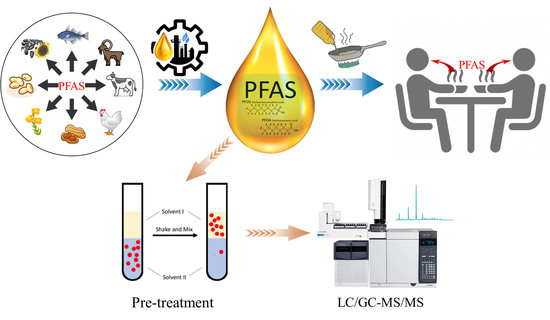
Research Progress of Perfluoroalkyl Substances in Edible Oil—A Review
Abstract
1. Introduction
2. Classification
3. Legislation
4. Sources of PFASs in Edible Oil
4.1. PFAS Accumulation in Oil Crops
4.2. PFAS Accumulation in Animal Edible Oil Raw Materials
4.3. Contamination of PFASs during Edible Oil Production
4.4. Migration from Oil Contact Materials to Edible Oil
5. PFAS Contamination in Edible Oil
6. Pre-Treatment Methods for PFAS Analysis in Edible Oil
6.1. Liquid-Liquid Extraction (LLE)
6.2. Ion Pair Extraction (IPE)
6.3. Dispersive Liquid-Liquid Microextraction (DLLME)
6.4. Alkaline Digestion
6.5. Liquid-Solid Extraction (LSE)
6.6. Solid Phase Extraction (SPE)
6.6.1. Dispersive Solid-Phase Extraction (d-SPE)
6.6.2. Magnetic Solid-Phase Extraction (MSPE)
6.7. QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe)
7. Determination of PFAS in Edible Oil
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahrens, L.; Felizeter, S.; Sturm, R.; Xie, Z.; Ebinghaus, R. Polyfluorinated compounds in waste water treatment plant effluents and surface waters along the river elbe, germany. Mar. Pollut. Bull. 2009, 58, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Chohan, A.; Petaway, H.; Rivera-Diaz, V.; Day, A.; Colaianni, O.; Keramati, M. Per and polyfluoroalkyl substances scientific literature review: Water exposure, impact on human health, and implications for regulatory reform. Rev. Environ. Health 2021, 36, 235–259. [Google Scholar] [CrossRef]
- Liu, Y.; D’Agostino, L.A.; Qu, G.; Jiang, G.; Martin, J.W. High-resolution mass spectrometry (hrms) methods for nontarget discovery and characterization of poly- and per-fluoroalkyl substances (pfass) in environmental and human samples. TrAC, Trends Anal. Chem. 2019, 121, 115420. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmaraja, R.; Chadalavada, S.; Naidu, R.; Chalker, J.M.; Fang, C. Recent advances in the analysis of per- and polyfluoroalkyl substances (pfas)—A review. Environ. Technol. Innov. 2020, 19, 100879. [Google Scholar] [CrossRef]
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; Dewitt, J.C.; Knappe, D.R.U.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific basis for managing pfas as a chemical class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jurkovic-Mlakar, S.; Lindh, C.H.; Scott, K.; Fletcher, T.; Jakobsson, K.; Engström, K. Associations between serum concentrations of perfluoroalkyl substances and dna methylation in women exposed through drinking water: A pilot study in ronneby, sweden. Environ. Int. 2020, 145, 106148. [Google Scholar] [CrossRef]
- Emerce, E.; Çetin, Ö. Genotoxicity assessment of perfluoroalkyl substances on human sperm. Toxicol. Ind. Health 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Ducatman, A.; Fenton, S.E. Invited perspective: Pfas and liver disease: Bringing all the evidence together. Environ. Health Perspect. 2022, 130, 041303. [Google Scholar] [CrossRef]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (pfas) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef]
- Calvert, L.; Green, M.P.; De Iuliis, G.N.; Dun, M.D.; Turner, B.D.; Clarke, B.O.; Eamens, A.L.; Roman, S.D.; Nixon, B. Assessment of the emerging threat posed by perfluoroalkyl and polyfluoroalkyl substances to male reproduction in humans. Front. Endocrinol. 2021, 12, 799043. [Google Scholar] [CrossRef]
- Cui, Q.; Pan, Y.; Wang, J.; Liu, H.; Yao, B.; Dai, J. Exposure to per- and polyfluoroalkyl substances (pfass) in serum versus semen and their association with male reproductive hormones. Environ. Pollut. 2020, 266, 115330. [Google Scholar] [CrossRef] [PubMed]
- Foguth, R.; Sepúlveda, M.S.; Cannon, J. Per- and polyfluoroalkyl substances (pfas) neurotoxicity in sentinel and non-traditional laboratory model systems: Potential utility in predicting adverse outcomes in human health. Toxics 2020, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Cui, Q.; Yao, B.; Wang, J.; Dai, J. Penetration of pfass across the blood cerebrospinal fluid barrier and its determinants in humans. Environ. Sci. Technol. 2018, 52, 13553–13561. [Google Scholar] [CrossRef]
- Gaballah, S.; Swank, A.; Sobus, J.R.; Howey, X.M.; Schmid, J.; Catron, T.; Mccord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to genx and other pfas. Environ. Health Perspect. 2020, 128, 047005. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Rericha, Y.; Thunga, P.; Marvel, S.; Wallis, D.; Simonich, M.T.; Field, J.A.; Cao, D.; Reif, D.M.; Tanguay, R.L. Systematic developmental toxicity assessment of a structurally diverse library of pfas in zebrafish. J. Hazard. Mater. 2022, 431, 128615. [Google Scholar] [CrossRef]
- Grandjean, P. Delayed discovery, dissemination, and decisions on intervention in environmental health: A case study on immunotoxicity of perfluorinated alkylate substances. Environ. Health 2018, 17, 62. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.; Wang, D.; Gao, N.; Ding, Y.; Chen, C. The role of interleukin family in perfluorooctanoic acid (pfoa)-induced immunotoxicity. J. Hazard. Mater. 2014, 280, 552–560. [Google Scholar] [CrossRef]
- Di Nisio, A.; Lopez-Espinosa, M.; Foresta, C. Editorial: Emerging chemical risks for human health: Endocrine disruption by per- and poly-fluorinated alkyl substances (pfas). Front. Endocrinol. 2022, 12, 813785. [Google Scholar] [CrossRef]
- Han, X.; Meng, L.; Zhang, G.; Li, Y.; Shi, Y.; Zhang, Q.; Jiang, G. Exposure to novel and legacy per- and polyfluoroalkyl substances (pfass) and associations with type 2 diabetes: A case-control study in east china. Environ. Int. 2021, 156, 106637. [Google Scholar] [CrossRef]
- Liu, S.; Yin, N.; Faiola, F. Pfoa and pfos disrupt the generation of human pancreatic progenitor cells. Environ. Sci. Technol. Lett. 2018, 5, 237–242. [Google Scholar] [CrossRef]
