Single-Walled Carbon Nanohorn-Based Fluorescence Energy Resonance Transfer Aptasensor Platform for the Detection of Aflatoxin B1
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Optimization of System Conditions
2.4. Detection of AFB1
2.5. Performance Analysis
2.6. Detection AFB1 in Soybean Oil Samples
3. Results and Discussion
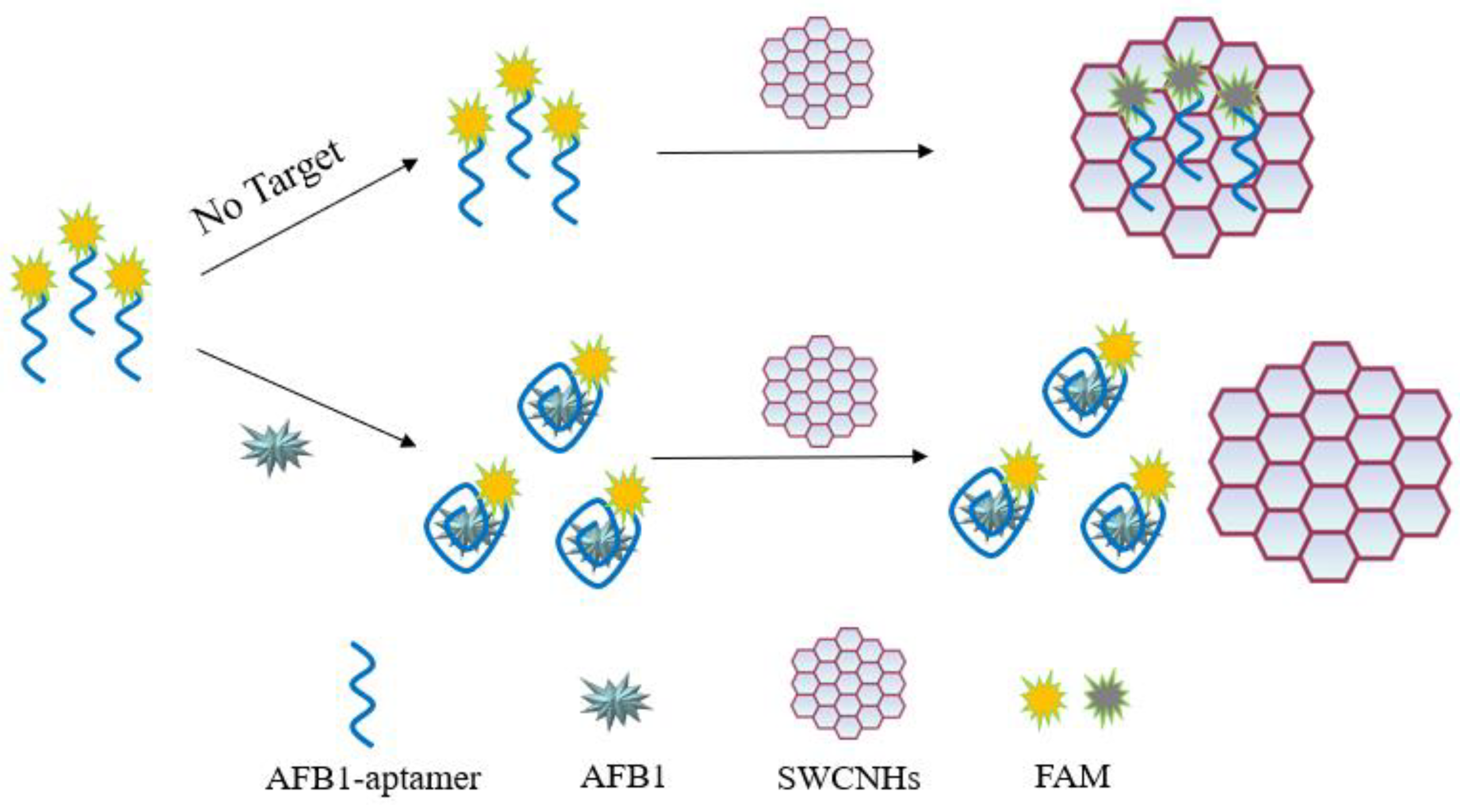
3.1. Sensing Strategy for AFB1 Detection
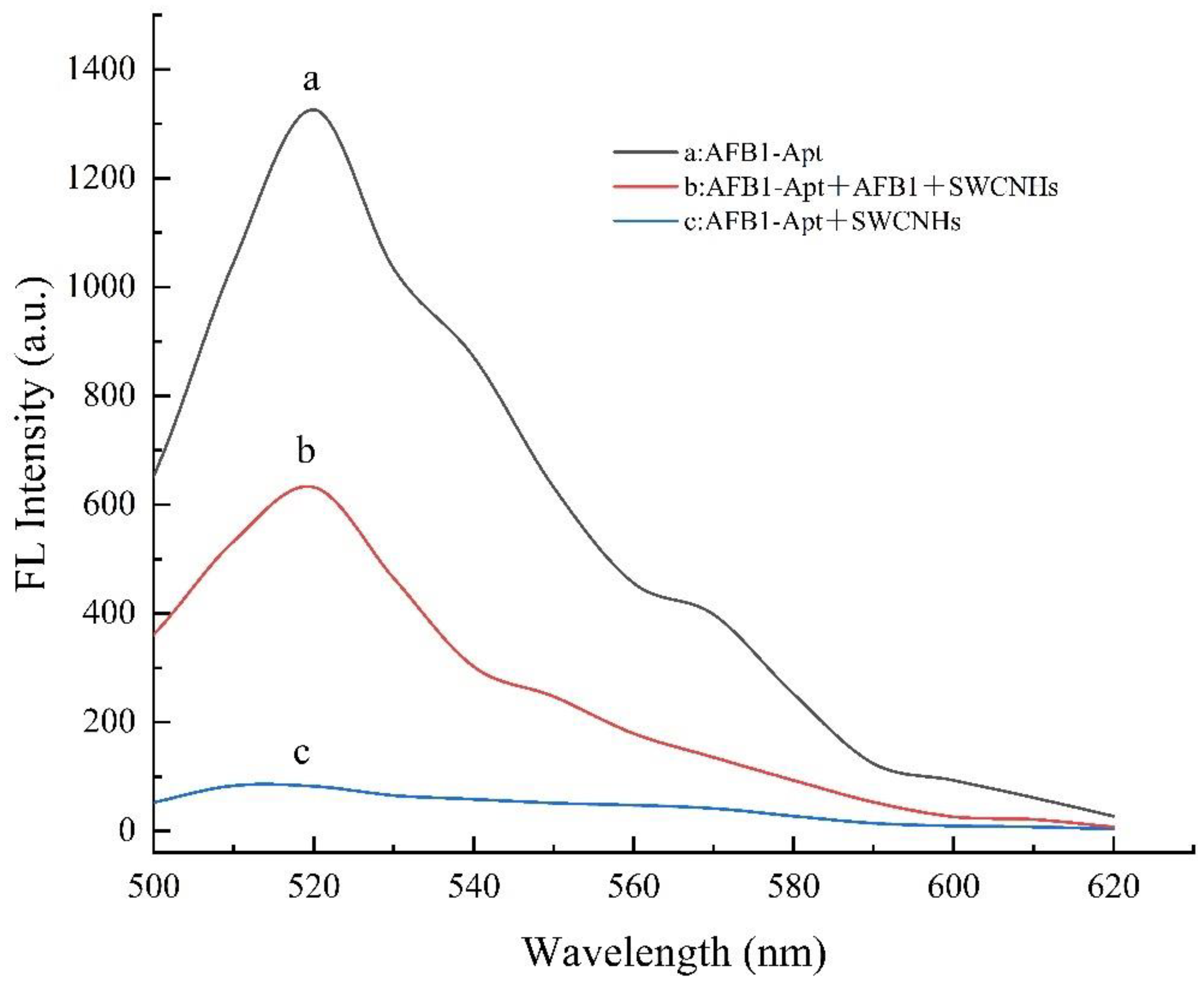
3.2. Feasibility Study
3.3. Characterizations of SWCNHs
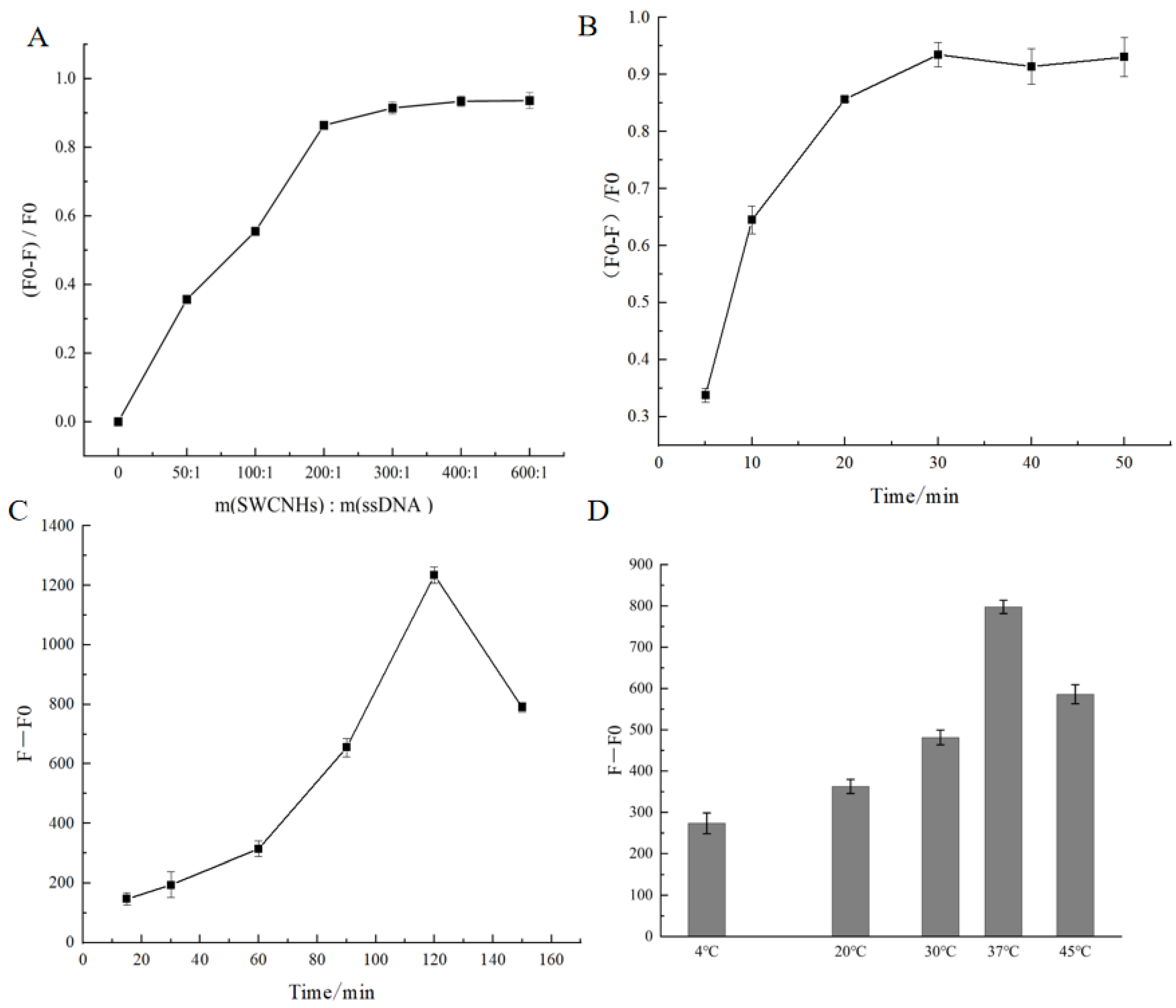
3.4. Optimization of the System Condition
3.4.1. Optimized SWCNH Concentration
3.4.2. Optimized Incubation Time
3.4.3. Optimized Incubation Temperature
3.5. Detection Performance of the AFB1 Aptasensor
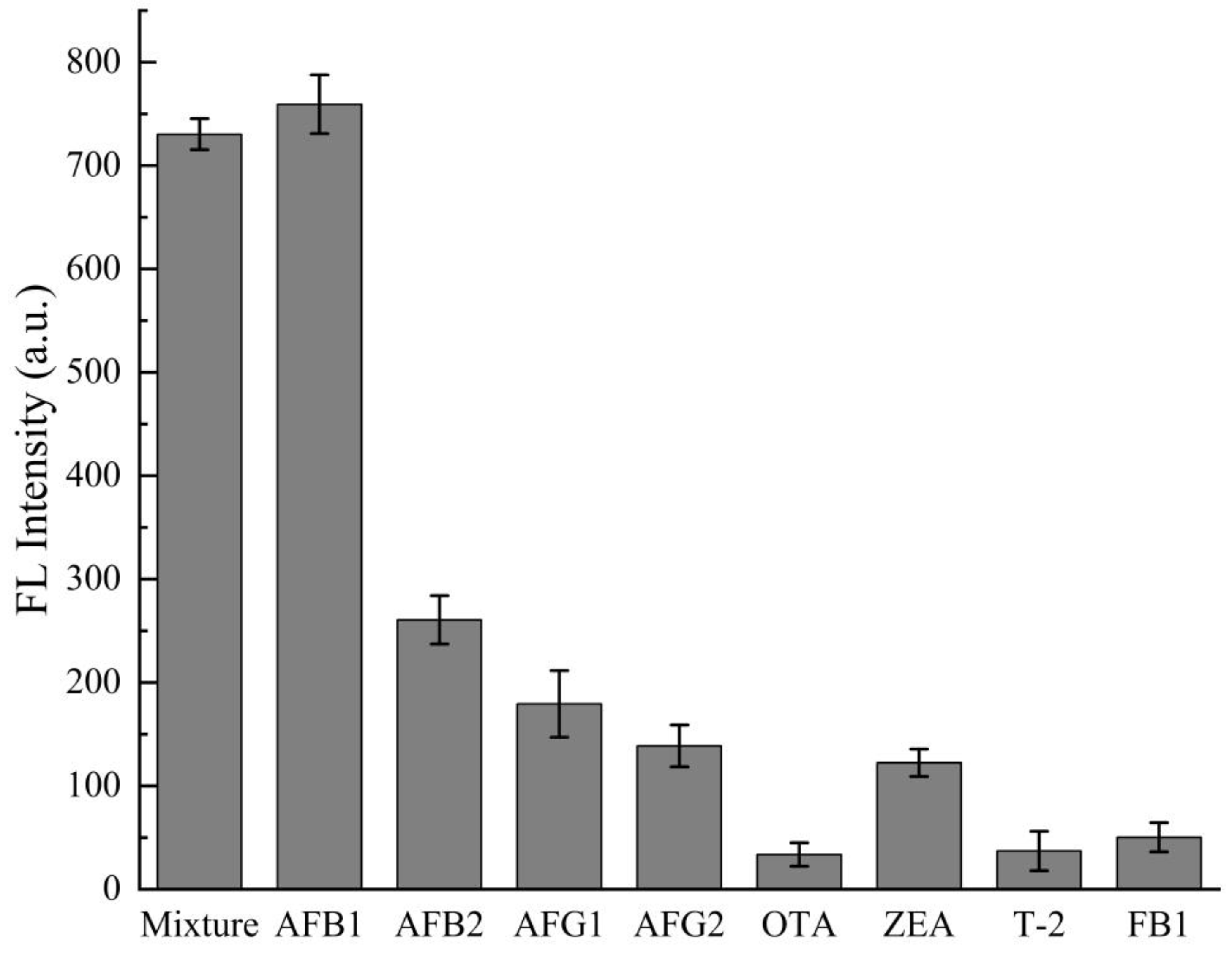
3.6. Performance Analysis
3.7. Analysis of AFB1 in Soybean Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef]
- Zhong, T.; Li, S.; Li, X.; Jiye, Y.; Mo, Y.; Chen, L.; Zhang, Z.; Wu, H.; Li, M.; Luo, Q. A label-free electrochemical aptasensor based on AuNPs-loaded zeolitic imidazolate framework-8 for sensitive determination of aflatoxin B1. Food Chem. 2022, 384, 132495. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, R.; Xie, Y.; Liu, Y. Reduction of aflatoxin B1 by magnetic graphene oxide/TiO2 nanocomposite and its effect on quality of corn oil. Food Chem. 2021, 343, 128521. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Akram, A.; Hanif, N.Q.; Mukhtar, T.; Arshad, M. Mycobiota Isolation and Aflatoxin B1 Contamination in Fresh and Stored Sesame Seeds from Rainfed and Irrigated Zones of Punjab, Pakistan. J. Food Prot. 2021, 84, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, G.A.; El-Fayoumi, R.I.; Smith, M.J.; Heckmann, R.A.; O’Neill, K.L. A comparative evaluation of aflatoxin B1 genotoxicity in fish models using the Comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 446, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tania, A.; Akram, A.; Hanif, N.Q.; Ajmal, M.; Seerat, W.; Nijabat, A.; Mehak, A. Proximate composition, fungal isolation and contamination of aflatoxin B1 in chickpea seeds from the Punjab, Pakistan. Nat. Prod. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Chen, P.; Li, C.; Ma, X.; Wang, Z.; Zhang, Y. A surface-enhanced Raman scattering aptasensor for ratiometric detection of aflatoxin B1 based on graphene oxide-Au@Ag core-shell nanoparticles complex. Food Control 2022, 134, 108748. [Google Scholar] [CrossRef]
- Wang, W.; Heitschmidt, G.W.; Ni, X.; Windham, W.R.; Hawkins, S.; Chu, X. Identification of aflatoxin B1 on maize kernel surfaces using hyperspectral imaging. Food Control 2014, 42, 78–86. [Google Scholar] [CrossRef]
- Xia, L.; Rasheed, H.; Routledge, M.N.; Wu, H.; Gong, Y.Y. Super-Sensitive LC-MS Analyses of Exposure Biomarkers for Multiple Mycotoxins in a Rural Pakistan Population. Toxins 2022, 14, 193. [Google Scholar] [CrossRef]
- Khayoon, W.S.; Saad, B.; Lee, T.P.; Salleh, B. High performance liquid chromatographic determination of aflatoxins in chilli, peanut and rice using silica based monolithic column. Food Chem. 2012, 133, 489–496. [Google Scholar] [CrossRef]
- Wu, W.; Xia, S.; Zhao, M.; Ping, J.; Lin, J.-M.; Hu, Q. Colorimetric liquid crystal-based assay for the ultrasensitive detection of AFB1 assisted with rolling circle amplification. Anal. Chim. Acta 2022, 1220, 340065. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, F.; Liu, R.; Liu, M.; Sang, Y.; Wang, S.; Wang, X. A Novel Colorimetric Nano Aptasensor for Ultrasensitive Detection of Aflatoxin B1 Based on the Exonuclease III-Assisted Signal Amplification Approach. Foods 2021, 10, 2568. [Google Scholar] [CrossRef]
- Zhan, S.; Hu, J.; Li, Y.; Huang, X.; Xiong, Y. Direct competitive ELISA enhanced by dynamic light scattering for the ultrasensitive detection of aflatoxin B1 in corn samples. Food Chem. 2021, 342, 128327. [Google Scholar] [CrossRef]
