Heme Oxygenase 1-Mediated Anti-Inflammatory Effect of Extract from the Aerial Part of Heracleum moellendorffii Hance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of H. moellendorffii Hance Extracts
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Quantification of NO
2.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR) and Western Blot Analyses
2.6. Subcellular Localization Assay
2.7. High Performance Liquid Chromatography (HPLC) Analysis
2.8. Statistical Analysis
3. Results
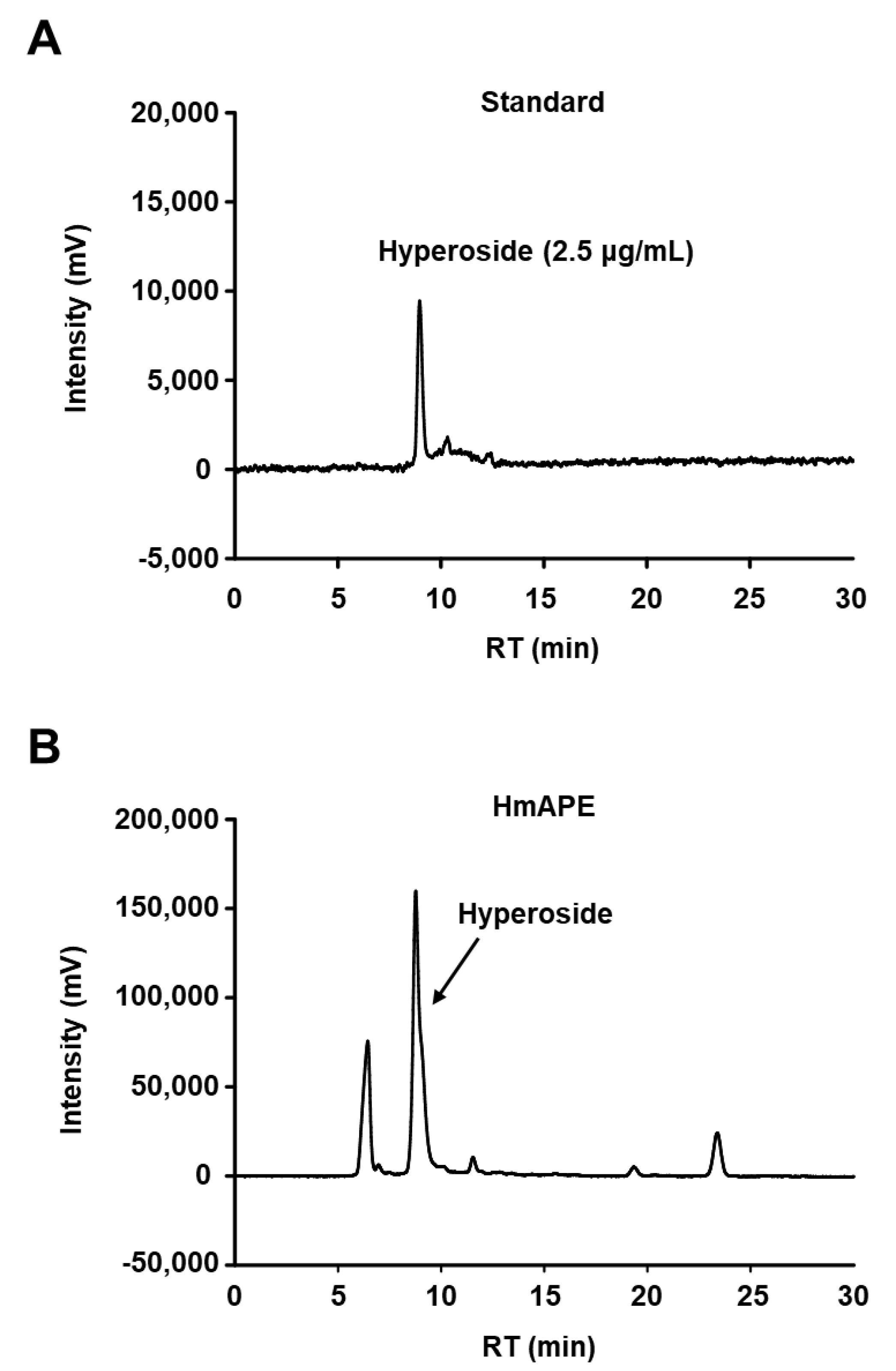
3.1. The HPLC Analysis of HmAPE
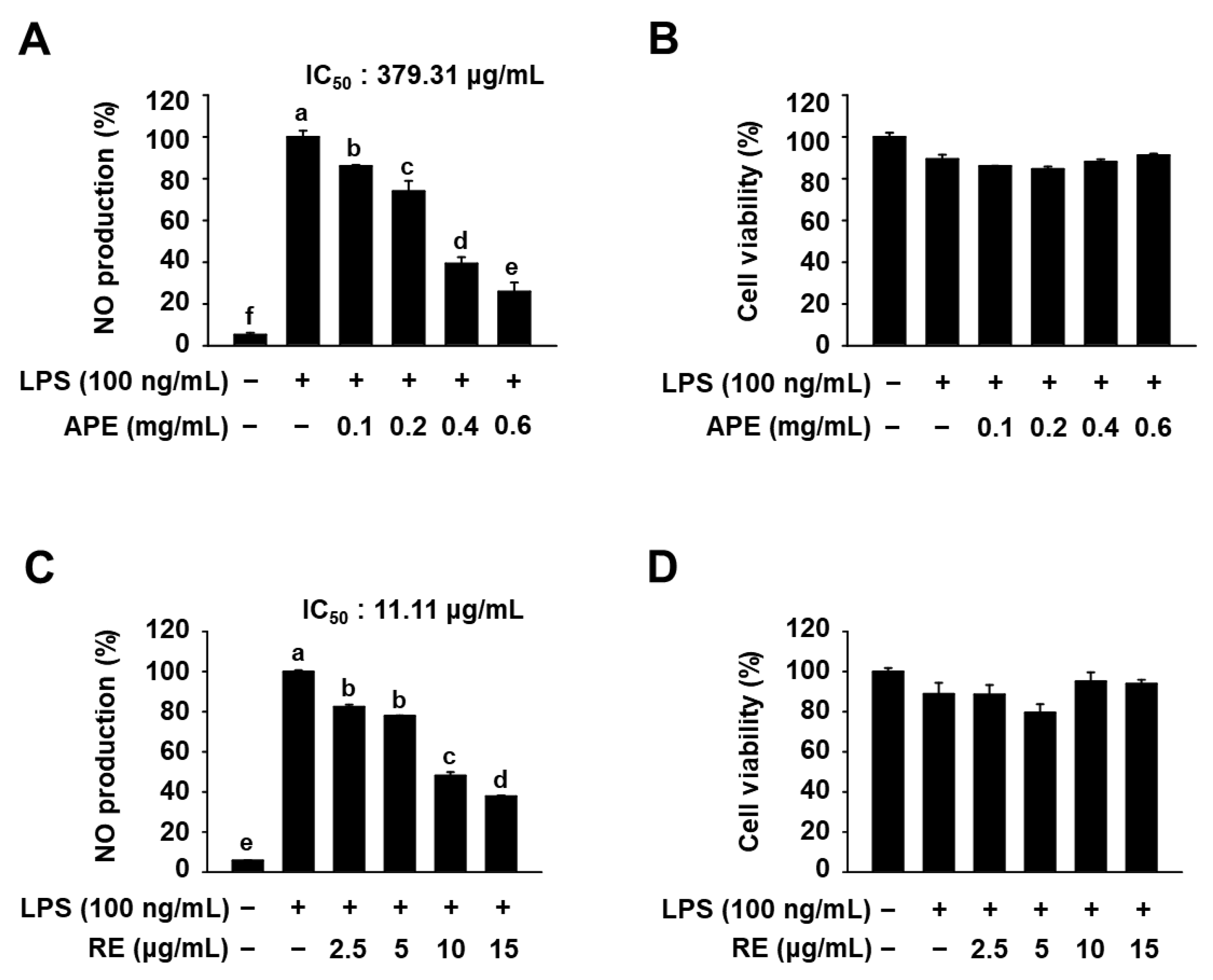
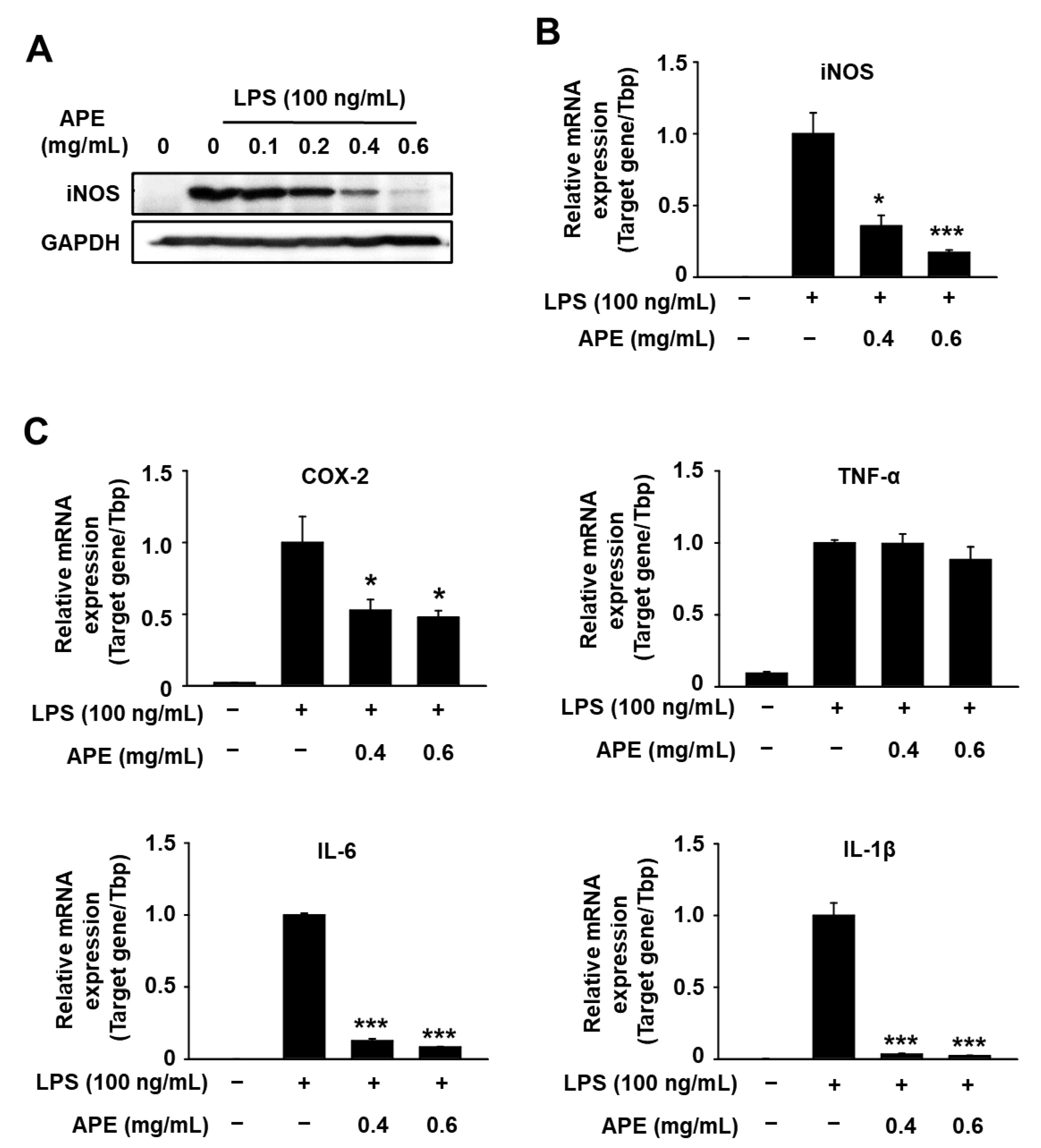
3.2. Anti-Inflammatory Effects of HmAPE in LPS-Treated RAW264.7 Cells
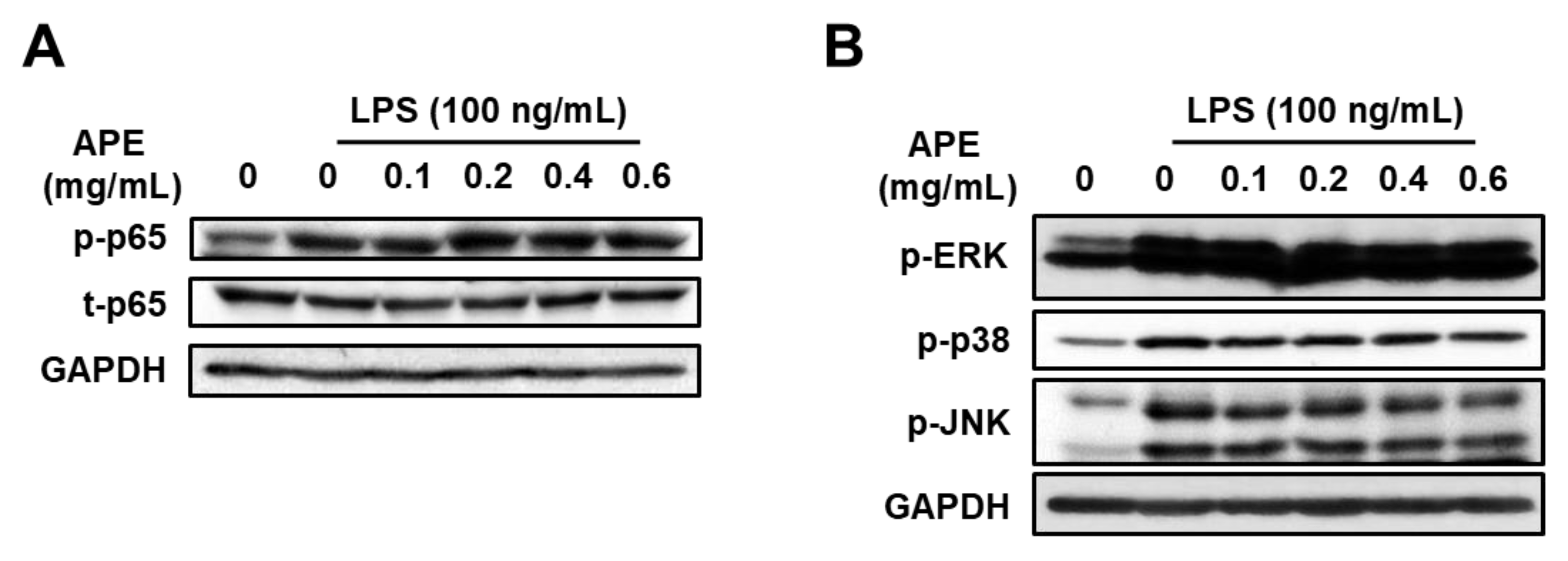
3.3. Anti-Inflammatory Mechanisms of HmAPE: Effect on the MAPKs/NF-κB Signaling Pathways
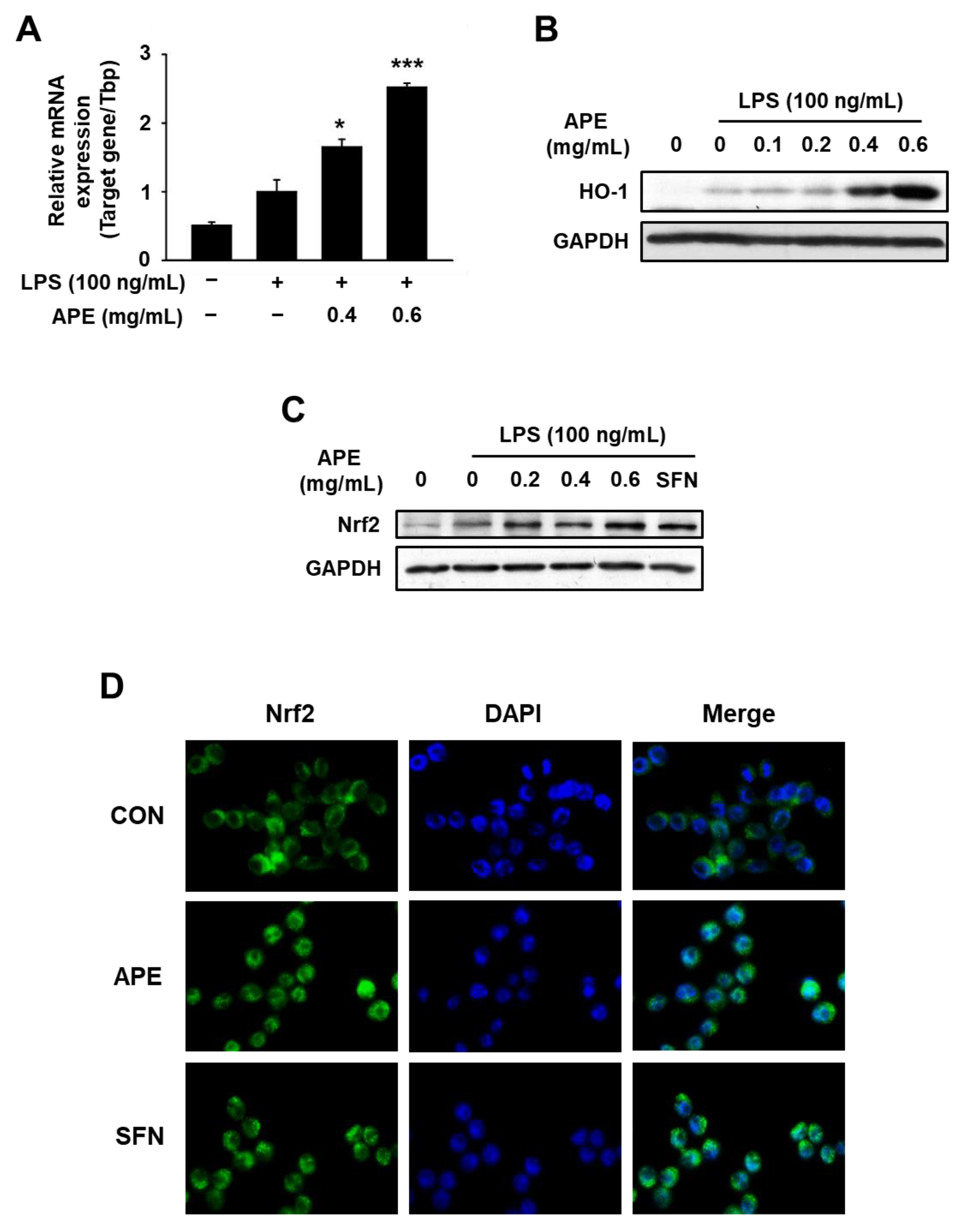
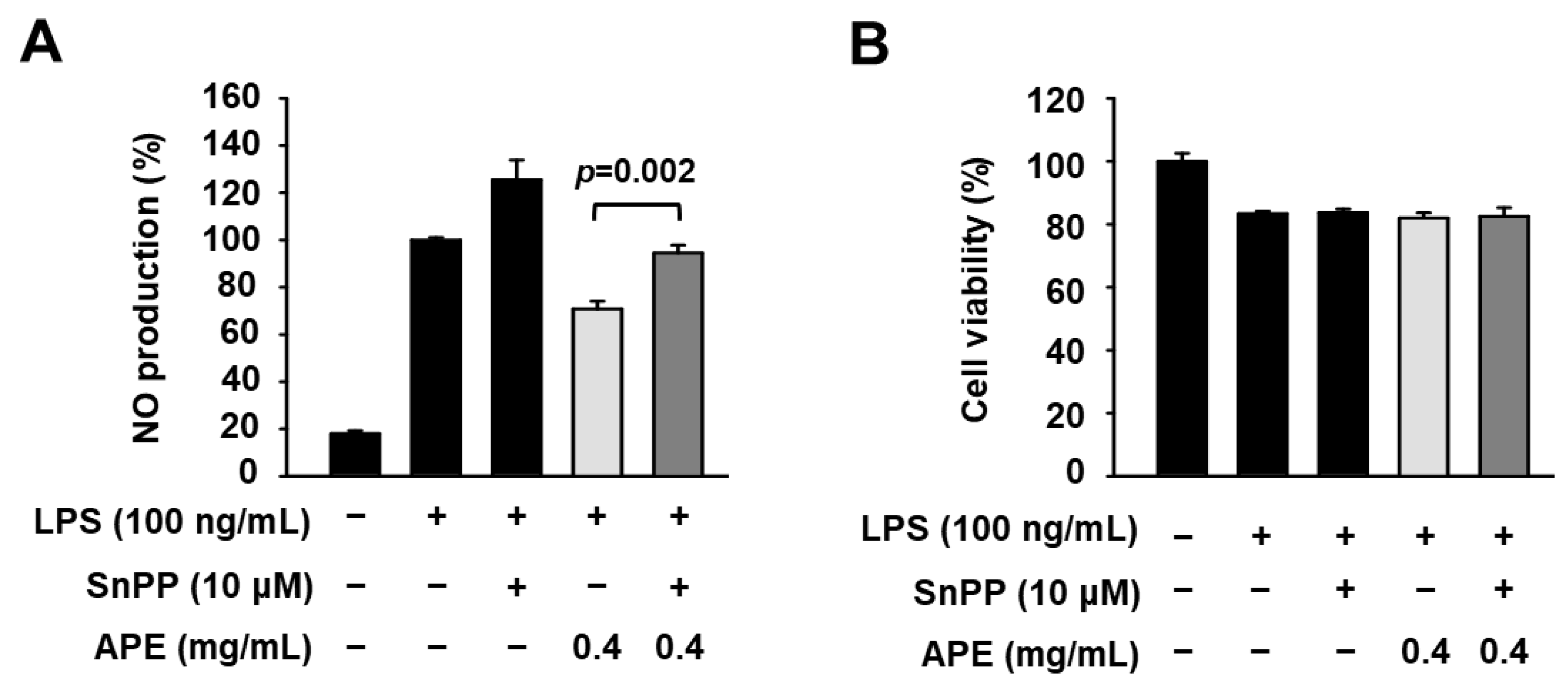
3.4. Anti-Inflammatory Mechanisms of HmAPE: Effect on the Nrf2/HO-1 Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, C.; Yang, L.; Luo, J.; Zhang, C.; Xia, Y.; Ma, T.; Kong, L. Sophoraflavanone G from Sophora alopecuroides inhibits lipopolysaccharide-induced inflammation in RAW264.7 cells by targeting PI3K/Akt, JAK/STAT and Nrf2/HO-1 pathways. Int. Immunopharmacol. 