- Jane, L.; Espartero, L.; Yamada, M.; Ford, J.; Owens, G.; Prow, T.; Juhasz, A. Health-related toxicity of emerging per- and polyfluoroalkyl substances: Comparison to legacy pfos and pfoa. Environ. Res. 2022, 212, 113431. [Google Scholar] [CrossRef]
- Wen, Z.J.; Wei, Y.J.; Zhang, Y.F.; Zhang, Y.F. A review of cardiovascular effects and underlying mechanisms of legacy and emerging per- and polyfluoroalkyl substances (pfas). Arch. Toxicol. 2023, 97, 1195–1245. [Google Scholar] [CrossRef] [PubMed]
- Girardi, P.; Merler, E. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ. Res. 2019, 179, 108743. [Google Scholar] [CrossRef]
- Mastrantonio, M.; Bai, E.; Uccelli, R.; Cordiano, V.; Screpanti, A.; Crosignani, P. Drinking water contamination from perfluoroalkyl substances (pfas): An ecological mortality study in the veneto region, italy. Eur. J. Public Health 2018, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (pfoa) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef]
- Catelan, D.; Biggeri, A.; Russo, F.; Gregori, D.; Pitter, G.; Da Re, F.; Fletcher, T.; Canova, C. Exposure to perfluoroalkyl substances and mortality for covid-19: A spatial ecological analysis in the veneto region (italy). Int. J. Environ. Res. Public Health 2021, 18, 2734. [Google Scholar] [CrossRef]
- Grandjean, P.; Timmermann, C.A.G.; Kruse, M.; Nielsen, F.; Vinholt, P.J.; Boding, L.; Heilmann, C.; Mølbak, K. Severity of covid-19 at elevated exposure to perfluorinated alkylates. PLoS ONE 2020, 15, e244815. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, M.; Xu, F.; Chen, D. Characterization of the binding of per- and poly-fluorinated substances to proteins: A methodological review. TrAC Trends Anal. Chem. 2019, 116, 177–185. [Google Scholar] [CrossRef]
- Sznajder-Katarzyńska, K.; Surma, M.; Wiczkowski, W.; Piskuła, M. Determination of perfluoroalkyl substances (pfass) in fats and oils by quechers/micro-hplc-ms/ms. Food Res. Int. 2020, 137, 109583. [Google Scholar] [CrossRef]
- Choi, H.; Bae, I.; Choi, J.C.; Park, S.; Kim, M. Perfluorinated compounds in food simulants after migration from fluorocarbon resin-coated frying pans, baking utensils, and non-stick baking papers on the korean market. Food Addit. Contam. Part B-Surveill. 2018, 11, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible plant oil: Global status, health issues, and perspectives. Front. Plant Sci. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.A.; Davis, K.L. Evaluating pfas cross contamination issues. Remediat. J. 2018, 28, 53–57. [Google Scholar] [CrossRef]
- Ogunbiyi, O.D.; Ajiboye, T.O.; Omotola, E.O.; Oladoye, P.O.; Olanrewaju, C.A.; Quinete, N. Analytical approaches for screening of per- and poly fluoroalkyl substances in food items: A review of recent advances and improvements. Environ. Pollut. 2023, 329, 121705. [Google Scholar] [CrossRef]
- Fan, C.; Wang, H.; Liu, Y.; Cao, X. New deep eutectic solvent based superparamagnetic nanofluid for determination of perfluoroalkyl substances in edible oils. Talanta 2021, 228, 122214. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (pfas) from water by adsorption: Role of pfas chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (pfass) in drinking water treatment: A review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef]
- Zheng, G.; Boor, B.E.; Schreder, E.; Salamova, A. Indoor exposure to per- and polyfluoroalkyl substances (pfas) in the childcare environment. Environ. Pollut. 2020, 258, 113714. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Dreyer, A.; Dietrich, S.; Fembacher, L.; Lahrz, T.; Völkel, W. Neutral polyfluorinated compounds in indoor air in germany-the lupe 4 study. Chemosphere 2015, 139, 572–578. [Google Scholar] [CrossRef]
- Nakayama, S.; Harada, K.; Inoue, K.; Sasaki, K.; Seery, B.; Saito, N.; Koizumi, A. Distributions of perfluorooctanoic acid (pfoa) and perfluorooctane sulfonate (pfos) in japan and their toxicities. Environ. Sci. 2005, 12, 293–313. [Google Scholar]
- Ritter, S.K. Fluorochemicals go short. Chem. Eng. News 2010, 88, 12–17. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Buck, R.C.; Hungerbühler, K. Global emission inventories for c4-c14 perfluoroalkyl carboxylic acid (pfca) homologues from 1951 to 2030, part i: Production and emissions from quantifiable sources. Environ. Int. 2014, 70, 62–75. [Google Scholar] [CrossRef]
- Unep. Sc-4/17: Listing of Perfluorooctane Sulfonic Acid, Its Salts and Perfluorooctane Sulfonyl Fluoride. Available online: https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexB/tabid/5850/Default.aspx (accessed on 12 June 2023).
- Unep. Sc-9/12: Listing of Perfluorooctanoic Acid (pfoa), Its Salts and Pfoa-Related Compounds. Available online: https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/tabid/5837/Default.aspx (accessed on 12 June 2023).
- Unep. Tenth Meeting of the Conference of the Parties to the Stockholm Convention. Available online: https://chm.pops.int/TheConvention/ConferenceoftheParties/Meetings/COP10/tabid/8397/Default.aspx (accessed on 12 June 2023).
- European, F.S.A.E. Perfluorooctane sulfonate (pfos), perfluorooctanoic acid (pfoa) and their salts scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 6, 653. [Google Scholar]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl Kraupp, B.; et al. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, e05194. [Google Scholar] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar]
- EU. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) no 1881/2006 (Text with EEA Relevance). Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023R0915 (accessed on 2 July 2023).
- EPA. Provisional Health Advisories for Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS). Available online: https://www.epa.gov/sites/default/files/2015-09/documents/pfoa-pfos-provisional.pdf (accessed on 17 May 2023).
- EPA. PFOA & PFOS Drinking Water Health Advisories. Available online: https://www.epa.gov/sites/default/files/2016-06/documents/drinkingwaterhealthadvisories_pfoa_pfos_updated_5.31.16.pdf (accessed on 17 May 2023).