- Bacaloni, A.; Cavaliere, C.; Cucci, F.; Foglia, P.; Samperi, R.; Lagana, A. Determination of aflatoxins in hazelnuts by various sample preparation methods and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1179, 182–189. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Zhang, Q.; Zhang, W.; Yu, L.; Wang, D.; Li, H.; Li, P. An On-Site Simultaneous Semi-quantification of Aflatoxin B1, Zearalenone, and T-2 Toxin in Maize- and Cereal-based Feed via Multicolor Immunochromatographic Assay. Toxins 2018, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Leng, Y.; Li, X.; Huang, X.; Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. TrAC Trends Anal. Chem. 2020, 126, 115861. [Google Scholar] [CrossRef]
- Liu, J.; Suo, Z.; Liu, Y.; He, B.; Wei, M. An electrochemical apta-assay based on hybridization chain reaction and aflatoxin B1-driven Ag-DNAzyme as amplification strategy. Bioelectrochemistry 2023, 149, 108322. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Acquah, C.; Agyei, D.; Obeng, E.M.; Pan, S.; Tan, K.X.; Danquah, M.K. Aptamers: An emerging class of bioaffinity ligands in bioactive peptide applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 1195–1206. [Google Scholar] [CrossRef]
- Guo, H.; Ma, P.; Li, K.; Zhang, S.; Zhang, Y.; Guo, H.; Wang, Z. A novel ratiometric aptasensor based on dual-emission fluorescent signals and the conformation of G-quadruplex for OTA detection. Sens. Actuators B Chem. 2022, 358, 131484. [Google Scholar] [CrossRef]
- Zhao, L.; Suo, Z.; Liu, Y.; Wei, M.; Jin, H. An amplifiable ratiometric fluorescent aptasensor for aflatoxin B1 detection based on dendrimer-like DNA nanostructures coupled with catalytic hairpin self-assembly. Sens. Actuators B Chem. 2023, 380, 133328. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, J.; Li, X.; Hu, Q.; Qian, J.; Hao, N.; Wang, K. Region separation type bio-photoelectrode based all-solid-state self-powered aptasensor for ochratoxin A and aflatoxin B1 detection. Sens. Actuators B Chem. 2022, 364, 131897. [Google Scholar] [CrossRef]
