Toxic Elements in Beans from Zhejiang, Southeast China: Distribution and Probabilistic Health Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals
2.3. Analysis and Quality Control
2.4. Exposure Assessment
2.5. Non-Carcinogenic Risk
2.6. Carcinogenic Risk
2.7. Monte Carlo Simulation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Method Validation for Elemental Analysis
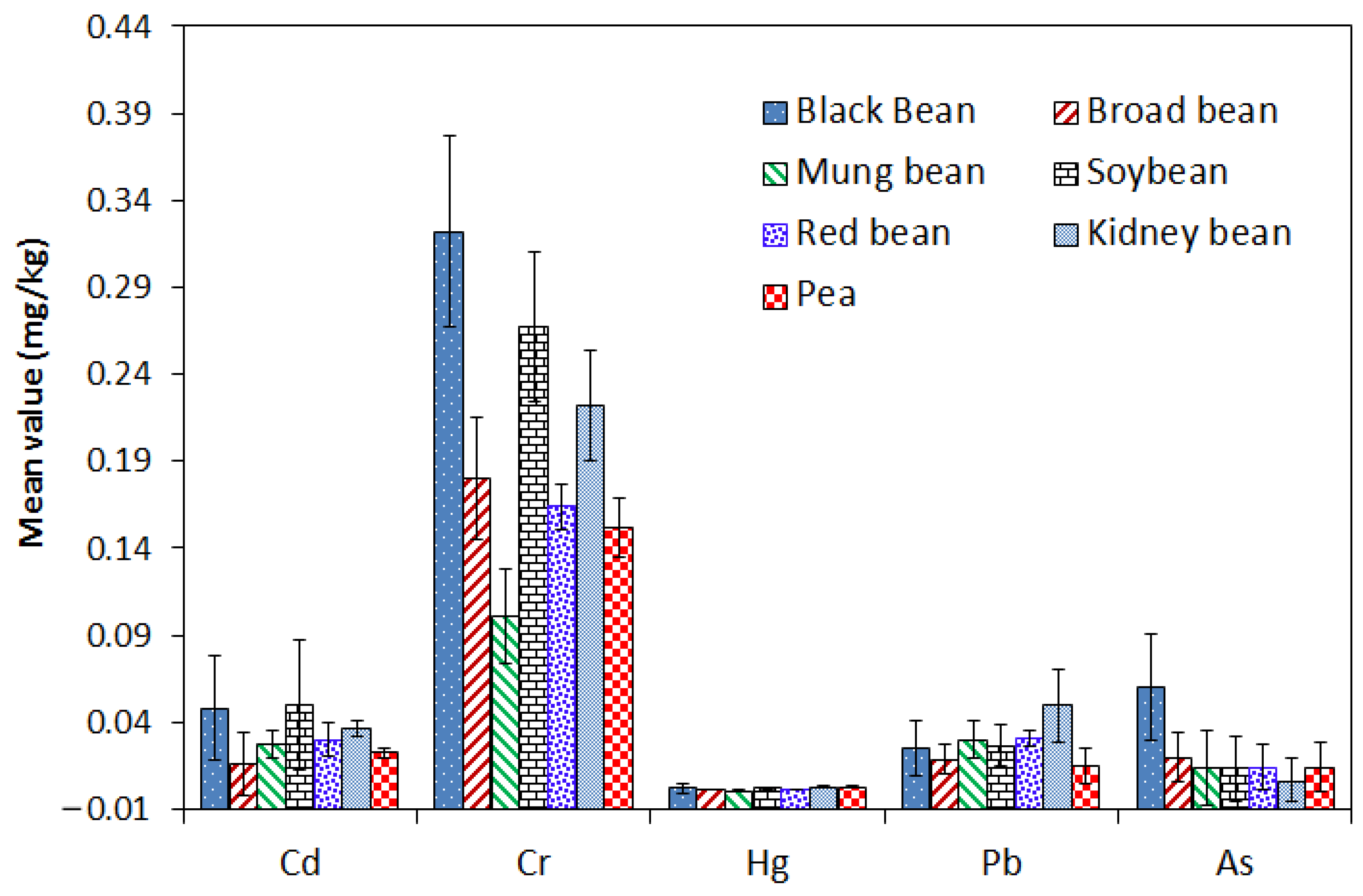
3.2. Levels of Potential Toxic Elements in Bean Samples
3.3. The Correlation Analysis of Different Elements
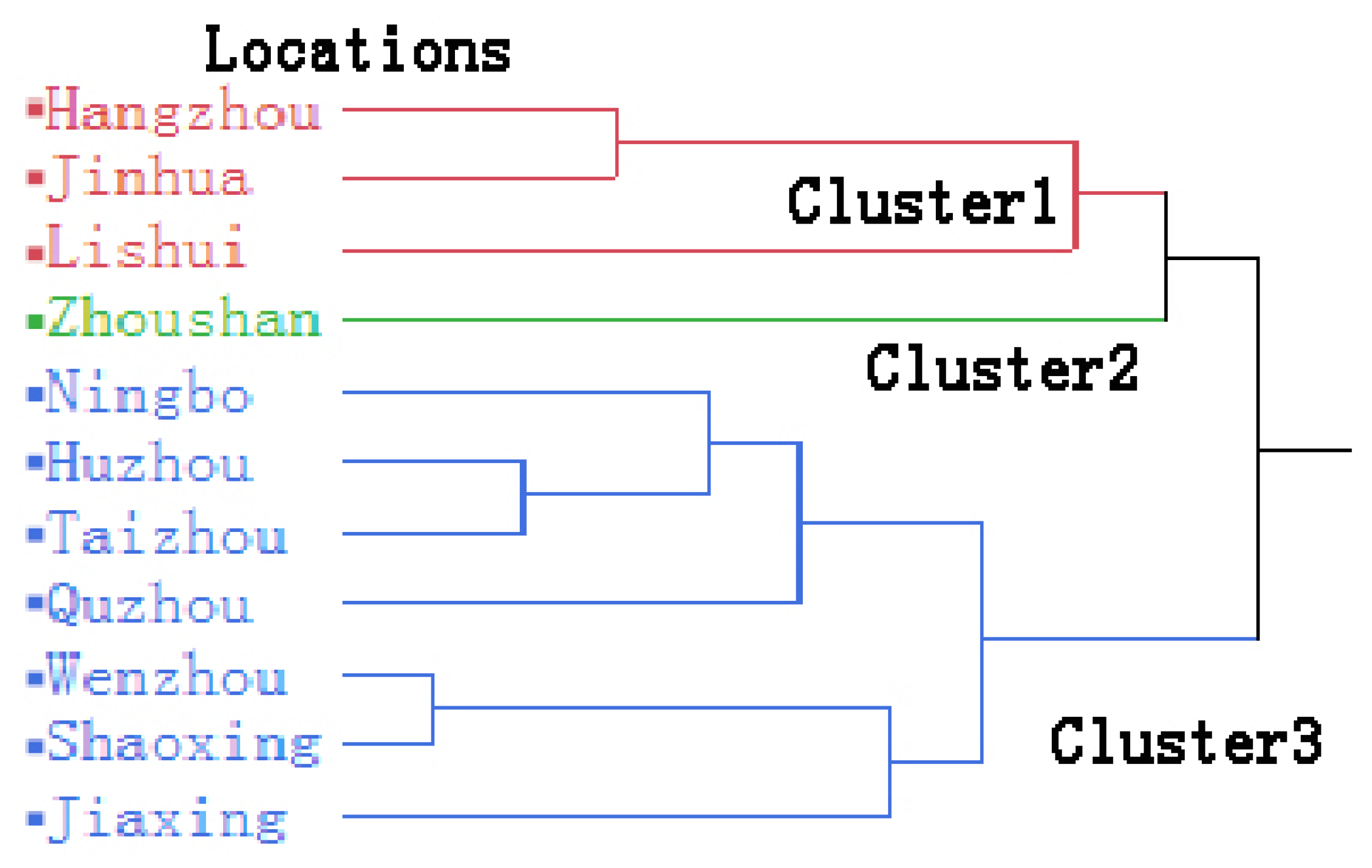
3.4. Spatial Distribution of Sampling Areas
3.5. Health Risk Assessment
3.5.1. Non-Carcinogenic Risks
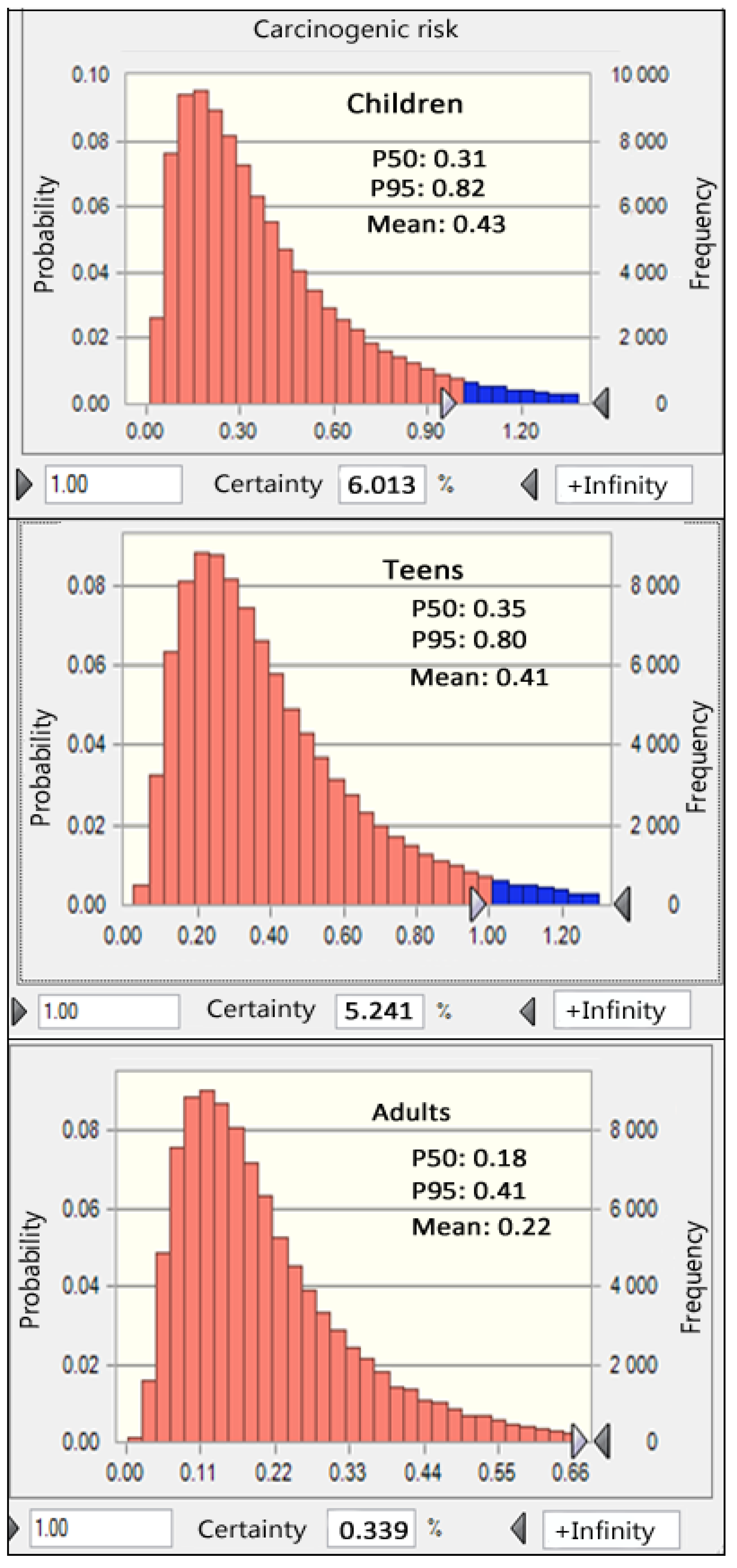
3.5.2. Carcinogenic Risks
3.6. Uncertainty
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, X.-D.; Han, J.-L. Distribution of cadmium in fresh vegetables marketed in southeast China and its dietary exposure assessment. Foods 2023, 12, 1204. [Google Scholar] [CrossRef]