- EPA. Drinking Water Health Advisories for pfas Fact Sheet for Public Water Systems. Available online: https://www.epa.gov/system/files/documents/2022-06/drinking-water-ha-pfas-factsheet-water-system.pdf (accessed on 17 May 2023).
- Tpch. Toxics in Packaging Clearinghouse Model Legislation 2021 Update. Available online: https://toxicsinpackaging.org/model-legislation/model (accessed on 17 May 2023).
- FDA. FDA Announces the Voluntary Phase-Out by Industry of Certain pfas Used in Food Packaging. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-announces-voluntary-phase-out-industry-certain-pfas-used-food-packaging (accessed on 17 May 2023).
- Mepc. List of Key Regulated New Pollutants (Version 2023). Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk02/202212/W020221230613338823204.pdf (accessed on 17 May 2023).
- Cscl. Cabinet Decision on the Cabinet Order for the Partial Revision of the Order for Enforcement of the Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc. Available online: https://www.meti.go.jp/english/press/2021/0416_001.html (accessed on 17 May 2023).
- EPA. Proposed PFAS National Primary Drinking Water Regulation. Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed on 17 May 2023).
- Mepc. Announcement on the Ban on the Production, Circulation, Use and Import and Export of Lindane and Other Persistent Organic Pollutants. Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/201903/t20190312_695462.html (accessed on 17 May 2023).
- Yang, L.; Jin, F.; Zhang, P.; Zhang, Y.; Wang, J.; Shao, H.; Jin, M.; Wang, S.; Zheng, L.; Wang, J. Simultaneous determination of perfluorinated compounds in edible oil by gel-permeation chromatography combined with dispersive solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2015, 63, 8364–8371. [Google Scholar] [CrossRef] [PubMed]
- Evich, M.G.; Davis, M.J.B.; Mccord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Recent progress and challenges on the removal of per- and poly-fluoroalkyl substances (pfas) from contaminated soil and water. Environ. Sci. Pollut. Res. 2022, 29, 58405–58428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Wang, M.; He, Q.; Niu, X.; Liang, Y. Distribution of perfluoroalkyl substances (pfass) in aquatic plant-based systems: From soil adsorption and plant uptake to effects on microbial community. Environ. Pollut. 2020, 257, 113575. [Google Scholar] [CrossRef]
- Zhi, Y.; Liu, J. Sorption and desorption of anionic, cationic and zwitterionic polyfluoroalkyl substances by soil organic matter and pyrogenic carbonaceous materials. Chem. Eng. J. 2018, 346, 682–691. [Google Scholar] [CrossRef]
- Xu, B.; Qiu, W.; Du, J.; Wan, Z.; Zhou, J.L.; Chen, H.; Liu, R.; Magnuson, J.T.; Zheng, C. Translocation, bioaccumulation, and distribution of perfluoroalkyl and polyfluoroalkyl substances (pfass) in plants. iScience 2022, 25, 104061. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xiao, R.; Du, L.; Zhang, G.; Yin, L.; Deng, R.; Wang, G. Phytoremediation of poly- and perfluoroalkyl substances: A review on aquatic plants, influencing factors, and phytotoxicity. J. Hazard. Mater. 2021, 418, 126314. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, Y.; Tang, Y.; Gong, H.; Guo, F.; Sun, W.; Liu, S.; Tan, H.; Chen, F. Antioxidant defence system is responsible for the toxicological interactions of mixtures: A case study on pfos and pfoa in daphnia magna. Sci. Total Environ. 2019, 667, 435–443. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, Y.; Chang, S.; Zhao, Z.; Zhao, Y.; Yuan, X.; Wu, F.; Sun, H. Occurrence and phase distribution of neutral and ionizable per- and polyfluoroalkyl substances (pfass) in the atmosphere and plant leaves around landfills: A case study in tianjin, china. Environ. Sci. Technol. 2018, 52, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, G.; Rao, Z.; Guo, F.; Li, Z.; Xie, F.; Tan, H. Occurrence and incidence of 18 per- and polyfluoroalkyl compounds in edible oils commonly consumed in guiyang, china. Food Addit. Contam. Part A-Chem. 2017, 34, 1573–1583. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, J.; Ye, T.; Li, X.; Gao, K.; Xue, Q.; Lv, J.; Zhang, A.; Fu, J. Occurrence, profiles, and ecotoxicity of poly- and perfluoroalkyl substances and their alternatives in global apex predators: A critical review. J. Environ. Sci. 2021, 109, 219–236. [Google Scholar] [CrossRef]
- Ng, C.A.; Hungerbuehler, K. Exploring the use of molecular docking to identify bioaccumulative perfluorinated alkyl acids (pfaas). Environ. Sci. Technol. 2015, 49, 12306–12314. [Google Scholar] [CrossRef]
- Li, X.; Gao, K.; Dong, S.; Liu, X.; Fu, K.; Wang, P.; Zhang, A.; Su, X.; Fu, J. Length-specific occurrence and profile of perfluoroalkyl acids (pfaas) in animal protein feeds. J. Hazard. Mater. 2019, 373, 224–231. [Google Scholar] [CrossRef]
- Barhoumi, B.; Sander, S.G.; Driss, M.R.; Tolosa, I. Survey of legacy and emerging per- and polyfluorinated alkyl substances in mediterranean seafood from a north african ecosystem. Environ. Pollut. 2022, 292, 118398. [Google Scholar] [CrossRef]
- Miranda, D.D.A.; Peaslee, G.F.; Zachritz, A.M.; Lamberti, G.A. A worldwide evaluation of trophic magnification of per- and polyfluoroalkyl substances in aquatic ecosystems. Integr. Environ. Assess. Manag. 2022, 18, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lu, Y.; Su, H.; Su, C.; Johnson, A.C.; Yu, L.; Jenkins, A. Managing health risks of perfluoroalkyl acids in aquatic food from a river-estuary-sea environment affected by fluorochemical industry. Environ. Int. 2020, 138, 105621. [Google Scholar] [CrossRef] [PubMed]
