Wild Fruits of Crataegus monogyna Jacq. and Sorbus aria (L.) Crantz: From Traditional Foods to Innovative Sources of Pigments and Antioxidant Ingredients for Food Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Methods
2.2.1. Characterization of Fresh Fruits
2.2.2. Analysis of Dried Fruit Epidermis
- Color
- Q-Total Phenolic Content
- Q-Hydroxybenzoic acids
- Q-Hydroxycinnamic acids
- Q-Flavonols
- Q-Total monomeric anthocyanins
- In vitro antioxidant capacity
2.2.3. Individual Anthocyanin Profile
- Extraction procedure
- Identification of anthocyanins in the extracts by HPLC/MS
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Fresh Fruits
3.2. Color
3.3. Phenolic Compounds Content
3.4. In Vitro Antioxidant Capacity
3.5. Characterization of Anthocyanins in Extracts Obtained from the Epidermis of the Fruits
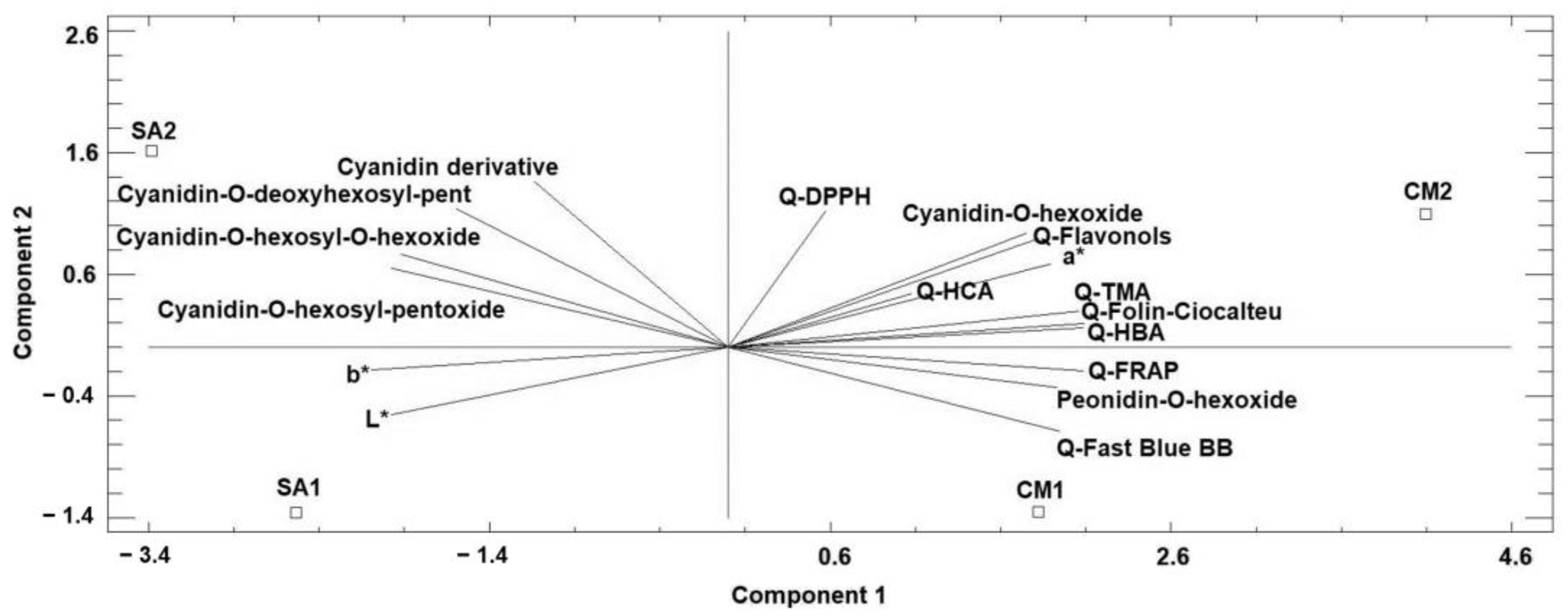
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Lourenço-Lopez, C.; Jimenes-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Scientific basis for the industrialization of traditionally used plants of the Rosaceae family. Food Chem. 2020, 330, 127–197. [Google Scholar] [CrossRef] [PubMed]
- Tardío, J.; Sánchez-Mata, M.C.; Morales, R.; Molina, M.; García-Herrera, P.; Morales, P.; Díez-Marqués, C.; Fernández-Ruiz, V.; Cámara, M.; Pardo-de-Santayana, M.; et al. Ethnobotanical and food composition monographs of selected Mediterranean wild edible plants. In Book Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables, 1st ed.; Sánchez-Mata, M.C., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 273–470. [Google Scholar]
- Natić, M.; Pavlović, A.; Bosco, F.L.; Stanisavljević, N.S.; Dabić-Zagorac, D.; Fotirić-Akšić, M.M.; Papetti, A. Nutraceutical properties and phytochemical characterization of wild Serbian fruits. Eur. Food Res. Technol. 2019, 245, 469–478. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; de Ancos, B.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Tardío, J. Wild blackthorn (Prunus spinosa L.) and hawthorn (Crataegus monogyna Jacq.) fruits as valuable sources of antioxidants. Foods 2014, 69, 61–73. [Google Scholar]
- Pawlaczyk-Graja, I. Polyphenolic-polysaccharide conjugates from flowers and fruits of single-seeded hawthorn (Crataegus monogyna Jacq.): Chemical profiles and mechanisms of anticoagulant activity. Int. J. Biol. Macromol. 2018, 116, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Dordević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef]
- Obikeze, K.; Garth, G.; Maajidah, M.; Mbewe, R.; Palmer, M.; Wilsnach, N. Crataegus monogyna and Centella asiatica Extracts as Inhibitors of Cathepsin S. Adv. Pharmacol. Pharm. 2022, 10, 247–252. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Khaneghah, A.M.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [Green Version]