2016, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, S.; Xuan, Z.; Ge, D.; Chen, X.; Zhang, J.; Wang, Q.; Wu, Y.; Liu, B. The phenolic fraction of Mentha haplocalyx and its constituent linarin ameliorate inflammatory response through inactivation of NF-κB and MAPKs in lipopolysaccharide-induced RAW264.7 cells. Molecules 2017, 22, 811–828. [Google Scholar] [CrossRef]
- Cao, Y.; Li, F.; Luo, Y.; Zhang, L.; Lu, S.; Xing, R.; Yan, B.; Zhang, H.; Hu, W. 20-Hydroxy-3-oxolupan-28-oic acid attenuates inflammatory responses by regulating PI3K–Akt and MAPKs signaling pathways in LPS-stimulated RAW264.7 macrophages. Molecules 2019, 24, 386–399. [Google Scholar] [CrossRef]
- Chang, Y.; Li, P.; Chen, B.; Chang, M.; Wang, J.; Chiu, W.; Lin, C. Lipoteichoic acid-induced nitric oxide synthase expression in RAW264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-κB pathways. Cell. Signal. 2006, 18, 1235–1243. [Google Scholar] [CrossRef]
- Hyung, J.H.; Ahn, C.B.; Kim, B.I.; Kim, K.H.; Je, J.Y. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. Eur. J. Pharmacol. 2016, 793, 43–48. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef]
- Karan, T.; Genc, N. Antioxidant activities of Heracleum platytaenium extracts and essential oil. TURJAF 2018, 6, 1274–1278. [Google Scholar] [CrossRef]
- Park, H.J.; Nugroho, A.; Jung, B.R.; Won, Y.H.; Jung, Y.J.; Kim, W.B.; Choi, J.S. Isolation and quantitative analysis of flavonoids with peroxynitrite scavenging effect from the young leaves of Heracleum moellendorffii. Korean J. Plant Res. 2010, 23, 393–398. [Google Scholar]