- Valdersnes, S.; Nilsen, B.M.; Breivik, J.F.; Borge, A.; Maage, A. Geographical trends of pfas in cod livers along the norwegian coast. PLoS ONE 2017, 12, e177947. [Google Scholar] [CrossRef]
- Groffen, T.; Wepener, V.; Malherbe, W.; Bervoets, L. Distribution of perfluorinated compounds (pfass) in the aquatic environment of the industrially polluted vaal river, south africa. Sci. Total Environ. 2018, 627, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, J.; Xing, Z.; Li, S.; Liu, Z.; Tong, Y. Occurrence, distribution, and risk assessment of perfluoroalkyl acids (pfaas) in muscle and liver of cattle in xinjiang, china. Int. J. Environ. Res. Public Health 2017, 14, 970. [Google Scholar] [CrossRef]
- Sadia, M.; Yeung, L.W.Y.; Fiedler, H. Trace level analyses of selected perfluoroalkyl acids in food: Method development and data generation. Environ. Pollut. 2020, 263, 113721. [Google Scholar] [CrossRef]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and polyfluoroalkyl substances (pfas) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar] [CrossRef]
- Pasecnaja, E.; Bartkevics, V.; Zacs, D. Occurrence of selected per- and polyfluorinated alkyl substances (pfass) in food available on the european market-a review on levels and human exposure assessment. Chemosphere 2022, 287, 132378. [Google Scholar] [CrossRef]
- Kedikoglou, K.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L. Preliminary assessment of general population exposure to perfluoroalkyl substances through diet in Greece. Environ. Res. 2019, 177, 108617. [Google Scholar] [CrossRef]
- Sungur, Ş.; Köroğlu, M.; Turgut, F. Determination of perfluorooctanoic acid (pfoa) and perfluorooctane sulfonic acid (pfos) in food and beverages. Int. J. Environ. Anal. Chem. 2018, 98, 360–368. [Google Scholar] [CrossRef]
- Sznajder-Katarzyńska, K.; Surma, M.; Wiczkowski, W.; Cieślik, E. The perfluoroalkyl substance (pfas) contamination level in milk and milk products in poland. Int. Dairy J. 2019, 96, 73–84. [Google Scholar] [CrossRef]
- Li, X.; Dong, S.; Zhang, W.; Fan, X.; Wang, R.; Wang, P.; Su, X. The occurrence of perfluoroalkyl acids in an important feed material (fishmeal) and its potential risk through the farm-to-fork pathway to humans. J. Hazard. Mater. 2019, 367, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yin, Y.; Wang, P.; Su, X. Occurrence of some legacy and emerging contaminants in feed and food and their ranking priorities for human exposure. Chemosphere 2023, 321, 138117. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Li, Y.; Hao, Y.; Li, J.; Zhang, L.; Wang, P.; Yin, Y.; Zhang, S.; Li, T.; et al. Occurrence of per- and polyfluoroalkyl substances (pfass) in raw milk and feed from nine chinese provinces and human exposure risk assessment. Chemosphere 2022, 300, 134521. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, L.S.; Hoskins, T.D.; Gharehveran, M.M.; Sepúlveda, M.S. Occurrence and implications of per and polyfluoroalkyl substances in animal feeds used in laboratory toxicity testing. Sci. Total Environ. 2023, 867, 161583. [Google Scholar] [CrossRef] [PubMed]
- Van Doosselaere, P. Production of oils. In Edible Oil Processing, 2nd ed.; Hamm, W., Hamilton, R.J., Calliauw, G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 55–96. [Google Scholar]
- Begley, T.H.; White, K.; Honigfort, P.; Twaroski, M.L.; Neches, R.; Walker, R.A. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit Contam 2005, 22, 1023–1031. [Google Scholar] [CrossRef]
- Yang, L. Study on Analysis Methodology of Perfluorinated Compounds in Edible Oils and Its Application. Master’s Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- De Greyt, W. Edible oil refining: Current and future technologies. In Edible Oil Processing, 2nd ed.; Hamm, W., Hamilton, R.J., Calliauw, G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 127–151. [Google Scholar]
- Vaisali, C.; Charanyaa, S.; Belur, P.D.; Regupathi, I. Refining of edible oils: A critical appraisal of current and potential technologies. Int. J. Food Sci. Technol. 2015, 50, 13–23. [Google Scholar] [CrossRef]
- Babin, H.; Dickinson, E.; Chisholm, H.; Beckett, S. Interactions in dispersions of sugar particles in food oils: Influence of emulsifier. Food Hydrocoll. 2005, 19, 513–520. [Google Scholar] [CrossRef]
- Johansson, D.; Bergenståhl, B. The influence of food emulsifiers on fat and sugar dispersions in oils. Iii. Water content, purity of oils. J. Am. Oil Chem. Soc. 1992, 69, 728–733. [Google Scholar] [CrossRef]
- Tereshchuk, L.V.; Starovoytova, K.V.; Ivashina, O.A. Practical aspects of the use of emulsifiers in manufacturing emulsion fat-and-oil products. Food Raw Mater. 2018, 6, 30–39. [Google Scholar] [CrossRef]
- Xu, Y.; Noonan, G.O.; Begley, T.H. Migration of perfluoroalkyl acids from food packaging to food simulants. Food Addit. Contam. Part A-Chem. 2013, 30, 899–908. [Google Scholar] [CrossRef]
- Begley, T.H.; Hsu, W.; Noonan, G.; Diachenko, G. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam. Part A-Chem. 2008, 25, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M.; Fengler, R.; Mbog, G.; Nguyen, K.H.; Granby, K. Food simulants and real food-what do we know about the migration of pfas from paper based food contact materials? Food Packag. Shelf Life 2023, 35, 100992. [Google Scholar] [CrossRef]