- Ahmed, S.; Fatema Tuj, Z.; Mahdi, M.M.; Nurnabi, M.; Alam, M.Z.; Choudhury, T.R. Health risk assessment for heavy metal accumulation in leafy vegetables grown on tannery effluent contaminated soil. Toxicol. Rep. 2022, 9, 346–355. [Google Scholar]
- Setia, R.; Dhaliwal, S.S.; Singh, R.; Kumar, V.; Taneja, S.; Kukal, S.S.; Pateriya, B. Phytoavailability and human risk assessment of heavy metals in soils and food crops around Sutlej river, India. Chemosphere 2021, 263, 128321. [Google Scholar]
- Antoniadis, V.; Shaheen, S.M.; Boersch, J.; Frohne, T.; Du Laing, G.; Rinklebe, J. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in germany. J. Environ. Manag. 2017, 186, 192–200. [Google Scholar]
- Conversa, G.; Miedico, O.; Chiaravalle, A.E.; Elia, A. Heavy metal contents in green spears of asparagus (Asparagus officinalis L.) grown in Southern Italy: Variability among farms, genotypes and effect of soil mycorrhizal inoculation. Sci. Hortic. 2019, 256, 108559. [Google Scholar]
- Miedico, O.; Iammarino, M.; Paglia, G.; Tarallo, M.; Mangiacotti, M.; Chiaravalle, A.E. Environmental monitoring of the area surrounding oil wells in Val d’Agri (Italy): Element accumulation in bovine and ovine organs. Environ. Monit. Assess. 2016, 188, 338. [Google Scholar]
- Silva-Gigante, M.; Hinojosa-Reyes, L.; Rosas-Castor, J.M.; Quero-Jiménez, P.C.; Pino-Sandoval, D.A.; Guzmán-Mar, J.L. Heavy metals and metalloids accumulation in common beans (Phaseolus vulgaris L.): A review. Chemosphere 2023, 335, 139010. [Google Scholar]
- Qureshi, Y. Impact of heavy metals consumption on human health: A literature review. J. Pharm. Res. Int. 2021, 33, 412–421. [Google Scholar] [CrossRef]
- Han, J.-L.; Pan, X.-D.; Chen, Q.; Huang, B.-F. Health risk assessment of heavy metals in marine fish to the population in Zhejiang, China. Sci. Rep. 2021, 11, 11079. [Google Scholar]
- Han, J.L.; Pan, X.D.; Chen, Q. Distribution and safety assessment of heavy metals in fresh meat from Zhejiang, China. Sci. Rep. 2022, 12, 3241. [Google Scholar]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar]
- Huang, Z.; Pan, X.-D.; Wu, P.-G.; Han, J.-L.; Chen, Q. Heavy metals in vegetables and the health risk to population in Zhejiang, China. Food Control 2014, 36, 248–252. [Google Scholar]
- Pan, X.-D.; Han, J.-L. Heavy metals accumulation in bivalve mollusks collected from coastal areas of southeast China. Mar. Pollut. Bull. 2023, 189, 114808. [Google Scholar]
- Pan, X.-D.; Wu, P.-G.; Jiang, X.-G. Levels and potential health risk of heavy metals in marketed vegetables in Zhejiang, China. Sci. Rep. 2016, 6, 20317. [Google Scholar] [PubMed]
- WHO. Reliable Evaluation of Low-Level Contaminants of Food. Workshop in the Frame of Gems/Food-Euro. Kulmbach, Germany, 1995; Available online: http://www.Who.Int/foodsafety/publications/chem/en/lowlevel_may1995.Pdf (accessed on 10 October 2021).
- ZJFDA. A Report on the Dietary Intake in Zhejiang Province, China. Hangzhou: Zjfda. 2008. Available online: http://www.Zjfda.Gov.Cn/news/detail/13556.Html (accessed on 10 October 2021).