- Bea, D.S.; Kim, C.Y.; Lee, J.K. Anti-inflammatory effects of dehydrogeijerin in LPS-stimulated murine macrophages. Int. Immunopharmacol. 2012, 14, 734–739. [Google Scholar]
- Nakano, Y.; Matsunaga, H.; Saita, T.; Mori, M.; Katano, M.; Okabe, H. Antiproliferative constituents in Umbelliferae plants II. Screening for polyacetylenes in some Umbelliferae plants, and isolation of panaxynol and falcarindiol from the root of Heracleum moellendorffii. Biol. Pharm. Bull. 1998, 21, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.S.; Cao, J.; Liu, Q.Z.; Du, S.S.; Deng, Z.W.; Liu, Z.L. Chemical composition and insecticidal activity of Heracleum moellendorffii Hance essential oil. Chemija 2012, 23, 108–112. [Google Scholar]
- Alam, M.; Seo, B.J.; Zhao, P.; Lee, S.H. Anti-melanogenic activities of Heracleum moellendorffii via ERK1/2-mediated MITF downregulation. Int. J. Mol. Sci. 2016, 17, 1844–1858. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Duenas, M.; Estrella, I.; Hernandez, T.; Benitez, V.; Esteban, R.M.; Martin-Cabrejas, M.A. Evaluation of phenolic profile and antioxidant properties of Pardina lentil as affected by industrial dehydration. J. Agric. Food Chem. 2010, 58, 10101–10108. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lee, S.H.; Chun, S.Y.; Kin, D.H.; Jang, B.I.; Han, M.H.; Lee, S.O. In Vitro and In Vivo Protective Effects of Lentil (Lens culinaris) Extract against Oxidative Stress-Induced Hepatotoxicity. Molecules 2021, 27, 59. [Google Scholar] [CrossRef]