- Zabaleta, I.; Blanco-Zubiaguirre, L.; Baharli, E.N.; Olivares, M.; Prieto, A.; Zuloaga, O.; Elizalde, M.P. Occurrence of per- and polyfluorinated compounds in paper and board packaging materials and migration to food simulants and foodstuffs. Food Chem. 2020, 321, 126746. [Google Scholar] [CrossRef] [PubMed]
- Poothong, S.; Boontanon, S.K.; Boontanon, N. Determination of perfluorooctane sulfonate and perfluorooctanoic acid in food packaging using liquid chromatography coupled with tandem mass spectrometry. J. Hazard. Mater. 2012, 205–206, 139–143. [Google Scholar] [CrossRef]
- Yuan, J.; Ye, L.; Zhang, J.; Du, X.; Ma, A.; Pan, J. Nonaqueous electroextraction with tunable selectivity for direct, fast, and exhaustive enrichment of per- and polyfluoroalkyl acids from oils and food contact materials. Anal. Chem. 2022, 94, 15663–15670. [Google Scholar] [CrossRef]
- Guillén, M.D.; Sopelana, P.; Palencia, G. Polycyclic aromatic hydrocarbons and olive pomace oil. J. Agric. Food Chem. 2004, 52, 2123–2132. [Google Scholar] [CrossRef]
- Zhou, R.; Jiang, J.; Mao, T.; Zhao, Y.; Lu, Y. Multiresidue analysis of environmental pollutants in edible vegetable oils by gas chromatography-tandem mass spectrometry. Food Chem. 2016, 207, 43–50. [Google Scholar] [CrossRef]
- Toptancı, I.; Ketenoglu, O.; Kıralan, M. Assessment of the migration of perfluorinated compounds and primary aromatic amines from ptfe-coated non-stick cookware marketed in turkey. Environ. Sci. Pollut. Res. 2022, 29, 38535–38549. [Google Scholar] [CrossRef]
- Yuan, G.; Peng, H.; Huang, C.; Hu, J. Ubiquitous occurrence of fluorotelomer alcohols in eco-friendly paper-made food-contact materials and their implication for human exposure. Environ. Sci. Technol. 2016, 50, 942–950. [Google Scholar] [CrossRef]
- Gebbink, W.A.; Berger, U.; Cousins, I.T. Estimating human exposure to pfos isomers and pfca homologues: The relative importance of direct and indirect (precursor) exposure. Environ. Int. 2015, 74, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Alapaty, K.; Zartarian, V.; Poulakos, A.; Strynar, M.; Buckley, T. Per- and polyfluoroalkyl substances exposure science: Current knowledge, information needs, future directions. Int. J. Environ. Sci. Technol. 2022, 19, 10393–10408. [Google Scholar] [CrossRef]
- Haug, L.S.; Salihovic, S.; Jogsten, I.E.; Thomsen, C.; van Bavel, B.; Lindström, G.; Becher, G. Levels in food and beverages and daily intake of perfluorinated compounds in norway. Chemosphere 2010, 80, 1137–1143. [Google Scholar] [CrossRef]
- Noorlander, C.W.; van Leeuwen, S.P.J.; Te Biesebeek, J.D.; Mengelers, M.J.B.; Zeilmaker, M.J. Levels of perfluorinated compounds in food and dietary intake of pfos and pfoa in the netherlands. J. Agric. Food Chem. 2011, 59, 7496–7505. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Rubio, S.; van Leeuwen, S. Tetrahydrofuran-water extraction, in-line clean-up and selective liquid chromatography/tandem mass spectrometry for the quantitation of perfluorinated compounds in food at the low picogram per gram level. J. Chromatogr. A 2010, 1217, 5913–5921. [Google Scholar] [CrossRef]
- Domingo, J.L.; Jogsten, I.E.; Eriksson, U.; Martorell, I.; Perelló, G.; Nadal, M.; Bavel, B.V. Human dietary exposure to perfluoroalkyl substances in catalonia, spain. Temporal trend. Food Chem. 2012, 135, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Suominen, K.; Hallikainen, A.; Ruokojärvi, P.; Airaksinen, R.; Koponen, J.; Rannikko, R.; Kiviranta, H. Occurrence of pcdd/f, pcb, pbde, pfas, and organotin compounds in fish meal, fish oil and fish feed. Chemosphere 2011, 85, 300–306. [Google Scholar] [CrossRef]
- Vestergren, R.; Berger, U.; Glynn, A.; Cousins, I.T. Dietary exposure to perfluoroalkyl acids for the swedish population in 1999, 2005 and 2010. Environ. Int. 2012, 49, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Perra, G.; Corsolini, S.; Focardi, S.E. Pilot study on levels of perfluorooctane sulfonic acid (pfos) and perfluorooctanoic acid (pfoa) in selected foodstuffs and human milk from Italy. Food Chem. 2013, 140, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Vavrouš, A.; Ševčík, V.; Dvořáková, M.; Čabala, R.; Moulisová, A.; Vrbík, K. Easy and inexpensive method for multiclass analysis of 41 food contact related contaminants in fatty food by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2019, 67, 10968–10976. [Google Scholar] [CrossRef]
- Tang, C.; Tan, J.; Wang, C.; Peng, X. Determination of perfluorooctanoic acid and perfluorooctane sulfonate in cooking oil and pig adipose tissue using reversed-phase liquid-liquid extraction followed by high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1341, 50–56. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, T.; Liu, H.; Li, Y.; You, J. New stable isotope labeling strategy in quaternary ammonium-functionalized magnetic nanoparticles for the analysis of perfluorocarboxylic acid in cod liver oil. Food Anal. Meth. 2019, 12, 1771–1780. [Google Scholar] [CrossRef]
- Han, Y.; Fan, C.; Yin, Y.; Shan, Y.; Cao, X. Cooperative hydrogen- and halogen-bonding interaction promoted deep eutectic solvent-functionalized magnetic metal-organic framework for perfluoroalkyl iodides detection in edible oils. Food Control. 2023, 148, 109625. [Google Scholar] [CrossRef]