- Mikulic-Petkovsek, M.; Krska, B.; Kiprovski, B.; Veberic, R. Bioactive Components and Antioxidant Capacity of Fruits from Nine Sorbus Genotypes. J. Food Sci. 2017, 82, 647–658. [Google Scholar] [CrossRef]
- Petkova, N.T.; Ognyanov, M.H.; Vrancheva, R.Z.; Zhelev, P. Phytochemical, nutritional and antioxidant characteristics of whitebeam (Sorbus aria) fruits. Acta Sci. Pol. Technol. Aliment. 2020, 19, 219–229. [Google Scholar]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef] [Green Version]
- Ganhão, R.; Estévez, M.; Armenteros, M.; Morcuende, D. Mediterranean Berries as Inhibitors of Lipid Oxidation in Porcine Burger Patties Subjected to Cooking and Chilled Storage. J. Integr. Agric. 2013, 12, 1982–1992. [Google Scholar] [CrossRef]
- Del Pino-García, R.; García-Lomillo, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Adaptation and validation of QUick, Easy, New, CHEap, and Reproducible (QUENCHER) antioxidant capacity assays in model products obtained from residual wine pomace. J. Agric. Food Chem. 2015, 63, 6922–6931. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, 18th ed.; AOAC: Arlington, VA, USA, 2006. [Google Scholar]
- Vega, E.N.; García-Herrera, P.; Ciudad-Mulero, M.; Dias, M.I.; Matallana-González, M.C.; Cámara, M.; Tardía, J.; Molina, M.; Pinela, J.; Pires, T.C.S.P.; et al. Wild sweet cherry, strawberry and bilberry as underestimated sources of natural colorants and bioactive compounds with functional properties. Food Chem. 2023, 414, 135669. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Carbonera, F.; Montanher, P.F.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Optimization of a New Methodology for Determination of Total Phenolic Content in Rice Employing Fast Blue BB and QUENCHER Procedure. J. Braz. Chem. Soc. 2016, 27, 1188–1194. [Google Scholar] [CrossRef]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant phenols in Barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Radish Anthocyanin Extract as a Natural Red Colorant for Maraschino Cherries. J. Food Sci. 1996, 61, 688–694. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1997, 28, 49–55. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primo da Silva, L.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical, and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.; Santos-Buelga, C.; Ferreira, I.C. Chemical characterisation, and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W. Effects of Production and Processing Factors on Major Fruit and Vegetable Antioxidants. J. Food Sci. 2005, 70, R11–R19. [Google Scholar] [CrossRef]
- Egea, I.; Sánchez-Bel, P.; Romojaro, F.; Pretel, M.T. Six edible Wild Fruits as Potential Antioxidant Additives or Nutritional Supplements. Plants Foods Hum. Nutr. 2010, 65, 121–129. [Google Scholar] [CrossRef]