- Berridge, M.; Tan, A. Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; He, D.; He, Y.; Yang, Y.; Chen, J.; Wang, X. Inhibition of vascular smooth muscle cell proliferation by serum from rats treated orally with Gastrodia and Uncaria decoction, a traditional Chinese formulation. J. Ethnopharm. 2007, 114, 458–462. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, D.H.; Lee, I.S.; Park, J.H.; Martin, G.; Safe, S.; Kim, K.J.; Kim, J.H.; Jang, B.I.; Lee, S.O. Plant alkaloid tetrandrine is a nuclear receptor 4A1 antagonist and inhibits Panc-1 cell growth in vitro and in vivo. Int. J. Mol. Sci. 2022, 23, 5280. [Google Scholar] [CrossRef]
- Cho, H.R.; Lee, S.O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, X.; Piao, M.; Fernando, P.M.D.J.; Kang, K.A.; Ryu, Y.S.; Jun, J.H.; Kim, K.G.; Hyun., J.W. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J. Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, J.D.; Yeo, J.H.; Son, H.J.; Park, S.B.; Park, G.H.; Eo, H.J.; Jeong, J.B. Heracleum moellendorffii roots inhibit the production of pro-inflammatory mediators through the inhibition of NF-κB and MAPK signaling, and activation of ROS/Nrf2/HO-1 signaling in LPS-stimulated RAW264.7 cells. BMC Complement Altern. Med. 2019, 19, 310–320. [Google Scholar] [CrossRef]