- Mahoney, H.; Xie, Y.; Brinkmann, M.; Giesy, J.P. Next generation per- and poly-fluoroalkyl substances: Status and trends, aquatic toxicity, and risk assessment. Eco-Environ. Health 2022, 1, 117–131. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbuehler, K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (pfaas) and their precursors: Status quo, ongoing challenges and possible solutions. Environ. Int. 2015, 75, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Z.; Wu, T.; Song, L.; Zhang, Y. Sorafenib-irinotecan sequential therapy augmented the anti-tumor efficacy of monotherapy in hepatocellular carcinoma cells hepg2. Neoplasma 2015, 62, 172–179. [Google Scholar] [CrossRef]
- Carlsson, P.; Herzke, D.; Kallenborn, R. Polychlorinated biphenyls (pcbs), polybrominated diphenyl ethers (pbdes) and perfluorinated alkylated substances (pfass) in traditional seafood items from western greenland. Environ. Sci. Pollut. Res. 2014, 21, 4741–4750. [Google Scholar] [CrossRef]
- Luque De Castro, M.D.; Priego-Capote, F. Ultrasound assistance to liquid–liquid extraction: A debatable analytical tool. Anal. Chim. Acta 2007, 583, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Tu, W.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Gure, A.; Megersa, N.; Retta, N. Ion-pair assisted liquid-liquid extraction for selective separation and analysis of multiclass pesticide residues in environmental waters. Anal. Methods 2014, 6, 4633–4642. [Google Scholar] [CrossRef]
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and opportunities of experimental design for the dispersive liquid-liquid microextraction method—A review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef]
- Perovani, I.S.; Barbetta, M.F.S.; Duarte, L.O.; de Oliveira, A.R.M. Determination of polyfluoroalkyl substances in biological matrices by chromatography techniques: A review focused on the sample preparation techniques-review. J. Chromatogr. Open 2023, 3, 100082. [Google Scholar] [CrossRef]
- Grau, J.; Azorín, C.; Benedé, J.L.; Chisvert, A.; Salvador, A. Use of green alternative solvents in dispersive liquid-liquid microextraction: A review. J. Sep. Sci. 2022, 45, 210–222. [Google Scholar] [CrossRef] [PubMed]
- So, M.K.; Taniyasu, S.; Lam, P.K.S.; Zheng, G.J.; Giesy, J.P.; Yamashita, N. Alkaline digestion and solid phase extraction method for perfluorinated compounds in mussels and oysters from south china and japan. Arch. Environ. Contam. Toxicol. 2006, 50, 240–248. [Google Scholar] [CrossRef]
- Kuklenyik, Z.; Ye, X.; Needham, L.L.; Calafat, A.M. Automated solid-phase extraction approaches for large scale biomonitoring studies. J. Chromatogr. Sci. 2009, 47, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Islas, G.; Ibarra, I.S.; Hernandez, P.; Miranda, J.M.; Cepeda, A. Dispersive solid phase extraction for the analysis of veterinary drugs applied to food samples: A review. Int. J. Anal. Chem. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Aguirre, M.Á.; Canals, A. Magnetic deep eutectic solvents in microextraction techniques. TrAC Trends Anal. Chem. 2022, 146, 116500. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, G.; Gao, M.; Huang, X.; Xu, D. Recent advances and applications of magnetic metal-organic frameworks in adsorption and enrichment removal of food and environmental pollutants. Crit. Rev. Anal. Chem. 2020, 50, 472–484. [Google Scholar] [CrossRef]
- Sarraf, M.; Beig-Babaei, A.; Naji-Tabasi, S. Application of quechers method for extraction of functional compounds. SN Appl. Sci. 2020, 2, 1858. [Google Scholar] [CrossRef]
- Wu, R.; Lin, H.; Yamazaki, E.; Taniyasu, S.; Sörengård, M.; Ahrens, L.; Lam, P.K.S.; Eun, H.; Yamashita, N. Simultaneous analysis of neutral and ionizable per- and polyfluoroalkyl substances in air. Chemosphere 2021, 280, 130607. [Google Scholar] [CrossRef]
- Rehman, A.U.; Crimi, M.; Andreescu, S. Current and emerging analytical techniques for the determination of pfas in environmental samples. Trends Environ. Anal. Chem. 2023, 37, e198. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, P.; Xiong, J.; Gao, L.; Tan, K. A new dual-recognition strategy for hybrid ratiometric and ratiometric sensing perfluorooctane sulfonic acid based on high fluorescent carbon dots with ethidium bromide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117362. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, J.; Su, Y.; Zhao, H. A facile solvothermal synthesis of 3d magnetic mos2/fe3o4 nanocomposites with enhanced peroxidase-mimicking activity and colorimetric detection of perfluorooctane sulfonate. Microchem J. 2019, 149, 104019. [Google Scholar] [CrossRef]
- Ranaweera, R.; Ghafari, C.; Luo, L. Bubble-nucleation-based method for the selective and sensitive electrochemical detection of surfactants. Anal. Chem. 2019, 91, 7744–7748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Fang, T.; Zhang, L.; Gong, J. Disposable photoelectrochemical sensing strip for highly sensitive determination of perfluorooctane sulfonyl fluoride on functionalized screen-printed carbon electrode. Talanta 2018, 181, 147–153. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Recent advances in photoelectrochemical sensing: From engineered photoactive materials to sensing devices and detection modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]



| PFASs | Classification | R | Examples | Structural Formula | CAS |
|---|---|---|---|---|---|