- Wu, B.; Zhang, Y.; Zhang, X.-X.; Cheng, S.-P. Health risk assessment of polycyclic aromatic hydrocarbons in the source water and drinking water of China: Quantitative analysis based on published monitoring data. Sci. Total Environ. 2011, 410, 112–118. [Google Scholar]
- Sanaei, F.; Amin, M.M.; Alavijeh, Z.P.; Esfahani, R.A.; Sadeghi, M.; Bandarrig, N.S.; Fatehizadeh, A.; Taheri, E.; Rezakazemi, M. Health risk assessment of potentially toxic elements intake via food crops consumption: Monte carlo simulation-based probabilistic and heavy metal pollution index. Environ. Sci. Pollut. Res. 2021, 28, 1479–1490. [Google Scholar]
- Liu, L.; Han, J.; Xu, X.; Xu, Z.; Abeysinghe, K.S.; Atapattu, A.J.; De Silva, P.M.C.S.; Lu, Q.; Qiu, G. Dietary exposure assessment of cadmium, arsenic, and lead in market rice from Sri Lanka. Environ. Sci. Pollut. Res. 2020, 27, 42704–42712. [Google Scholar]
- United States Environmental Protection Agency. EPA Region 3 Riskbased Concentration Table. 2014. Available online: https://cfpub.Epa.Gov/ncea/iris/search/index.Cfm (accessed on 24 December 2021).
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants: Forty-First Report of the Joint Fao/Who Expert Committee on Food Additives. 2011. Available online: https://apps.Who.Int/iris/handle/10665/36981 (accessed on 10 October 2021).
- Liang, F.; Li, Y.; Zhang, G.; Tan, M.; Lin, J.; Liu, W.; Li, Y.; Lu, W. Total and speciated arsenic levels in rice from China. Food Addit. Contam. 2010, 27, 810–816. [Google Scholar]
- Ma, L.; Wang, L.; Tang, J.; Yang, Z. Arsenic speciation and heavy metal distribution in polished rice grown in Guangdong province, southern China. Food Chem. 2017, 233, 110–116. [Google Scholar]
- Rajkumar, H.; Naik, P.K.; Singh, G.; Rishi, M. Hydrogeochemical characterization, multi-exposure deterministic and probabilistic health hazard evaluation in groundwater in parts of northern India. Toxin Rev. 2022, 42, 204–227. [Google Scholar]
- Ades, A.; Cliffe, S. Markov chain monte carlo estimation of a multiparameter decision model: Consistency of evidence and the accurate assessment of uncertainty. Med. Decis. Mak. 2002, 22, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Du, Q.; Guan, Q.; Luo, H.; Shan, Y.; Shao, W. A monte carlo simulation-based health risk assessment of heavy metals in soils of an oasis agricultural region in northwest China. Sci. Total Environ. 2023, 857, 159543. [Google Scholar] [PubMed]
- FAO/WHO. Evaluation of certain food additives and contaminants. Geneva. In World Health Organization, Joint Fao/Who Expert Committee on Food Additives; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; Volume 859, pp. e29–e35. [Google Scholar]
- MHPRC (Ministry of Health of the People’s Republic of China). Maximum Levels of Contaminants in Foods (Gb2762–2019); Ministry of Health of the People’s Republic of China: Beijing, China, 2019. (In Chinese)
- Smith, M.; Mohammed, F.K. Heavy metal intake and health risk implications from consumption of dried pulses in trinidad and tobago, W.I. Food. Addit. Contam. B 2020, 13, 207–214. [Google Scholar]