- Park, J.Y.; Kwon, Y.W.; Lee, S.C.; Park, S.D.; Lee, J.H. Herbal formula SC-E1 suppresses lipopolysaccharide-stimulated inflammatory responses through activation of Nrf2/HO-1 signaling pathway in RAW 264.7 macrophages. BMC Complement Altern. Med. 2017, 17, 374–385. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa. 2019, 5, e08. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Jung, H.A.; Roy, A.; Abdul, Q.A.; Kim, H.R.; Park, H.J.; Choi, J.S. Leteolin 5-O-glucoside from Korean Milk Thistle, Crisium maackii, exhibits anti-inflammatory activity via activation of the Nrf2/HO-1 pathway. Nat. Prod. Sci. 2017, 23, 183–191. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Wang, Y.; Zhai, J.; Chen, G.; Ju, K. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NF-κB and MAPK activation to reduce inflammation in LPS-activated RAW264.7 cells. Biomed. Pharmacother. 2019, 109, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dai, Q.; Zhang, S.; Liu, Y.; Yu, Q.; Tan, F.; Lu, S.; Wang, Q.; Chen, J.; Huang, H.; et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol. Sin. 2018, 39, 1294–1304. [Google Scholar] [CrossRef]
- Intayoung, P.; Limtrakul, P.; Yodkeeree, S. Antiinflammatory activities of Crebanine by inhibition of NF-κB and AP-1 activation through suppressing MAPKs and Akt signaling in LPS-induced RAW264.7 macrophages. Biol. Pharm. Bull. 2016, 39, 54–61. [Google Scholar] [CrossRef]
