Preparation of Green Anti-Staphylococcus aureus Inclusion Complexes Containing Hinoki Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ICs
2.3. Characterization of ICs
2.3.1. Complexation Efficiency
2.3.2. Morphological Observation
2.3.3. GC-MS Analysis
2.3.4. XRD Analysis
2.3.5. FT-IR Analysis
2.3.6. Determination of Water Contact Angle
2.3.7. Anti-S. aureus Analysis
2.3.8. Statistical Analysis
3. Results and Discussion
3.1. Complexation Efficiency Analysis
3.2. SEM Analysis
3.3. GC-MS Analysis
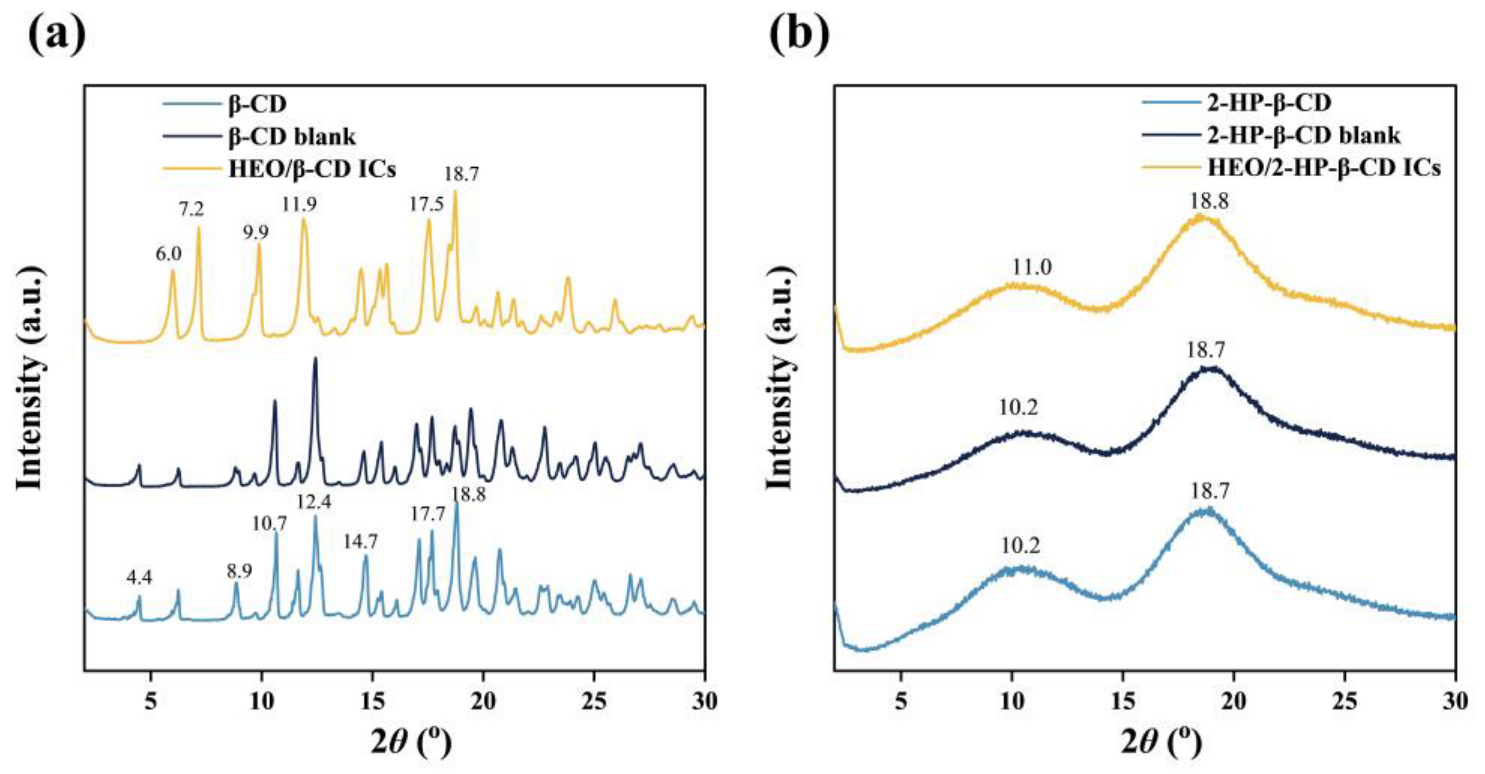
3.4. XRD Analysis
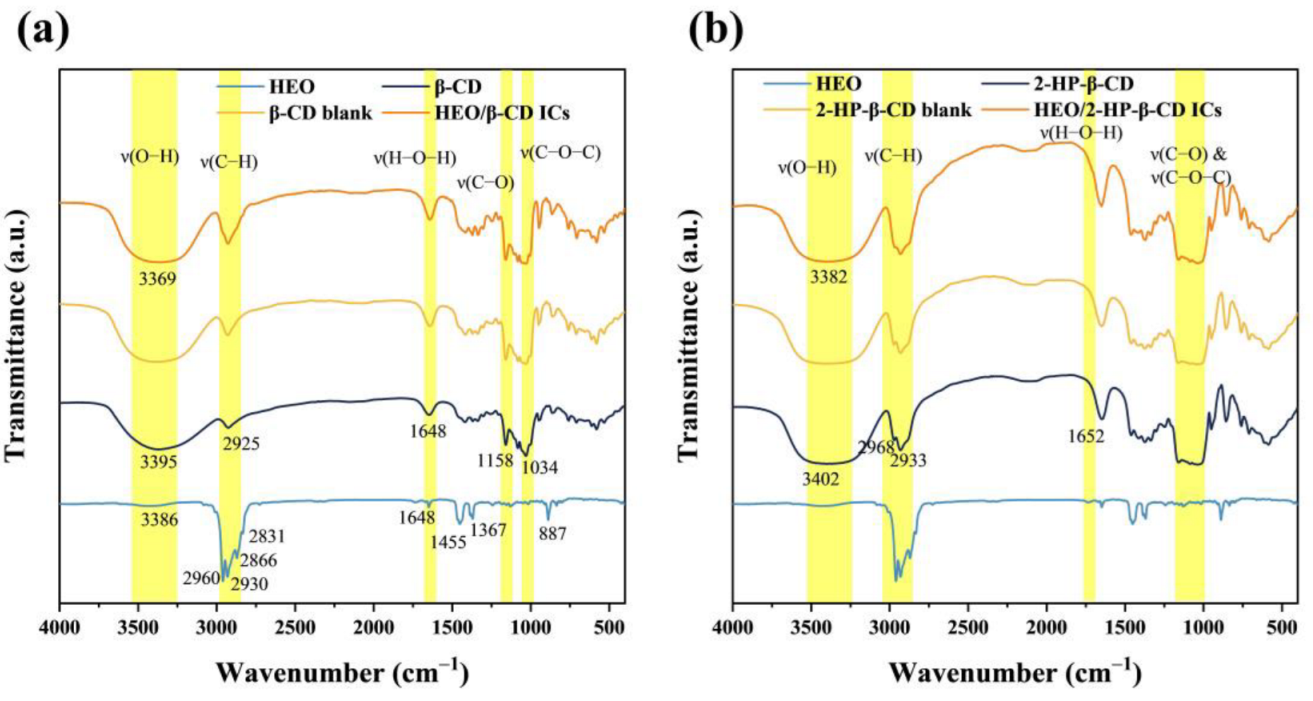
3.5. FT-IR Analysis
3.6. Water Contact Angle Analysis
3.7. Anti-S. aureus Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hennekinne, J.-A.; De Buyser, M.-L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xia, D.; Ma, P.; Gao, X.; Kang, W.; Wei, J. Advances in the detection of virulence genes of Staphylococcus aureus originate from food. Food Sci. Hum. Wellness 2020, 9, 40–44. [Google Scholar] [CrossRef]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc oxide nanoparticles in meat packaging: A systematic review of recent literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. New insight into melanin for food packaging and biotechnology applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 4629–4655. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Rafflisman Zaidi, N.S.; Mah, S.-K. Poly(vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant extract mediated silver nanoparticles and their applications as antimicrobials and in sustainable food packaging: A state-of-the-art review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef]
- Lee, S.-H.; Do, H.-S.; Min, K.-J. Effects of Essential Oil from Hinoki Cypress, Chamaecyparis obtusa, on Physiology and Behavior of Flies. PLoS ONE 2015, 10, e0143450. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Lee, J.; Miyazaki, Y. Comparison of the effects of olfactory stimulation by air-dried and high-temperature-dried wood chips of hinoki cypress (Chamaecyparis obtusa) on prefrontal cortex activity. J. Wood Sci. 2015, 61, 537–540. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of forest-related visual, olfactory, and combined stimuli on humans: An additive combined effect. Urban For. Urban Green. 2019, 44, 126437. [Google Scholar] [CrossRef]