- He, Y.; Wen, C.-Y.; Guo, Z.-J.; Huang, Y.-F. Noble metal nanomaterial-based aptasensors for microbial toxin detection. J. Food Drug Anal. 2020, 28, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Setlem, S.K.; Mondal, B.; Ramlal, S. A fluorescent aptasensor for the detection of Aflatoxin B1 by graphene oxide mediated quenching and release of fluorescence. J. Microbiol. Methods 2022, 193, 106414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, F.; Yang, Q.; Wu, W. Graphene oxide quantum dots based nanotree illuminates AFB1: Dual signal amplified aptasensor detection AFB1. Sens. Actuators B Chem. 2021, 345, 130387. [Google Scholar] [CrossRef]
- Young, S.-J.; Liu, Y.-H.; Lin, Z.-D.; Ahmed, K.; Shiblee, M.D.N.I.; Romanuik, S.; Sekhar, P.K.; Thundat, T.; Nagahara, L.; Arya, S.; et al. Multi-Walled Carbon Nanotubes Decorated with Silver Nanoparticles for Acetone Gas Sensing at Room Temperature. J. Electrochem. Soc. 2020, 167, 167519. [Google Scholar] [CrossRef]
- Zhu, G.; Sun, H.; Zou, B.; Liu, Z.; Sun, N.; Yi, Y.; Wong, K.Y. Electrochemical sensing of 4-nitrochlorobenzene based on carbon nanohorns/graphene oxide nanohybrids. Biosens. Bioelectron. 2018, 106, 136–141. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Liu, Y.; Ye, Y.; Yao, S. Electrochemical sensing of nitenpyram based on the binary nanohybrid of hydroxylated multiwall carbon nanotubes/single-wall carbon nanohorns. J. Electroanal. Chem. 2020, 862, 113955. [Google Scholar] [CrossRef]
- Gao, J.; He, S.; Nag, A.; Wong, J.W.C. A Review of the Use of Carbon Nanotubes and Graphene-Based Sensors for the Detection of Aflatoxin M1 Compounds in Milk. Sensors 2021, 21, 3602. [Google Scholar] [CrossRef]
- Lv, L.; Cui, C.; Liang, C.; Quan, W.; Wang, S.; Guo, Z. Aptamer-based single-walled carbon nanohorn sensors for ochratoxin A detection. Food Control 2016, 60, 296–301. [Google Scholar] [CrossRef]