- Vareille, M.; Rannou, F.; Thélier, N.; Glasser, A.L.; de Sablet, T.; Martin, C.; Gobert, A.P. Heme oxygenase-1 is a critical regulator of nitric oxide production in enterohemorrhagic Escherichia coli-infected human enterocytes. J. Immunol. 2008, 180, 5720–5726. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, H.; Hou, Z.; Li, D.; He, S.; Wan, C.; Yin, P.; Liu, M.; Liu, F.; Xu, J. Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/Akt pathway and inhibits LPS-induced oxidative stress in RAW264.7 macrophages. Madiators Inflamm. 2015, 2015, 380218. [Google Scholar] [CrossRef]
- Shin, J.E.; Lee, K.M.; Kim, J.H.; Madhi, I.; Kim, Y.H. A formulated Korean Red Ginseng extract inhibited nitric oxide production through Akt- and mitogen activated protein kinase-dependent heme oxygenase-1 upregulation in lipoteichoic acid-stimulated microglial cells. J. Life Sci. 2019, 29, 402–409. [Google Scholar]
- Choi, S.L.; Nguten, V.T.; Tae, N.R.; Lee, S.H.; Ryoo, S.W.; Min, B.S.; Lee, J.H. Anti-inflamamtory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264.7 cells. Toxicol. Appl. Pharmacol. 2014, 280, 434–442. [Google Scholar] [CrossRef]
- Won, A.N.; Kim, S.A.; Ahn, Y.; Han, J.H.; Kim, C.H.; Lee, J.H.; Kim, D.I. HO-1 induction by Selaginella tamariscina extract inhibitss inflammatory response in lipopolysaccharide-stimulated RAW264.7 macrophages. Evid. Based Complement. Alternat. Med. 2018, 2018, 7816923. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Park, C.M. Alleviated oxidative damage by Taraxacum officinale through the induction of Nrf2-MAPK/PI3K mediated HO-1 activation in murine macrophages RAW264.7 cell line. Biomolecules 2019, 9, 288–298. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Fernández, P.; Guillén, M.I. Anti-inflammatory actions of the heme oxygenas-1 pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, J.H.; Shin, J.Y.; Kang, E.S.; Cho, B.O. Anti-inflammatory effects of Peucedanum japonicum Thunberg leaves extract in lipopolysaccharide-stimulated RAW264.7 cells. J. Ethnopharmacol. 2023, 309, 116–362. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, U.; Seo, S.; Wibowo, A.E.; Pongtuluran, O.B.; Lee, K.J.; Han, S.B.; Cho, S. Anti-inflammatory and antioxidant activities of methanol extract of Piper betle Linn. (Piper betle L.) leaves and stems by inhibiting NF-κB/MAPK/Nrf2 signaling pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 2022, 155, 113734. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, J.Y.; Hong, S.H.; Lee, J.Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am. J. Chin. Med. 2011, 39, 171–181. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primer Sequences | |
|---|---|---|
| Tbp | Sense | 5′-GTGAAGGGTACAAGGGGGTG-3′ |
| Antisense | 5′-ACATCTCAGCAACCCACACA-3′ | |
| Nos2 | Sense | 5′-GTCGATGTCATGCAGCTT-3′ |
| Antisense | 5′-GAAGAAAACCCCTTGTGCTG-3′ | |
| Cox-2 | Sense | 5′-GGCGCAGTTTATGTTGTCTG-3′ |
| Antisense | 5′-CAGCACTTCACCCATCAGTT-3′ | |
| Il-1β | Sense | 5′-AGGTCAAAGGTTTGGAAGCA-3′ |
| Antisense | 5′-TGAAGCAGCTATGGCAACTG-3′ | |