| Ionic PFASs | Perfluoroalkyl carboxylic acid, PFCAs | –COOH | Pentadecafluorooctanoic acid, PFOA |  | 335-67-1 |
| Perfluoroalkane sulfonic acids, PFSAs | –SO3H | Perfluorooctane sulfonic acid, PFOS | 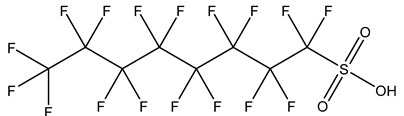 | 1763-23-1 | |
| Perfluoroalkane sulfinic acids, PFSIAs | –SO2H | Perfluorooctane sulfinic acid | 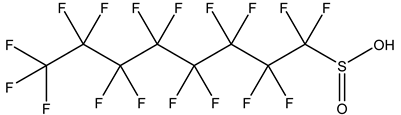 | 647-29-0 | |
| Perfluoroalkyl phosphonic acids, PFPAs | –P(=O)(OH)2 | Perfluorooctyl phosphonic acid, PFOPA | 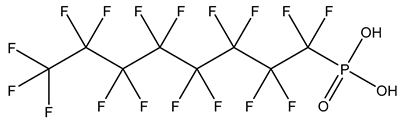 | 40143-78-0 | |
| Perfluoroalkyl phosphinic acids, PFPIAs | –P(=OH)(CnF2n+1) | Bis(heptadecafluorooctyl) phosphinic acid | 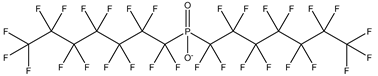 | 500776-69-2 | |
| Perfluoroalkane sulfonamides, FASAs | –SO2NH2 | Perfluorooctane sulfonamide, FOSA | 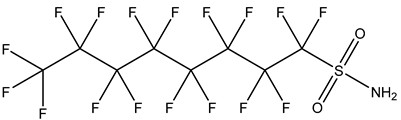 | 754-91-6 | |
| Non-Ionic PFASs | Perfluoroalkane sulfonyl fluorides, PASFs | –SO2F | Perfluorooctane sulfonyl fluoride, PFOSF | 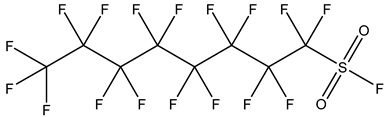 | 307-35-7 |
| Perfluoroalkanoyl fluorides, PAFs | –COF | Perfluorooctanoyl fluoride | 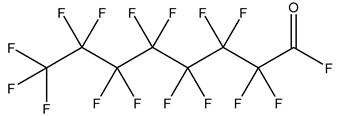 | 335-66-0 | |
| Perfluoroalkyl iodides, PFAIs | –I | Perfluorooctyl iodide, PFOI | 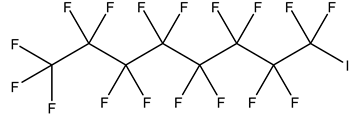 | 507-63-1 | |
| Perfluoroalkyl aldehydes, PFALs | –CHO | (Perfluorooctane)-1-carbaldehydle | 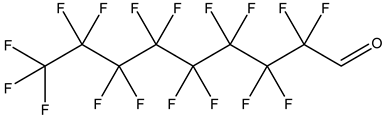 | 63967-40-8 | |
| Perfluoroalkyl esters, PFEs | –COOR | Perfluorooctanoic acid methyl ester |  | 376-27-2 | |
| Fluorotelomer alcohol, FTOHs | –OH | 1H,1H,2H,2H-Perfluorooctanol, 6:2 FTOH |  | 647-42-7 |
| District | Year | Regulation | Restriction | Reference |
|---|---|---|---|---|
| Global | 2009 | Stockholm Convention on POPs | PFOS and its salts were listed in Annex B (restriction). | [44] |
| 2019 | Stockholm Convention on POPs | PFOA, its salts, and PFOA-related compounds were listed in Annex A (elimination). | [45] | |
| 2022 | Stockholm Convention on POPs | PFHxS, its salts, and PFHxS-related compounds were listed in Annex A (elimination). | [46] | |
| Europe | 2008 | EFSA | The tolerable daily intake (TDI) of 150 ng kg−1 bw.d−1 (body weight/day) for PFOS and 1500 ng kg−1 bw.d−1 for PFOA was established. | [47] |
| 2018 | EFSA | The tolerable weekly intake (TWI) for PFOS is 13 ng kg−1 bw. wk−1 (body weight/per week) and for PFOA is 6 ng kg−1 bw. wk −1. | [48] | |
| 2020 | EFSA | The TWI of 4.4 ng kg−1 b.w. for the sum of PFOA, perfluorononanoic acid (PFNA), PFHxS, and PFOS was suggested. | [49] | |
| 2023 | EU | The maximum levels for the sum of PFOS, PFOA, PFNA, and PFHxS in meat and eggs were set to be 1.3–45 μg kg−1 wet weight. | [50] | |
| USA | 2009 | USEPA | The minimum risk levels of PFOA and PFOS in drinking water were set to be 0.4 and 0.2 μg L−1. | [51] |
| 2015 | USEPA | The minimum risk levels of PFOA and PFOS in drinking water were set to be 0.07 μg L−1. When both PFOA and PFOS are found in drinking water, the combined concentrations of PFOA and PFOS should be below 0.07 μg L−1. | [52] | |
| 2021 | TPCH | TPCH has proclaimed that packing materials and their components must not incorporate PFASs. | [54] | |
| 2021 | FDA | The FDA declared it would phase out certain short-chain PFASs in the food market by 2024. | [55] | |
| 2022 | USEPA | The minimum risk levels of PFOA, PFOS, GenX chemicals, and PFBS in drinking water were advised to be 0.000004, 0.00002, 0.01, and 2 μg L−1. | [53] | |
| 2023 | USEPA | The USEPA announced the proposed action of PFOS, PFOA, PFHxS, PFNA, GenX chemicals, and PFBS into the National Primary Drinking Water Regulation (NPDWR). | [58] | |
| Asia | 2019 | MEPC | Prohibit the production, circulation, use, and import or export of PFOS, its salts, and PFOSF except for acceptable uses. | [59] |
| 2023 | MEPC | PFOS, its salts, PFOSF, PFOA, its salts, PFOA-related compounds, PFHxS, its salts, and PFHxS-related compounds were included in the list of key regulated new pollutants (version 2023). | [56] | |
| 2021 | CSCL | PFOA and its salts were added to the list of Class I Specified Chemical Substances (the import, manufacture, or sale of products containing Class I substances is prohibited). | [57] |
| District | Matrix | PFSAs | PFCAs | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C4a | C6a | C7a | C8a | C4b | C5 | C6b | C7b | C8b | C9 | C10 | C11 | C12 | C13 | C14 | C16 | C18 | |||
| Norway | Margarine | <1.6 | 1.3 | - | 2.3 | 51 | 51 | 2.5 | <5.6 | 12 | <13 | <8.6 | <16 | <16 | - | - | - | - | [109] |
| Netherlands | Vegetable oil | <0.9 | <2 | - | <3 | <32 | <28 | <3 | 1 | <3 | <0.1 | <0.6 | <2 | <1 | - | - | - | - | [110] |
| Butter | <3 | 16 | - | 33 | <31 | <43 | 20 | 5 | 16 | 2 | 6 | <3 | 2 | - | - | - | - | ||
| Spain | Sunflower oil | - | ND | - | ND | ND | - | ND | - | ND | ND | ND | ND | - | - | - | - | - | [111] |
| Olive oil | <1.2 | <0.7 | - | 1.1 | <43 | <5.9 | <14 | <140 | <140 | <38 | <3.8 | <14 | <4.2 | <6.1 | <6.2 | <48 | <41 | [112] | |
| Finland | Fish oil | - | - | - | ND | - | - | - | - | ND | - | - | ND | - | ND | ND | - | - | [113] |
| Sweden | Fats (butter, margarine, cooking oil, and mayonnaise) | - | <2.3 | - | 13 | - | - | 4.3 | <2.3 | <5.4 | <3.0 | <3.6 | 5.8 | <2.3 | <2.3 | <2.3 | - | - | [114] |
| Butter | - | 2.6–18 | - | 8.1–21.3 | - | - | - | - | 8.1–56 | - | - | - | - | - | - | - | - | [79] | |
| Italy | Olive oil | - | - | - | <500 | - | - | - | - | <500 | - | - | - | - | - | - | - | - | [115] |
