Skandamis, P.N. Variability of Listeria monocytogenes strains in biofilm formation on stainless steel and polystyrene materials and resistance to peracetic acid and quaternary ammonium compounds. Int. J. Food Microbiol. 2016, 237, 164–171. [Google Scholar] [CrossRef]
- Nowak, J.; Cruz, C.D.; Tempelaars, M.; Abee, T.; van Vliet, A.H.M.; Fletcher, G.C.; Hedderley, D.; Palmer, J.; Flint, S. Persistent Listeria monocytogenes strains isolated from mussel production facilities form more biofilm but are not linked to specific genetic markers. Int. J. Food Microbiol. 2017, 256, 45–53. [Google Scholar] [CrossRef]
- Rodríguez-Campos, D.; Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Persistent Listeria monocytogenes isolates from a poultry-processing facility form more biofilm but do not have a greater resistance to disinfectants than sporadic strains. Pathogens 2019, 8, 250. [Google Scholar] [CrossRef]
- Harrand, A.S.; Jagadeesan, B.; Baert, L.; Wiedmann, M.; Orsi, R.H. Evolution of Listeria monocytogenes in a Food Processing Plant Involves Limited Single-Nucleotide Substitutions but Considerable Diversification by Gain and Loss of Prophages. Appl. Environ. Microbiol. 2020, 86, e02493-19. [Google Scholar] [CrossRef]
- Harvey, J.; Keenan, K.P.; Gilmour, A. Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol. 2007, 24, 380–392. [Google Scholar] [CrossRef]
- Jensen, A.; Larsen, M.H.; Ingmer, H.; Vogel, B.F.; Gram, L. Sodium Chloride Enhances Adherence and Aggregation and Strain Variation Influences Invasiveness of Listeria monocytogenes Strains. J. Food Prot. 2007, 70, 592–599. [Google Scholar] [CrossRef]
- Cruz, C.D.; Fletcher, G.C. Prevalence and biofilm-forming ability of Listeria monocytogenes in New Zealand mussel (Perna canaliculus) processing plants. Food Microbiol. 2011, 28, 1387–1393. [Google Scholar] [CrossRef]
- Szlavik, J.; Paiva, D.S.; Mørk, N.; van den Berg, F.; Verran, J.; Whitehead, K.; Knøchel, S.; Nielsen, D.S. Initial adhesion of Listeria monocytogenes to solid surfaces under liquid flow. Int. J. Food Microbiol. 2012, 152, 181–188. [Google Scholar] [CrossRef]
- Costa, A.; Bertolotti, L.; Brito, L.; Civera, T. Biofilm Formation and Disinfectant Susceptibility of Persistent and Nonpersistent Listeria monocytogenes Isolates from Gorgonzola Cheese Processing Plants. Foodborne Pathog. Dis. 2016, 13, 602–609. [Google Scholar]
- Korenova, J.; Oravcova, K.; Veghova, J.; Karpiskova, R.; Kuchta, T. Biofilm formation in various conditions is not a key factor of persistence potential of Listeria monocytogenes in food-processing environment. J. Food. Nutr. Res 2016, 55, 189–193. [Google Scholar]
- In Lee, S.H.; Barancelli, G.V.; de Camargo, T.M.; Corassin, C.H.; Rosim, R.E.; da Cruz, A.G.; Cappato, L.P.; de Oliveira, C.A.F. Biofilm-producing ability of Listeria monocytogenes isolates from Brazilian cheese processing plants. Food Res. Int. 2017, 91, 88–91. [Google Scholar] [CrossRef]
- Cherifi, T.; Carrillo, C.; Lambert, D.; Miniaï, I.; Quessy, S.; Larivière-Gauthier, G.; Blais, B.; Fravalo, P. Genomic characterization of Listeria monocytogenes isolates reveals that their persistence in a pig slaughterhouse is linked to the presence of benzalkonium chloride resistance genes. BMC Microbiol. 2018, 18, 220. [Google Scholar] [CrossRef]
- Sereno, M.J.; Viana, C.; Pegoraro, K.; da Silva, D.A.L.; Yamatogi, R.S.; Nero, L.A.; Bersot, L.d.S. Distribution, adhesion, virulence and antibiotic resistance of persistent Listeria monocytogenes in a pig slaughterhouse in Brazil. Food Microbiol. 2019, 84, 103234. [Google Scholar] [CrossRef]