- Raha, S.; Kim, S.M.; Lee, H.J.; Lee, S.J.; Heo, J.D.; Venkatarame Gowda Saralamma, V.; Ha, S.E.; Kim, E.H.; Mun, S.P.; Kim, G.S. Essential oil from Korean Chamaecyparis obtusa leaf ameliorates respiratory activity in Sprague-Dawley rats and exhibits protection from NF-κB-induced inflammation in WI38 fibroblast cells. Int. J. Mol. Med. 2019, 43, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Choi, W.-S.; Kang, H.-Y.; Gwak, K.-S.; Lee, G.-S.; Jeung, E.-B.; Choi, I.-G. Inhibitory effect of the essential oil from Chamaecyparis obtusa on the growth of food-borne pathogens. J. Microbiol. 2010, 48, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, P.; Ye, K.Y.; He, Y.Z.; Chen, S.Q.; Yuan, A.Q.; Chen, F.; Yang, W.L. Preparation, Characterization, In Vitro Release, and Antibacterial Activity of Oregano Essential Oil Chitosan Nanoparticles. Foods 2022, 11, 3756. [Google Scholar] [CrossRef]
- Kong, I.N.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physico-Chemical, Mechanical Properties and Its Application in Food Preservation-A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef]
- Sun, C.; Cao, J.; Wang, Y.; Chen, J.; Huang, L.; Zhang, H.; Wu, J.; Sun, C. Ultrasound-mediated molecular self-assemble of thymol with 2-hydroxypropyl-β-cyclodextrin for fruit preservation. Food Chem. 2021, 363, 130327. [Google Scholar] [CrossRef]
- Liu, C.H.; Tian, Y.C.; Ma, Z.H.; Zhou, L.Y. Pickering Emulsion Stabilized by β-Cyclodextrin and Cinnamaldehyde/β-Cyclodextrin Composite. Foods 2023, 12, 2366. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, M. Characterizing the natural canthaxanthin/2-hydroxypropyl-β-cyclodextrin inclusion complex. Carbohydr. Polym. 2014, 101, 1147–1153. [Google Scholar] [CrossRef]
- Castro, J.C.; Pante, G.C.; de Souza, D.S.; Pires, T.Y.; Miyoshi, J.H.; Garcia, F.P.; Nakamura, C.V.; Mulati, A.C.N.; Mossini, S.A.G.; Machinski Junior, M.; et al. Molecular inclusion of Cymbopogon martinii essential oil with β-cyclodextrin as a strategy to stabilize and increase its bioactivity. Food Hydrocoll. Health 2022, 2, 100066. [Google Scholar] [CrossRef]
- Cetin Babaoglu, H.; Bayrak, A.; Ozdemir, N.; Ozgun, N. Encapsulation of clove essential oil in hydroxypropyl beta-cyclodextrin for characterization, controlled release, and antioxidant activity. J. Food Process. Preserv. 2017, 41, e13202. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Szakal, R.N.; Chirilă, C.A.; Lukinich-Gruia, A.T.; Păunescu, V.; Muntean, C.; Rusu, G.; Bujancă, G.; Hădărugă, D.I. Complexation of Danube common nase (Chondrostoma nasus L.) oil by β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2020, 303, 125419. [Google Scholar] [CrossRef] [PubMed]