- Hassan, R.O.; Othman, H.O.; Ali, D.S.; Abdullah, F.O.; Darwesh, D.A. Assessment of the health risk posed by toxic metals in commonly consumed legume brands in Erbil, Iraq. J. Food Compos. Anal. 2023, 120, 105282. [Google Scholar]
- Adepoju, F.A.; Adepoju, A.F.; Fadina, O.O. Heavy metal levels in beans (Vigna unguiculata) in selected markets in Ibadan, Nigeria. J. Exp. Agric. Int. 2018, 25, 1–8. [Google Scholar]
- Islam, M.S.; Khanam, M.S.; Sarker, N.I. Health risk assessment of metals transfer from soil to the edible part of some vegetables grown in Patuakhali province of Bangladesh. Arch. Agric. Environ. Sci. 2018, 3, 187–197. [Google Scholar] [CrossRef]
- Karimi, F.; Shariatifar, N.; Rezaei, M.; Alikord, M.; Arabameri, M. Quantitative measurement of toxic metals and assessment of health risk in agricultural products food from Markazi province of Iran. Int. J. Food Contam. 2021, 8, 2. [Google Scholar]
- Zhu, Y.; Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with cd alone and with multiple metals (cd, pb, and zn). J. Agric. Food. Chem. 2007, 55, 1045–1052. [Google Scholar]
- Yaashikaa, P.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar]
- Wang, L.; Ma, L.; Yang, Z. Spatial variation and risk assessment of heavy metals in paddy rice from Hunan province, southern China. Int. J. Environ. Sci. Technol. 2018, 15, 1561–1572. [Google Scholar]
- Yañez, L.M.; Alfaro, J.A.; Avila Carreras, N.M.E.; Bovi Mitre, G. Arsenic accumulation in lettuce (Lactuca sativa L.) and broad bean (Vicia faba L.) crops and its potential risk for human consumption. Heliyon 2019, 5, e01152. [Google Scholar] [CrossRef] [PubMed]

| Elements | Certified Values (mg/kg) | Tested (mg/kg) | LOQ (mg/kg) |
|---|---|---|---|
| As | 0.035 ± 0.012 | 0.032 ± 0.015 | 0.0006 |
| Cd | 0.011 | 0.010 ± 0.005 | 0.0008 |
| Cr | 0.28 ± 0.04 | 0.26 ± 0.03 | 0.0006 |
| Hg | 0.0015 | 0.0012 ± 0.0005 | 0.0001 |
| Pb | 0.07 ± 0.02 | 0.06 ± 0.03 | 0.0008 |
| Variance | Unit | Description | Distribution | Population | ||
|---|---|---|---|---|---|---|
| Children | Teens | Adults | ||||
| Cmetal | mg/kg | Concentration of element | Log normal | analyzed based on different metals | ||
| IR | g/day | Daily rice consumption | Normal | 32.9 | 58.87 | 42.75 |
| EF | day/year | Exposure frequency | Triangular | Min: 180; Mode: 345 Max: 365 | Min: 180; Mode: 345 Max: 365 | Min: 180; Mode: 345 Max: 365 |
| ED | year | Exposure duration | Uniform | 1–7 | 8–25 | 26–70 |
| BW | kg | Body weight | Normal | 16.68 ± 1.48 | 46.25 ± 1.18 | 57.03 ± 1.10 |
| AT | days | Averaging time | Fixed | 2190 | 5475 | 18,250 |
| Rfd | mg/kg/day | Reference dose | Fixed | Varied based on different elements | ||
| Type | Elements | Concentration (mg/kg) | No. of >ML | ||||
|---|---|---|---|---|---|---|---|
| Median | Mean | Maxium | P95 | SD | |||
| Black bean (n = 307) | Cd | 0.0336 | 0.0481 | 0.98 | 0.113 | 0.0481 | 4 |
| Cr | 0.16 | 0.322 | 4.6 | 1.06 | 0.3220 | 11 | |
| Hg | — | 0.0019 | 0.037 | 0.00437 | 0.0019 | — | |
| Pb | 0.0045 | 0.0253 | 0.3 | 0.103 | 0.0253 | 3 | |
| As | 0.00987 | 0.0601 | 5.71 | 0.052 | 0.0601 | — | |
| Broad bean (n = 88) | Cd | 0.005 | 0.0159 | 0.29 | 0.0448 | 0.0159 | 1 |
| Cr | 0.1 | 0.18 | 1.05 | 0.67 | 0.1800 | 2 | |