- Guo, Z.; Tian, J.; Cui, C.; Wang, Y.; Yang, H.; Yuan, M.; Yu, H. A label-free aptasensor for turn-on fluorescent detection of ochratoxin a based on SYBR gold and single walled carbon nanohorns. Food Control 2021, 123, 107741. [Google Scholar] [CrossRef]
- Aoyagi, M.; Yudasaka, M.; Minamikawa, H.; Asakawa, M.; Masuda, M.; Shimizu, T.; Iijima, S. Quantitative analyses of PEGylated phospholipids adsorbed on single walled carbon nanohorns by high resolution magic angle spinning 1H NMR. Carbon 2016, 101, 213–217. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Qian, J.; Wang, L.; Ren, Y.; Wang, Y.; Xu, M.; Huang, X. A FRET aptasensor for sensitive detection of aflatoxin B1 based on a novel donor-acceptor pair between ZnS quantum dots and Ag nanocubes. Anal. Methods 2021, 13, 462–468. [Google Scholar] [CrossRef]
- Shim, W.-B.; Mun, H.; Joung, H.-A.; Ofori, J.A.; Chung, D.-H.; Kim, M.-G. Chemiluminescence competitive aptamer assay for the detection of aflatoxin B1 in corn samples. Food Control 2014, 36, 30–35. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, M.; Li, M.; Xiao, Z.; Ling, L. Hybridization chain reaction and DNAzyme-based dual signal amplification strategy for sensitive fluorescent sensing of aflatoxin B1 by using the pivot of triplex DNA. Food Res. Int. 2022, 158, 111538. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-Y.; Li, J.; Fan, L.; Wen, J.; Zhang, J.; Zhang, Z.-Q. A label-free aptasensor based on a dual-emission fluorescent strategy for aflatoxin B1 detection. Sens. Actuators B Chem. 2021, 346, 130561. [Google Scholar] [CrossRef]
- Sano, N.; Ishii, T.; Tamon, H. Transformation from single-walled carbon nanotubes to nanohorns by simple heating with Pd at 1600°C. Carbon 2011, 49, 3698–3700. [Google Scholar] [CrossRef]