- Adjali, A.; Pontillo, A.R.N.; Kavetsou, E.; Katopodi, A.; Tzani, A.; Grigorakis, S.; Loupassaki, S.; Detsi, A. Clove essential oil–hydroxypropyl-β-cyclodextrin inclusion complexes: Preparation, characterization and incorporation in biodegradable chitosan films. Micro 2022, 2, 212–224. [Google Scholar] [CrossRef]
- Cui, H.; Siva, S.; Lin, L. Ultrasound processed cuminaldehyde/2-hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization and antibacterial activity. Ultrason. Sonochem. 2019, 56, 84–93. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.; Cui, H.; Meenatchi, V.; Lin, L. Encapsulation of essential oil components with methyl-β-cyclodextrin using ultrasonication: Solubility, characterization, DPPH and antibacterial assay. Ultrason. Sonochem. 2020, 64, 104997. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Xu, P.; Qi, W.; Kong, Q.; Wang, J.; Liao, T.; Xiong, G. Preparation and characterization of a sustained-release bio-preservative based on β-cyclodextrin encapsulated eugenol. Food Biosci. 2021, 42, 101192. [Google Scholar] [CrossRef]
- Kong, P.; Abe, J.P.; Masuo, S.; Enomae, T. Preparation and characterization of tea tree oil-β-cyclodextrin microcapsules with super-high encapsulation efficiency. J. Bioresour. Bioprod. 2023, 8, 224–234. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Z.; Liu, P. Optimization of preparation process of β-cyclodextrin inclusion compound of clove essential oil and evaluation of heat stability and antioxidant activities in vitro. J. Food Meas. Charact. 2018, 12, 2057–2067. [Google Scholar] [CrossRef]
- Ma, J.; Fan, J.; Xia, Y.; Kou, X.; Ke, Q.; Zhao, Y. Preparation of aromatic β-cyclodextrin nano/microcapsules and corresponding aromatic textiles: A review. Carbohydr. Polym. 2023, 308, 120661. [Google Scholar] [CrossRef]
- Campos, C.A.; Lima, B.S.; Trindade, G.G.G.; Souza, E.P.B.S.S.; Mota, D.S.A.; Heimfarth, L.; Quintans, J.S.S.; Quintans-Júnior, L.J.; Sussuchi, E.M.; Sarmento, V.H.V.; et al. Anti-hyperalgesic and anti-inflammatory effects of citral with β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion complexes in animal models. Life Sci. 2019, 229, 139–148. [Google Scholar] [CrossRef]