| Peanut oil | - | - | - | <500 | - | - | - | - | <500 | - | - | - | - | - | - | - | - | ||
| Czech Republic | Edible oils | ND-1600 | ND-1800 | - | ND-1700 | - | - | ND-1400 | ND-2700 | ND-1500 | ND-2400 | ND-1700 | ND-1700 | ND-2100 | - | - | - | - | [116] |
| Butter | ND-1600 | ND-1800 | - | ND-1700 | - | - | ND-1400 | ND-2700 | ND-1500 | ND-2400 | ND-1700 | ND-1700 | ND-2100 | - | - | - | - | ||
| China Guangzhou | Cooking oil | - | - | - | ND-20 pg mL−1 | - | - | - | - | ND-20 pg mL−1 | - | - | - | - | - | - | - | - | [117] |
| China Guiyang | Rapeseed oil | - | 140 | ND | 390 | 130 | 120 | - | - | 90 | 940 | - | - | 90 | - | - | 100 | 160 | [69] |
| Blended oil | - | 240 | ND | 450 | 110 | ND | - | - | 80 | 1770 | - | - | ND | - | - | ND | ND | ||
| Peanut oil | - | 170 | ND | 290 | ND | ND | - | - | ND | ND | - | - | ND | - | - | ND | ND | ||
| Corn oil | - | 120 | ND | 210 | ND | ND | - | - | ND | ND | - | - | ND | - | - | 110 | 140 | ||
| Sunflower oil | - | ND | ND | 220 | ND | ND | - | - | ND | ND | - | - | ND | - | - | ND | ND | ||
| Lard oil | - | 230 | 210 | 330 | ND | ND | - | - | - | 710 | - | - | 90 | - | - | 100 | ND | ||
| Beef tallown | - | 130 | ND | 290 | ND | ND | - | - | - | ND | - | - | ND | - | - | 110 | ND | ||
| China Beijing | Blended oil | - | - | ND | - | - | - | ND-60 | ND | ND-430 | ND-4640 | ND | - | - | - | - | - | - | [60] |
| Soybean oil | - | - | ND-20 | - | - | - | ND-440 | ND-40 | 130–160 | 20–470 | ND-40 | - | - | - | - | - | - | ||
| Peanut oil | - | - | ND | - | - | - | 80–100 | ND | 180–240 | ND | ND | - | - | - | - | - | - | ||
| Sesame oil | - | - | 20–30 | - | - | - | ND-490 | 50–80 | 150–500 | 30–1060 | 160–510 | - | - | - | - | - | - | ||
| Corn oil | - | - | ND | - | - | - | ND-450 | ND | 150–170 | ND-20 | ND-40 | - | - | - | - | - | - | ||
| Sunflower oil | - | - | ND | - | - | - | 400–500 | ND | 400–500 | ND | 400–600 | - | - | - | - | - | - | ||
| Olive oil | - | - | - | <1.6 | - | ND | - | ND | - | ND | - | ND | [35] | ||||||
| Sesame oil | - | - | - | 405 | - | 112 | - | 2018 | - | 2458 | - | 1841 | |||||||
| Corn oil | - | - | - | ND | - | ND | - | <0.5 | - | 17 | - | 7 | |||||||
| Camellia seed oil | - | - | - | <1.6 | - | 57 | - | 21 | - | 9 | - | 5 | |||||||
| Soybean oil | - | - | - | ND | - | ND | - | ND | - | 8 | - | 7 | |||||||
| Blended oil (80% Corn; 20% sesame) | - | - | - | 432 | - | ND | - | 8 | - | ND | - | ND | |||||||
| Blended oil (70% Corn; 30% sesame) | - | - | - | 588 | - | 37 | - | 21 | - | 6 | - | <0.3 | |||||||
| Vegetable oil | - | - | - | ND | - | ND | - | <0.5 | - | ND | - | ND | |||||||
| China Shandong | Cod liver oil | - | - | - | - | - | - | ND | 2100–6200 | 40,000 | 9000 | 8200 | 3400–5800 | 3400–6300 | [118] | ||||
| Fish Oil | - | - | - | - | - | - | ND | ND | ND | ND | ND | ND | ND | - | - | - | - | ||
| Poland | Sunflower oil | ND | ND | - | ND | 1062 | ND | ND | ND | 640 | 565 | ND | - | - | - | - | - | - | [30] |
| Rapeseed oil | 3 | ND | - | 16 | ND | ND | ND | 250 | 110 | ND | ND | - | - | - | - | - | - | ||
| Olive oil | ND | ND | - | ND | 962 | ND | 49 | ND | 27 | ND | ND | - | - | - | - | - | - | ||
| Margarine | ND | ND | - | ND | ND | ND | ND | ND | 250 | ND | ND | - | - | - | - | - | - | ||
| Mix of margarine and butter | ND | ND | - | ND | ND | ND | ND | ND | 270 | ND | ND | - | - | - | - | - | - | ||
| Extraction | Extraction Solvent/Material | Clean-Up | Clean-Up Material | Recovery (%) | Analytical Instrumentation | LOD (pg g–1) | Reference |
|---|---|---|---|---|---|---|---|
| LLE | Basified water/methanol, dichloromethane | - | - | - | LC-MS/MS | 10–2500 pg mL– 1 | [117] |
| Acetonitrile, n-pentane | SPE | DSC-18 SPE cartridge (Sigma-Aldrich) | 37–120 | HPLC-MS/MS | 1400–2700 | [116] | |
| Acetonitrile | GPC+DSPE | C18, GCB | 60–129 | LC-ES-MS/MS | 4–400 | [60] | |
| Acetonitrile | d-SPE | ENV SPE bulk sorbent | 72–104 | micro-HPLC-MS/MS | 2–75 | [30] | |
| Basified methanol/water (1:1, v/v, containing 1% NH3H2O) | MSPE | Fe3O4@SiO2@ Quaternary ammonium (QTA) | 85.0–98.5 | LC-MS/MS | 1.5–20 | [118] | |
| Methanol | MSPE | Fe3O4@UiO-66-NH2@DES | 74.9–111 | GC-MS/MS | 2.81–34.3 | [119] | |
| DLLME | Superparamagnetic nanofluid | DES based nano Fe3O4 fluid | 90–109 | UPLC-QTOF-MS | 0.3–1.6 | [35] | |
| Ion pair extraction | MTBE | SPE | Florisil and graphitised carbon | 62–91 | UPLC-ESI-MS/MS | 2.3–5.4 | [114] |
| MTBE | - | - | - | HPLC-ESI-MS/MS | - | [115] | |
| Alkaline digestion | Sodium hydroxide in methanol | SPE | WAX, ENVI-carb | - | UPLC-ESI- MS/MS | - | [109,112] |
| LSE | Tetrahydrofuran and water | SPE | A weak anion exchange resin and ENVI-carb | - | LC-EI-MS/MS | - | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Cao, X. Research Progress of Perfluoroalkyl Substances in Edible Oil—A Review. Foods 2023, 12, 2624. https://doi.org/10.3390/foods12132624
Han Y, Cao X. Research Progress of Perfluoroalkyl Substances in Edible Oil—A Review. Foods. 2023; 12(13):2624. https://doi.org/10.3390/foods12132624
Chicago/Turabian StyleHan, Yingyi, and Xueli Cao. 2023. "Research Progress of Perfluoroalkyl Substances in Edible Oil—A Review" Foods 12, no. 13: 2624. https://doi.org/10.3390/foods12132624
APA StyleHan, Y., & Cao, X. (2023). Research Progress of Perfluoroalkyl Substances in Edible Oil—A Review. Foods, 12(13), 2624. https://doi.org/10.3390/foods12132624