| Il-6 | Sense | 5′-TCTGAAGGACTCTGGCTTTG-3′ |
| Antisense | 5′-GATGGATGCTACCAAACTGGA-3′ | |
| Tnf-α | Sense | 5′-GGTCTGGGCCATAGAACTGA-3′ |
| Antisense | 5′-CAGCCTCTTCTCATTCCTGC-3′ | |
| Ho-1 | Sense | 5′-ACAACCAGTGAGTGGAGCCT-3′ |
| Antisense | 5′-TCAAGGCCTCAGACAAATCC-3′ | |
| Instrument | Condition |
|---|---|
| Column | SunFire C18 (250 × 4.6 × 5 μm) |
| Injection volume | 10 μL |
| Detector wavelength | 280 nm |
| Flow rate | 0.8 mL/min |
| Column temperature | 30 °C |
| Mobile phase | MeOH containing 0.1% TFA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.Y.; Lee, S.-O. Heme Oxygenase 1-Mediated Anti-Inflammatory Effect of Extract from the Aerial Part of Heracleum moellendorffii Hance. Foods 2023, 12, 3309. https://doi.org/10.3390/foods12173309
Jang HY, Lee S-O. Heme Oxygenase 1-Mediated Anti-Inflammatory Effect of Extract from the Aerial Part of Heracleum moellendorffii Hance. Foods. 2023; 12(17):3309. https://doi.org/10.3390/foods12173309
Chicago/Turabian StyleJang, Hyun Young, and Syng-Ook Lee. 2023. "Heme Oxygenase 1-Mediated Anti-Inflammatory Effect of Extract from the Aerial Part of Heracleum moellendorffii Hance" Foods 12, no. 17: 3309. https://doi.org/10.3390/foods12173309
APA StyleJang, H. Y., & Lee, S.-O. (2023). Heme Oxygenase 1-Mediated Anti-Inflammatory Effect of Extract from the Aerial Part of Heracleum moellendorffii Hance. Foods, 12(17), 3309. https://doi.org/10.3390/foods12173309