- Arora, D.; Saneja, A.; Jaglan, S. Cyclodextrin-based delivery systems for dietary pharmaceuticals. Environ. Chem. Lett. 2019, 17, 1263–1270. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Liu, C.; Wang, D.; Wang, C.-Y. Fabrication of fucoxanthin/2-hydroxypropyl-β-cyclodextrin inclusion complex assisted by ultrasound procedure to enhance aqueous solubility, stability and antitumor effect of fucoxanthin. Ultrason. Sonochem. 2022, 90, 106215. [Google Scholar] [CrossRef]
- Barbieri, N.; Sanchez-Contreras, A.; Canto, A.; Cauich-Rodriguez, J.V.; Vargas-Coronado, R.; Calvo-Irabien, L.M. Effect of cyclodextrins and Mexican oregano (Lippia graveolens Kunth) chemotypes on the microencapsulation of essential oil. Ind. Crops Prod. 2018, 121, 114–123. [Google Scholar] [CrossRef]
- Anaya-Castro, M.A.; Ayala-Zavala, J.F.; Muñoz-Castellanos, L.; Hernández-Ochoa, L.; Peydecastaing, J.; Durrieu, V. β-Cyclodextrin inclusion complexes containing clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) essential oils: Preparation, physicochemical and antimicrobial characterization. Food Packag. Shelf Life 2017, 14, 96–101. [Google Scholar] [CrossRef]
- Pinheiro, J.G.d.O.; Tavares, E.D.A.; Silva, S.S.d.; Félix Silva, J.; Carvalho, Y.M.B.G.d.; Ferreira, M.R.A.; Araújo, A.A.d.S.; Barbosa, E.G.; Fernandes Pedrosa, M.D.F.; Soares, L.A.L.; et al. Inclusion Complexes of Copaiba (Copaifera multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity. Int. J. Mol. Sci. 2017, 18, 2388. [Google Scholar] [CrossRef] [PubMed]
- Michalska, P.; Wojnicz, A.; Ruiz-Nuño, A.; Abril, S.; Buendia, I.; León, R. Inclusion complex of ITH12674 with 2-hydroxypropyl-β-cyclodextrin: Preparation, physical characterization and pharmacological effect. Carbohydr. Polym. 2017, 157, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Huang, J.; Zhang, S.; Lu, Q.; Fang, Z.; Li, C.; Zhang, Q.; Liu, Y.; Chen, H.; Liu, A.; et al. Preparation and characterization of inclusion complex of Myristica fragrans Houtt. (nutmeg) essential oil with 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2023, 423, 136316. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Espinoza, J.; Urzúa, A.; Sanhueza, L.; Walter, M.; Fincheira, P.; Muñoz, P.; Mendoza, L.; Wilkens, M. Essential Oil, Extracts, and Sesquiterpenes Obtained From the Heartwood of Pilgerodendron uviferum Act as Potential Inhibitors of the Staphylococcus aureus NorA Multidrug Efflux Pump. Front. Microbiol. 2019, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, S.; Xu, L.; Fu, W.; Gibbons, S.; Mu, Q. Sesquiterpenoids with Anti-MDR Staphylococcus aureus Activities from Ferula ferulioides. Chem. Biodivers. 