- Ramires, T.; Kleinubing, N.R.; Iglesias, M.A.; Vitola, H.R.S.; Núncio, A.S.P.; Kroning, I.S.; Moreira, G.M.S.G.; Fiorentini, Â.M.; da Silva, W.P. Genetic diversity, biofilm and virulence characteristics of Listeria monocytogenes in salmon sushi. Food Res. Int. 2021, 140, 109871. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, Y.; Yamada, F.; Mochizuki, M.; Takano, T.; Hondo, R.; Ueda, F. Biofilm formation under different temperature conditions by a single genotype of persistent Listeria monocytogenes strains. J. Food Prot. 2014, 77, 133–140. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.A.L.; de Melo Tavares, R.; Camargo, A.C.; Yamatogi, R.S.; De Martinis, E.C.P.; Nero, L.A. Biofilm growth by Listeria monocytogenes on stainless steel and expression of biofilm-related genes under stressing conditions. World J. Microbiol. Biotechnol. 2021, 37, 119. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, R.; Ferreira, V.; Biscottini, G.; Brandao, T.R.S.; Almeida, G.; Teixeira, P. Biofilm formation by persistent and non-persistent listeria monocytogenes strains on abiotic surfaces. Acta Aliment. 2017, 46, 43–50. [Google Scholar]
- Nesse, L.L.; Sekse, C.; Berg, K.; Johannesen, K.C.; Solheim, H.; Vestby, L.K.; Urdahl, A.M. Potentially pathogenic Escherichia coli can form a biofilm under conditions relevant to the food production chain. Appl. Environ. Microbiol. 2014, 80, 2042–2049. [Google Scholar] [CrossRef]
- Upham, J.; Chen, S.; Boutilier, E.; Hodges, L.; Eisebraun, M.; Croxen, M.A.; Fortuna, A.; Mallo, G.V.; Garduño, R.A. Potential Ad Hoc Markers of Persistence and Virulence in Canadian Listeria monocytogenes Food and Clinical Isolates. J. Food Prot. 2019, 82, 1909–1921. [Google Scholar] [CrossRef]
- Park, M.; Kim, J.; Horn, L.; Haan, J.; Strickland, A.; Lappi, V.; Boxrud, D.; Hedberg, C.; Ryu, S.; Jeon, B. Sugar Modification of Wall Teichoic Acids Determines Serotype-Dependent Strong Biofilm Production in Listeria monocytogenes. Microbiol. Spectr. 2022, 10, e0276922. [Google Scholar] [CrossRef]
- Keeney, K.; Trmcic, A.; Zhu, Z.; Delaquis, P.; Wang, S. Stress survival islet 1 contributes to serotype-specific differences in biofilm formation in Listeria monocytogenes. Lett. Appl. Microbiol. 2018, 67, 530–536. [Google Scholar] [CrossRef]
- Takahashi, H.; Miya, S.; Igarashi, K.; Suda, T.; Kuramoto, S.; Kimura, B. Biofilm Formation Ability of Listeria monocytogenes Isolates from Raw Ready-to-Eat Seafood. J. Food Prot. 2009, 72, 1476–1480. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. The differential adherence capabilities of two Listeria monocytogenes strains in monoculture and multispecies biofilms as a function of temperature. Lett. Appl. Microbiol. 2001, 33, 320–324. [Google Scholar] [CrossRef]
- Nakamura, H.; Takakura, K.-I.; Sone, Y.; Itano, Y.; Nishikawa, Y. Biofilm formation and resistance to benzalkonium chloride in Listeria monocytogenes isolated from a fish processing plant. J. Food Prot. 2013, 76, 1179–1186. [Google Scholar] [CrossRef]
- Maury, M.M.; Bracq-Dieye, H.; Huang, L.; Vales, G.; Lavina, M.; Thouvenot, P.; Disson, O.; Leclercq, A.; Brisse, S.; Lecuit, M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat. Commun. 2019, 10, 2488. [Google Scholar] [CrossRef] [PubMed]
- Renier, S.; Hébraud, M.; Desvaux, M. Molecular biology of surface colonization by Listeria monocytogenes: An additional facet of an opportunistic Gram-positive foodborne pathogen. Environ. Microbiol. 2011, 13, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Vatanyoopaisarn, S.; Aisha, N.; Christine, E.R.D.; Catherine, E.D.R.; Will, M.W. Effect of Flagella on Initial Attachment of Listeria monocytogenes to Stainless Steel. Appl. Environ. Microbiol. 2000, 66, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar Motility Is Critical for Listeria monocytogenes Biofilm Formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef]