- Ortolani, T.S.; Pereira, T.S.; Assumpção, M.H.M.T.; Vicentini, F.C.; Gabriel de Oliveira, G.; Janegitz, B.C. Electrochemical sensing of purines guanine and adenine using single-walled carbon nanohorns and nanocellulose. Electrochim. Acta 2019, 298, 893–900. [Google Scholar] [CrossRef]
- Hitabatuma, A.; Pang, Y.H.; Yu, L.H.; Shen, X.F. A competitive fluorescence assay based on free-complementary DNA for ochratoxin A detection. Food Chem. 2021, 342, 128303. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS(2) NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Dadmehr, M.; Shahi, S.C.; Malekkiani, M.; Korouzhdehi, B.; Tavassoli, A. A stem-loop like aptasensor for sensitive detection of aflatoxin based on graphene oxide/AuNPs nanocomposite platform. Food Chem. 2023, 402, 134212. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, D.W.; Pu, H.; Wei, Q. A novel fluorescence biosensor based on CRISPR/Cas12a integrated MXenes for detecting Aflatoxin B1. Talanta 2023, 252, 123773. [Google Scholar] [CrossRef] [PubMed]
- Anukul, N.; Vangnai, K.; Mahakarnchanakul, W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 2013, 21, 227–241. [Google Scholar] [CrossRef]
- Vijitvarasan, P.; Cheunkar, S.; Oaew, S. A point-of-use lateral flow aptasensor for naked-eye detection of aflatoxin B1. Food Control 2022, 134, 108767. [Google Scholar] [CrossRef]
- Jia, B.; Liao, X.; Sun, C.; Fang, L.; Zhou, L.; Kong, W. Development of a quantum dot nanobead-based fluorescent strip immunosensor for on-site detection of aflatoxin B(1) in lotus seeds. Food Chem. 2021, 356, 129614. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, S.; Wang, S.; Liu, J.; Dong, Y. Development of lateral flow immunochromatographic strips for micropollutant screening using colorants of aptamer-functionalized nanogold particles, part II: Experimental verification with aflatoxin B1 and chloramphenicol. J. AOAC Int. 2018, 101, 1408–1414. [Google Scholar] [CrossRef]
- Setlem, K.; Mondal, B.; Shylaja, R.; Parida, M. Dual Aptamer-DNAzyme based colorimetric assay for the detection of AFB1 from food and environmental samples. Anal. Biochem. 2020, 608, 113874. [Google Scholar] [CrossRef]