2015, 12, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Inoue, Y.; Ikeda, N.; Murata, I.; Kanamoto, I. Improvement of stability due to a cyclamen aldehyde/β-cyclodextrin inclusion complex. J. Mol. Struct. 2020, 1215, 128161. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Uyar, T. Solid Inclusion Complexes of Vanillin with Cyclodextrins: Their Formation, Characterization, and High-Temperature Stability. J. Agric. Food. Chem. 2011, 59, 11772–11778. [Google Scholar] [CrossRef] [PubMed]
- Nuchuchua, O.; Saesoo, S.; Sramala, I.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. Physicochemical investigation and molecular modeling of cyclodextrin complexation mechanism with eugenol. Food Res. Int. 2009, 42, 1178–1185. [Google Scholar] [CrossRef]




| RY (%) | EF (%) | LC (%) | |
|---|---|---|---|
| HEO/β-CD ICs | 92.5 ± 1.0 a | 78.0 ± 2.7 a | 11.9 ± 0.5 a |
| HEO/β-CD ICs control | 90.1 ± 1.7 b | 52.3 ± 2.0 b | 8.2 ± 0.4 b |
| HEO/2-HP-β-CD ICs | 80.8 ± 1.2 c | 73.7 ± 3.2 a | 12.9 ± 0.4 c |
| HEO/2-HP-β-CD ICs control | 78.9 ± 0.6 c | 58.9 ± 1.7 c | 10.6 ± 0.3 d |
| Inhibitory Rate (%) | |||
|---|---|---|---|
| 5 g L−1 | 10 g L−1 | 20 g L−1 | |
| HEO | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| β-CD | 16.2 ± 0.6 b | 18.2 ± 1.5 b | 20.9 ± 1.2 b |
| 2-HP-β-CD | 6.0 ± 5.9 c | 12.1 ± 1.9 c | 17.9 ± 1.3 c |
| HEO/β-CD ICs | 58.0 ± 3.1 d | 86.5 ± 1.1 d | 99.8 ± 0.2 a |
| HEO/2-HP-β-CD ICs | 72.8 ± 3.6 e | 93.0 ± 0.8 e | 100.0 ± 0.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, P.; Thangunpai, K.; Zulfikar, A.; Masuo, S.; Abe, J.P.; Enomae, T. Preparation of Green Anti-Staphylococcus aureus Inclusion Complexes Containing Hinoki Essential Oil. Foods 2023, 12, 3104. https://doi.org/10.3390/foods12163104
Kong P, Thangunpai K, Zulfikar A, Masuo S, Abe JP, Enomae T. Preparation of Green Anti-Staphylococcus aureus Inclusion Complexes Containing Hinoki Essential Oil. Foods. 2023; 12(16):3104. https://doi.org/10.3390/foods12163104
Chicago/Turabian StyleKong, Peifu, Kotchaporn Thangunpai, Ainun Zulfikar, Shunsuke Masuo, Junichi Peter Abe, and Toshiharu Enomae. 2023. "Preparation of Green Anti-Staphylococcus aureus Inclusion Complexes Containing Hinoki Essential Oil" Foods 12, no. 16: 3104. https://doi.org/10.3390/foods12163104
APA StyleKong, P., Thangunpai, K., Zulfikar, A., Masuo, S., Abe, J. P., & Enomae, T. (2023). Preparation of Green Anti-Staphylococcus aureus Inclusion Complexes Containing Hinoki Essential Oil. Foods, 12(16), 3104. https://doi.org/10.3390/foods12163104