- Kumar, S.; Parvathi, A.; George, J.; Krohne, G.; Karunasagar, I.; Karunasagar, I. A study on the effects of some laboratory-derived genetic mutations on biofilm formation by Listeria monocytogenes. World J. Microbiol. Biotechnol. 2008, 25, 527–531. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Young, G.M. Loss of Flagellum-Based Motility by Listeria monocytogenes Results in Formation of Hyperbiofilms. J. Bacteriol. 2008, 190, 6030–6034. [Google Scholar] [CrossRef]
- Lemon, K.P.; Freitag, N.E.; Kolter, R. The Virulence Regulator PrfA Promotes Biofilm Formation by Listeria monocytogenes. J. Bacteriol. 2010, 192, 3969–3976. [Google Scholar] [CrossRef]
- Zhou, Q.; Feng, F.; Wang, L.; Feng, X.; Yin, X.; Luo, Q. Virulence Regulator PrfA is Essential for Biofilm Formation in Listeria monocytogenes but not in Listeria innocua. Curr. Microbiol. 2011, 63, 186–192. [Google Scholar] [CrossRef]
- Knudsen, G.M.; Olsen, J.E.; Dons, L. Characterization of DegU, a response regulator in Listeria monocytogenes, involved in regulation of motility and contributes to virulence. FEMS Microbiol. Lett. 2004, 240, 171–179. [Google Scholar] [CrossRef]
- Gueriri, I.; Cyncynatus, C.; Dubrac, S.; Arana, A.T.; Dussurget, O.; Msadek, T. The DegU orphan response regulator of Listeria monocytogenes autorepresses its own synthesis and is required for bacterial motility, virulence and biofilm formation. Microbiology 2008, 154, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Travier, L.; Guadagnini, S.; Gouin, E.; Dufour, A.; Chenal-Francisque, V.; Cossart, P.; Olivo-Marin, J.-C.; Ghigo, J.-M.; Disson, O.; Lecuit, M. ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage. PLoS Pathog. 2013, 9, e1003131. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Hu, Y.; Wiedmann, M.; Boor, K.J. Alternative sigma factor sigma(B) is not essential for Listeria monocytogenes surface attachment. J. Food Prot. 2005, 68, 311–317. [Google Scholar] [CrossRef]
- van der Veen, S.; Abee, T. Importance of SigB for Listeria monocytogenes Static and Continuous-Flow Biofilm Formation and Disinfectant Resistance. Appl. Environ. Microbiol. 2010, 76, 7854–7860. [Google Scholar] [CrossRef] [PubMed]
- Janež, N.; Škrlj, B.; Sterniša, M.; Klančnik, A.; Sabotič, J. The role of the Listeria monocytogenes surfactome in biofilm formation. Microb. Biotechnol. 2021, 14, 1269–1281. [Google Scholar] [CrossRef]
- Jordan, S.J.; Perni, S.; Glenn, S.; Fernandes, I.; Barbosa, M.; Sol, M.; Tenreiro, R.P.; Chambel, L.; Barata, B.; Zilhao, I.; et al. Listeria monocytogenes Biofilm-Associated Protein (BapL) May Contribute to Surface Attachment of L. monocytogenes but Is Absent from Many Field Isolates. Appl. Environ. Microbiol. 2008, 74, 5451–5456. [Google Scholar] [CrossRef]
- Chen, B.Y.; Kim, T.J.; Jung, Y.S.; Silva, J.L. Attachment Strength of Listeria monocytogenes and its Internalin-Negative Mutants. Food Biophys. 2008, 3, 329–332. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Kim, T.-J.; Silva, J.L.; Jung, Y.-S. Positive Correlation Between the Expression of inlA and inlB Genes of Listeria monocytogenes and Its Attachment Strength on Glass Surface. Food Biophys. 2009, 4, 304–311. [Google Scholar] [CrossRef]
- Franciosa, G.; Maugliani, A.; Scalfaro, C.; Floridi, F.; Aureli, P. Expression of Internalin A and Biofilm Formation among Listeria monocytogenes Clinical Isolates. Int. J. Immunopathol. Pharmacol. 2009, 22, 183–193. [Google Scholar] [CrossRef]
- Popowska, M.; Krawczyk-Balska, A.; Ostrowski, R.; Desvaux, M. InlL from Listeria monocytogenes is involved in biofilm formation and adhesion to mucin. Front Microbiol. 2017, 8, 660. [Google Scholar] [CrossRef]