| Hg | — | 0.00146 | 0.0059 | 0.00156 | 0.0015 | — | |
| Pb | — | 0.0188 | 0.135 | 0.0687 | 0.0188 | 0 | |
| As | 0.003 | 0.0197 | 0.29 | 0.083 | 0.0197 | — | |
| Mung bean (n = 43) | Cd | 0.00409 | 0.0274 | 0.463 | 0.077 | 0.0274 | 2 |
| Cr | 0.0721 | 0.101 | 0.379 | 0.241 | 0.1010 | 0 | |
| Hg | — | 0.000632 | 0.00165 | 0.00085 | 0.0006 | — | |
| Pb | 0.0139 | 0.0302 | 0.353 | 0.0677 | 0.0302 | 1 | |
| As | — | 0.0137 | 0.1 | 0.09 | 0.0137 | — | |
| Soybean (n = 104) | Cd | 0.0226 | 0.0498 | 0.489 | 0.169 | 0.0498 | 4 |
| Cr | 0.186 | 0.267 | 1.51 | 0.72 | 0.2670 | 3 | |
| Hg | — | 0.00213 | 0.0371 | 0.0088 | 0.0021 | — | |
| Pb | — | 0.0267 | 0.196 | 0.138 | 0.0267 | 0 | |
| As | — | 0.0133 | 0.17 | 0.05 | 0.0133 | — | |
| Red bean (n = 51) | Cd | 0.00453 | 0.0301 | 0.648 | 0.177 | 0.0301 | 1 |
| Cr | 0.126 | 0.164 | 0.965 | 0.436 | 0.1640 | 0 | |
| Hg | — | 0.0012 | 0.0143 | 0.00513 | 0.0012 | — | |
| Pb | 0.00861 | 0.0308 | 0.19 | 0.17 | 0.0308 | 0 | |
| As | 0.00613 | 0.0138 | 0.14 | 0.042 | 0.0138 | — | |
| Kidney bean (n = 12) | Cd | 0.0165 | 0.0364 | 0.16 | 0.16 | 0.0364 | 0 |
| Cr | 0.132 | 0.222 | 1.6 | 1.6 | 0.2220 | 1 | |
| Hg | — | 0.00258 | 0.0194 | 0.0194 | 0.0026 | — | |
| Pb | 0.0274 | 0.0498 | 0.183 | 0.183 | 0.0498 | 0 | |
| As | — | 0.0064 | 0.018 | 0.018 | 0.0064 | — | |
| Pea (n = 87) | Cd | 0.019 | 0.0223 | 0.252 | 0.052 | 0.0223 | 1 |
| Cr | 0.0669 | 0.152 | 1.18 | 0.66 | 0.1520 | — | |
| Hg | — | 0.00274 | 0.038 | 0.0079 | 0.0027 | — | |
| Pb | — | 0.0145 | 0.0757 | 0.05 | 0.0145 | 0 | |
| As | — | 0.0139 | 0.12 | 0.066 | 0.0139 | — | |
| Group | Type | As | Cd | Cr | Hg | Pb | Total (Sum) |
|---|---|---|---|---|---|---|---|
| Children | Mean | 0.14 | 0.43 | 0 | 0.02 | 0.01 | 0.59 |
| P50 | 0.10 | 0.30 | 0 | 0.01 | 0.01 | 0.45 | |
| P70 | 0.14 | 0.47 | 0 | 0.02 | 0.01 | 0.66 | |
| P95 | 0.25 | 0.88 | 0 | 0.04 | 0.01 | 1.1 | |
| Teens | Mean | 0.13 | 0.41 | 0.02 | 0.01 | 0.56 | |
| P50 | 0.11 | 0.34 | 0 | 0.01 | 0.01 | 0.5 | |
| P70 | 0.15 | 0.49 | 0 | 0.02 | 0.01 | 0.68 | |
| P95 | 0.25 | 0.82 | 0 | 0.04 | 0.01 | 1.05 | |
| Adults | Mean | 0.07 | 0.22 | 0 | 0.01 | 0 | 0.31 |
| P50 | 0.05 | 0.17 | 0 | 0.01 | 0 | 0.26 | |
| P70 | 0.08 | 0.25 | 0 | 0.01 | 0 | 0.35 | |
| P95 | 0.13 | 0.42 | 0 | 0.02 | 0.01 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Pan, X.-D.; Han, J.-L. Toxic Elements in Beans from Zhejiang, Southeast China: Distribution and Probabilistic Health Risk Assessment. Foods 2023, 12, 3300. https://doi.org/10.3390/foods12173300
Yu S, Pan X-D, Han J-L. Toxic Elements in Beans from Zhejiang, Southeast China: Distribution and Probabilistic Health Risk Assessment. Foods. 2023; 12(17):3300. https://doi.org/10.3390/foods12173300
Chicago/Turabian StyleYu, Sha, Xiao-Dong Pan, and Jian-Long Han. 2023. "Toxic Elements in Beans from Zhejiang, Southeast China: Distribution and Probabilistic Health Risk Assessment" Foods 12, no. 17: 3300. https://doi.org/10.3390/foods12173300
APA StyleYu, S., Pan, X.-D., & Han, J.-L. (2023). Toxic Elements in Beans from Zhejiang, Southeast China: Distribution and Probabilistic Health Risk Assessment. Foods, 12(17), 3300. https://doi.org/10.3390/foods12173300