- Xiong, J.; He, S.; Zhang, S.; Qin, L.; Yang, L.; Wang, Z.; Zhang, L.; Shan, W.; Jiang, H. A label-free aptasensor for dual-mode detection of aflatoxin B1 based on inner filter effect using silver nanoparticles and arginine-modified gold nanoclusters. Food Control 2023, 144, 109397. [Google Scholar] [CrossRef]
- Xia, X.; Wang, H.; Yang, H.; Deng, S.; Deng, R.; Dong, Y.; He, Q. Dual-Terminal Stemmed Aptamer Beacon for Label-Free Detection of Aflatoxin B(1) in Broad Bean Paste and Peanut Oil via Aggregation-Induced Emission. J. Agric. Food Chem. 2018, 66, 12431–12438. [Google Scholar] [CrossRef]
- Qi, X.; Lv, L.; Wei, D.; Lee, J.J.; Niu, M.; Cui, C.; Guo, Z. Detection of aflatoxin B1 with a new label-free fluorescence aptasensor based on PVP-coated single-walled carbon nanohorns and SYBR Gold. Anal. Bioanal. Chem. 2022, 414, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wen, F.; Li, M.; Guo, X.; Li, S.; Zheng, N.; Wang, J. A simple aptamer-based fluorescent assay for the detection of Aflatoxin B1 in infant rice cereal. Food Chem. 2017, 215, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ’FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]



| Methods | Material | Linear Range (ng/mL) | LOD (ng/mL) | Samples | Detection Step | Detection Time | Reference |
|---|---|---|---|---|---|---|---|
| Fluorescenceimmunosensor | QBs | 1–19 | 1 | lotus seed | 5 | >12 h | [46] |
| Colorimetric lateral flow aptasensor | Polystyrene dyed particles | 5–1000 | 4.56 | peanut, corn, rice, and chili powder | 5 | 345 min | [45] |
| Colorimetric immunosensor | AuNPs | 10–50,000 | 10 | water | 4 | >24 h | [47] |
| Colorimetricaptasensor | DNAzyme | 0–200 | 22.6 | sorghum | 4 | 180 min | [48] |
| Colorimetric/Fluorescenceaptasensor | AgNPs/ AuNCs | 20–400 5–400 | 12.16/1.91 | corn/wheat | 4 | >24 h | [49] |