- Rieu, A.; Weidmann, S.; Garmyn, D.; Piveteau, P.; Guzzo, J. agr System of Listeria monocytogenes EGD-e: Role in Adherence and Differential Expression Pattern. Appl. Environ. Microbiol. 2007, 73, 6125–6133. [Google Scholar] [CrossRef]
- Riedel, C.U.; Monk, I.R.; Casey, P.G.; Waidmann, M.S.; Gahan, C.G.M.; Hill, C. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol. Microbiol. 2009, 71, 1177–1189. [Google Scholar] [CrossRef]
- Zetzmann, M.; Bucur, F.I.; Crauwels, P.; Borda, D.; Nicolau, A.I.; Grigore-Gurgu, L.; Seibold, G.M.; Riedel, C.U. Characterization of the biofilm phenotype of a Listeria monocytogenes mutant deficient in agr peptide sensing. Microbiologyopen 2019, 8, e00826-n/a. [Google Scholar] [CrossRef]
- Garmyn, D.; Gal, L.; Lemaître, J.-P.; Hartmann, A.; Piveteau, P. Communication and Autoinduction in the species Listeria monocytogenes: A central role for the agr system. Commun. Integr. Biol. 2009, 2, 371–374. [Google Scholar] [CrossRef]
- Belval, S.C.; Gal, L.; Margiewes, S.; Garmyn, D.; Piveteau, P.; Guzzo, J. Assessment of the Roles of LuxS, S-Ribosyl Homocysteine, and Autoinducer 2 in Cell Attachment during Biofilm Formation by Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 2006, 72, 2644–2650. [Google Scholar] [CrossRef]
- Sela, S.; Frank, S.; Belausov, E.; Pinto, R. A Mutation in the luxS Gene Influences Listeria monocytogenes Biofilm Formation. Appl. Environ. Microbiol. 2006, 72, 5653–5658. [Google Scholar] [CrossRef]
- Trémoulet, F.; Duché, O.; Namane, A.; Martinie, B.; The European Listeria Genome, C.; Labadie, J.C. Comparison of protein patterns of Listeria monocytogenes grown in biofilm or in planktonic mode by proteomic analysis. FEMS Microbiol. Lett. 2002, 210, 25–31. [Google Scholar] [CrossRef]
- Lourenço, A.; de Las Heras, A.; Scortti, M.; Vazquez-Boland, J.; Frank, J.F.; Brito, L. Comparison of Listeria monocytogenes Exoproteomes from Biofilm and Planktonic State: Lmo2504, a Protein Associated with Biofilms. Appl. Environ. Microbiol. 2013, 79, 6075–6082. [Google Scholar] [CrossRef]
- Verghese, B.; Lok, M.; Wen, J.; Alessandria, V.; Chen, Y.; Kathariou, S.; Knabel, S. comK Prophage Junction Fragments as Markers for Listeria monocytogenes Genotypes Unique to Individual Meat and Poultry Processing Plants and a Model for Rapid Niche-Specific Adaptation, Biofilm Formation, and Persistence (vol 77, pg 3279, 2011). Appl. Environ. Microbiol. 2011, 77, 5064. [Google Scholar] [CrossRef]
- Nielsen, E.M.; Björkman, J.T.; Kiil, K.; Grant, K.; Dallman, T.; Painset, A.; Amar, C.; Roussel, S.; Guillier, L.; Félix, B.; et al. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready-to-eat (RTE) foods: Activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis. EFSA Support. Publ. 2017, 14, 1151E. [Google Scholar] [CrossRef]
- Muhterem-Uyar, M.; Ciolacu, L.; Wagner, K.-H.; Wagner, M.; Schmitz-Esser, S.; Stessl, B. New Aspects on Listeria monocytogenes ST5-ECVI Predominance in a Heavily Contaminated Cheese Processing Environment. Front. Microbiol. 2018, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimou, L.; Fanning, S.; Fox, E.M. Genomic insights into persistence of Listeria species in the food processing environment. J. Appl. Microbiol. 2021, 131, 2082–2094. [Google Scholar] [CrossRef]
- Fagerlund, A.; Wagner, E.; Møretrø, T.; Heir, E.; Moen, B.; Rychli, K.; Langsrud, S. Pervasive Listeria monocytogenes Is Common in the Norwegian Food System and Is Associated with Increased Prevalence of Stress Survival and Resistance Determinants. Appl. Environ. Microbiol. 2022, 88, e0086122. [Google Scholar] [CrossRef]
- Yi, C.; Tobin, S.; Kari, P.; Qing, J.; Eric, B.; Luke, F.L.; Dumitru, M. Genetic Diversity of Listeria monocytogenes Isolated From Three Commercial Tree Fruit Packinghouses and Evidence of Persistent and Transient Contamination. Front. Microbiol. 2022, 12, 756688. [Google Scholar] [CrossRef]
- Stasiewicz, M.J.; Oliver, H.F.; Wiedmann, M.; den Bakker, H.C. Whole-genome sequencing allows for improved identification of persistent Listeria monocytogenes in food-associated environments. Appl. Environ. Microbiol. 2015, 81, 6024–6037. [Google Scholar] [CrossRef] [PubMed]
- Louha, S.; Meinersmann, R.J.; Abdo, Z.; Berrang, M.E.; Glenn, T.C. An Open-Source Program (Haplo-ST) for Whole-Genome Sequence Typing Shows Extensive Diversity among Listeria monocytogenes Isolates in Outdoor Environments and Poultry Processing Plants. Appl. Environ. Microbiol. 2020, 87, e02248-20. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Fang, Z.; Yu, Y.; Bi, J.; Wang, J.; Dong, Q.; Zhang, H. Persistence of Listeria monocytogenes ST5 in Ready-to-Eat Food Processing Environment. Foods 2022, 11, 2561. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Schirmer, B.C.T.; Møretrø, T.; Heir, E. Genome analysis of Listeria monocytogenes sequence type 8 strains persisting in salmon and poultry processing environments and comparison with related strains. PLoS ONE 2016, 11, e0151117. [Google Scholar] [CrossRef]
- Schmitz-Esser, S.; Müller, A.; Stessl, B.; Wagner, M. Genomes of sequence type 121 Listeria monocytogenes strains harbor highly conserved plasmids and prophages. Front. Microbiol. 2015, 6, 380. [Google Scholar] [CrossRef]
- Holch, A.; Webb, K.; Lukjancenko, O.; Ussery, D.; Rosenthal, B.M.; Gram, L. Genome Sequencing Identifies Two Nearly Unchanged Strains of Persistent Listeria monocytogenes Isolated at Two Different Fish Processing Plants Sampled 6 Years Apart. Appl. Environ. Microbiol. 2013, 79, 2944–2951. [Google Scholar] [CrossRef]
- Patel, J.; Singh, M.; Macarisin, D.; Sharma, M.; Shelton, D. Differences in biofilm formation of produce and poultry Salmonella enterica isolates and their persistence on spinach plants. Food Microbiol. 2013, 36, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ray, A.J.; Hammons, S.R.; Oliver, H.F. Persistent and transient Listeria monocytogenes strains from retail deli environments vary in their ability to adhere and form biofilms and rarely have inlA premature stop codons. Foodborne Pathog. Dis. 2015, 12, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Nyarko, E.B.; Donnelly, C.W. Listeria monocytogenes: Strain Heterogeneity, Methods, and Challenges of Subtyping. J. Food Sci. 2015, 80, M2868–M2878. [Google Scholar] [CrossRef]
- Morganti, M.; Scaltriti, E.; Cozzolino, P.; Bolzoni, L.; Casadei, G.; Pierantoni, M.; Foni, E.; Pongolini, S. Processing-dependent and clonal contamination patterns of Listeria monocytogenes in the cured ham food chain revealed by genetic analysis. Appl. Environ. Microbiol. 2016, 82, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.M.; Nielsen, J.B.; Marvig, R.L.; Ng, Y.; Worning, P.; Westh, H.; Gram, L. Genome-wide-analyses of Listeria monocytogenes from food-processing plants reveal clonal diversity and date the emergence of persisting sequence types. Environ. Microbiol. Rep. 2017, 9, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, B.; Baert, L.; Wiedmann, M.; Orsi, R.H. Comparative Analysis of Tools and Approaches for Source Tracking Listeria monocytogenes in a Food Facility Using Whole-Genome Sequence Data. Front. Microbiol. 2019, 10, 947. [Google Scholar] [CrossRef]