| Fluorescenceaptasensor | AIE dye | 40–300 | 27.3 | broad bean paste/ peanut oil | 3 | 230 min | [50] |
| Fluorescenceaptasensor | SYBR Gold | 5–200 | 1.89 | soybean sauce | 3 | 130 min | [51] |
| Fluorescenceaptasensor | FAM | 5–100 | 1.6 | rice cereal | 3 | 60 min | [52] |
| Fluorescenceaptasensor | SWCNHs | 10–100 | 4.1 | soybean oil | 3 | 185 min | This work |
| Soybean Oil | Addition Amount (ng/mL) | Measured Volume (ng/mL) | Recovery Rate (%) | RSD (%) | |||
|---|---|---|---|---|---|---|---|
| This Method | ELISA Method | This Method | ELISA Method | This Method | ELISA Method | ||
| 1 | 10 | 10.25 | 9.98 | 102.5 | 99.8 | 4.41 | 5.34 |
| 2 | 50 | 42.93 | 54.33 | 85.9 | 108.7 | 5.46 | 4.71 |
| 3 | 100 | 100.31 | 97.28 | 100.3 | 97.3 | 3.59 | 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Yang, H.; Li, J.; Amin, K.; Lyu, B.; Jing, W.; Wang, S.; Fu, H.; Yu, H.; Guo, Z. Single-Walled Carbon Nanohorn-Based Fluorescence Energy Resonance Transfer Aptasensor Platform for the Detection of Aflatoxin B1. Foods 2023, 12, 2880. https://doi.org/10.3390/foods12152880
Fan Y, Yang H, Li J, Amin K, Lyu B, Jing W, Wang S, Fu H, Yu H, Guo Z. Single-Walled Carbon Nanohorn-Based Fluorescence Energy Resonance Transfer Aptasensor Platform for the Detection of Aflatoxin B1. Foods. 2023; 12(15):2880. https://doi.org/10.3390/foods12152880
Chicago/Turabian StyleFan, Yiting, Huanhuan Yang, Jiaxin Li, Khalid Amin, Bo Lyu, Wendan Jing, Sainan Wang, Hongling Fu, Hansong Yu, and Zhijun Guo. 2023. "Single-Walled Carbon Nanohorn-Based Fluorescence Energy Resonance Transfer Aptasensor Platform for the Detection of Aflatoxin B1" Foods 12, no. 15: 2880. https://doi.org/10.3390/foods12152880
APA StyleFan, Y., Yang, H., Li, J., Amin, K., Lyu, B., Jing, W., Wang, S., Fu, H., Yu, H., & Guo, Z. (2023). Single-Walled Carbon Nanohorn-Based Fluorescence Energy Resonance Transfer Aptasensor Platform for the Detection of Aflatoxin B1. Foods, 12(15), 2880. https://doi.org/10.3390/foods12152880