- Ortiz, S.; López, V.; Martínez-Suárez, J.V. Control of Listeria monocytogenes contamination in an Iberian pork processing plant and selection of benzalkonium chloride-resistant strains. Food Microbiol. 2014, 39, 81–88. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Kalmokoff, M.L.; Austin, J.W.; Wan, X.D.; Sanders, G.; Banerjee, S.; Farber, J.M. Adsorption, attachment and biofilm formation among isolates of Listeria monocytogenes using model conditions. J. Appl. Microbiol. 2001, 91, 725–734. [Google Scholar] [CrossRef]
- Manso, B.; Melero, B.; Stessl, B.; Fernández-Natal, I.; Jaime, I.; Hernández, M.; Wagner, M.; Rovira, J.; Rodríguez-Lázaro, D. Characterization of Virulence and Persistence Abilities of Listeria monocytogenes Strains Isolated from Food Processing Premises. J. Food Prot. 2019, 82, 1922–1930. [Google Scholar] [CrossRef]
- Feng, J.; Lamour, G.; Xue, R.; Mirvakliki, M.N.; Hatzikiriakos, S.G.; Xu, J.; Li, H.; Wang, S.; Lu, X. Chemical, physical and morphological properties of bacterial biofilms affect survival of encased Campylobacter jejuni F38011 under aerobic stress. Int. J. Food Microbiol. 2016, 238, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Rahimi, S.; Fazlara, A.; Anvari, S.E. High biofilm-forming Pseudomonas strains isolated from poultry slaughterhouse surfaces: Their importance in the persistence of Salmonella enteritidis in slaughterhouses. Int. J Food. Microbiol. 2023, 390, 110126. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.T.; Nou, X.; Lefcourt, A.M.; Shelton, D.R.; Lo, Y.M. Dual-species biofilm formation by Escherichia coli O157:H7 and environmental bacteria isolated from fresh-cut processing facilities. Int. J. Food Microbiol. 2014, 171, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a microbial hazard in the food industry: A scoping review. J. Appl. Microbiol. 2022, 133, 2210–2234. [Google Scholar] [CrossRef]
- Fox, E.M.; Leonard, N.; Jordan, K. Physiological and Transcriptional Characterization of Persistent and Nonpersistent Listeria monocytogenes Isolates. Appl. Environ. Microbiol. 2011, 77, 6559–6569. [Google Scholar] [CrossRef]
- Rychli, K.; Grunert, T.; Ciolacu, L.; Zaiser, A.; Razzazi-Fazeli, E.; Schmitz-Esser, S.; Ehling-Schulz, M.; Wagner, M. Exoproteome analysis reveals higher abundance of proteins linked to alkaline stress in persistent Listeria monocytogenes strains. Int. J. Food Microbiol. 2016, 218, 17–26. [Google Scholar] [CrossRef]
- Harvey, J.; Gilmour, A. Application of multilocus enzyme electrophoresis and restriction fragment length polymorphism analysis to the typing of Listeria monocytogenes strains isolated from raw milk, nondairy foods, and clinical and veterinary sources. Appl. Environ. Microbiol. 1994, 60, 1547–1553. [Google Scholar] [CrossRef]
- Vogel, B.F.; Jørgensen, L.V.; Ojeniyi, B.; Huss, H.H.; Gram, L. Diversity of Listeria monocytogenes isolates from cold-smoked salmon produced in different smokehouses as assessed by Random Amplified Polymorphic DNA analyses. Int. J. Food Microbiol. 2001, 65, 83–92. [Google Scholar] [CrossRef]
- Simmons, C.; Stasiewicz, M.J.; Wright, E.; Warchocki, S.; Roof, S.; Kause, J.R.; Bauer, N.; Ibrahim, S.; Wiedmann, M.; Oliver, H.F. Listeria monocytogenes and Listeria spp. contamination patterns in retail delicatessen establishments in three U.S. states. J. Food Prot. 2014, 77, 1929–1939. [Google Scholar] [CrossRef]
- Lomonaco, S.; Decastelli, L.; Nucera, D.; Gallina, S.; Manila Bianchi, D.; Civera, T. Listeria monocytogenes in Gorgonzola: Subtypes, diversity and persistence over time. Int. J. Food Microbiol. 2009, 128, 516–520. [Google Scholar] [CrossRef]
- Almeida, G.; Magalhães, R.; Carneiro, L.; Santos, I.; Silva, J.; Ferreira, V.; Hogg, T.; Teixeira, P. Foci of contamination of Listeria monocytogenes in different cheese processing plants. Int. J. Food Microbiol. 2013, 167, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.; Ferreira, V.; Brandão, T.R.S.; Palencia, R.C.; Almeida, G.; Teixeira, P. Persistent and non-persistent strains of Listeria monocytogenes: A focus on growth kinetics under different temperature, salt, and pH conditions and their sensitivity to sanitizers. Food Microbiol. 2016, 57, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.; Moura, A.; Avillan, J.; Herman, N.; McFarland, A.P.; Sreevatsan, S.; Call, D.R.; Woodward, J.J.; Lecuit, M.; Nero, L.A. Whole-genome sequencing reveals Listeria monocytogenes diversity and allows identification of long-term persistent strains in Brazil. Environ. Microbiol. 2019, 21, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
| Gene(s) | Product | Role in Biofilm Formation | Reference |
|---|---|---|---|
| actA | Actin-assembly-inducing protein precursor (ActA) | Promotes bacterial aggregation. Required to reach wild-type biofilm levels on glass under static and continuous-flow conditions. | [122] |
| agrBDCA | Peptide-based sensing system | Involved in early adherence to glass and polystyrene. May act via quorum sensing. | [131,132] |
| bapL | BapL, a putative cell wall-anchored protein | Involved in adherence to polystyrene and stainless steel (although potentially only in specific strains and serotypes). Mechanism is unclear. | [113,125,126] |
| comK prophage | N/a | comK prophage associated with enhanced biofilm formation on abiotic surfaces. | [139] |
| degU | DegU, a putative response regulator | Involved in adherence to polystyrene. | [120,121] |
| flaA | Flagellin A, the main structural component of bacterial flagella | Involved in initial adherence under static and dynamic conditions but may inhibit biofilm formation under dynamic conditions. | [114,115,116,117] |
| inlA | InlA, a cell surface protein important for virulence | Involved in adherence to glass. Truncation is associated with increased biofilm formation on polystyrene. | [127,128,129] |
| inlB | InlB, a cell surface protein important for virulence | Involved in adherence to glass. | [127,128] |
| inlL | InlL, a cell surface protein | Involved in initial adherence to polystyrene. | [130] |
| lmo2504 | Lmo2504, a putative cell wall-binding protein | Unclear. Overexpressed in biofilm-associated, compared with planktonic cells. | [138] |
| luxS | LuxS, an AI-2 biosynthesis protein | Intact gene associated with repression of biofilm development on glass. | [135,136] |
| prfA | PrfA, a transcriptional regulator | Involved in the late stages of biofilm development. | [118,119] |
| recO | RecO, a DNA repair and protection protein | Overexpressed in biofilm-associated compared with planktonic cells. | [137] |
| sigB | SigB, a major transcriptional regulator of stress response genes | Required to obtain wild-type biofilm levels under static and continuous-flow conditions on stainless steel and polystyrene. | [123,124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finn, L.; Onyeaka, H.; O’Neill, S. Listeria monocytogenes Biofilms in Food-Associated Environments: A Persistent Enigma. Foods 2023, 12, 3339. https://doi.org/10.3390/foods12183339
Finn L, Onyeaka H, O’Neill S. Listeria monocytogenes Biofilms in Food-Associated Environments: A Persistent Enigma. Foods. 2023; 12(18):3339. https://doi.org/10.3390/foods12183339
Chicago/Turabian StyleFinn, Lawrence, Helen Onyeaka, and Sally O’Neill. 2023. "Listeria monocytogenes Biofilms in Food-Associated Environments: A Persistent Enigma" Foods 12, no. 18: 3339. https://doi.org/10.3390/foods12183339
APA StyleFinn, L., Onyeaka, H., & O’Neill, S. (2023). Listeria monocytogenes Biofilms in Food-Associated Environments: A Persistent Enigma. Foods, 12(18), 3339. https://doi.org/10.3390/foods12183339