- Rossi, G. Quantification of Total Phenolic, Anthocyanin, and Flavonoid Content in a Diverse Panel of Blueberry Cultivars and Ecotypes. HortScience 2022, 57, 901–909. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, V.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Optimization of Extraction Solvent and Fast Blue BB Assay for Comparative Analysis of Antioxidant Phenolics from Cucumis melo L. Plants 2021, 10, 1379. [Google Scholar] [CrossRef]
- Abuashwashi, M.A.; Palomino, O.M.; Gómez-Serranillos, M.P. Geographic origin influences the phenolic composition and antioxidant potential of wild Crataegus monogyna from Spain. Pharm. Biol. 2016, 11, 2708–2713. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.R.; Tanase, C.; Días, M.I.; Pereira, C.; et al. Development of an Optimized Drying Process for the Recovery of Bioactive Compounds from the Autumn Fruits of Berberis vulgaris L. and Crataegus monogyna Jacq. Antioxidants 2021, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara-Hurtado, M.; Pérez-Rodríguez, M.L. Evaluation of the antioxidant potential of mixed fruit-based beverages: A new insight on the folin-ciocalteu method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Tahirović, A.; Mehić, E.; Kjoseviski, N.; Bašić, N. Phenolics content and antioxidant activity of three Sorbus species. Bull. Chem. Technol. Bosnia Herzeg. 2019, 5, 15–21. [Google Scholar]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, J.C.; Torres-Moreno, H.; Vidal-Gutiérrez, M.; Cabrera-Cabrera, G.G.; Robles-Zepeda, R.E.; Rodríguez-Martínez, K.L.; Ortega-García, J.; Villegas-Ochoa, M.A.; Salazar-López, N.J.; Domínguez-Avila, J.A.; et al. Caesalpinia palmeri: First Report on the Phenolic Compounds Profile, Antioxidant and Cytotoxicity Effect. Chem. Biodivers. 2023, 20, e202200631. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 56–66. [Google Scholar]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant Activity of Synthetic Polymers of Phenolic Compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17. [Google Scholar] [CrossRef]
- Mraihi, F.; Hidalgo, M.; Pascual-Teresa, S.; Trabelsi-Ayadi, M.; Chéif, J.K. Wild grown red and yellow hawthorn fruits from Tunisia as source of antioxidants. Arab. J. Chem. 2015, 8, 570–578. [Google Scholar] [CrossRef]

| Site 1 | Site 2 | |
|---|---|---|
| Crataegus monogyna Jacq. | ||
| Municipality | “Finca El Encín”, | Monte de Valdelatas |
| Sotos del Henares | ||
| Province | Madrid (Spain) | Madrid (Spain) |
| Latitude | 40.517947 | 40.541579 |
| Longitude | −3.297917 | −3.683101 |
| Sorbus aria (L.) Crantz | ||
| Municipality | Zarzuela de Galve, | Puerto de la Quesera, |
| Valverde de los Arroyos | Riofrío de Riaza | |
| Province | Guadalajara (Spain) | Segovia (Spain) |
| Latitude | 41.155864 | 41.219553 |
| Longitude | −3.248964 | −3.418064 |
| Moisture (g/100 g fw) | °Brix | pH | Titratable Acidity (meq of NaOH/100 g fw) | Ripening Index | |
|---|---|---|---|---|---|
| Crataegus monogyna Jacq. (Hawthom) | |||||
| Site 1 Site 2 | 75.1 ± 0.3 c 74.2 ± 0.1 c | 5.33 ± 0.06 a 7.33 ± 0.06 b | 4.55 ± 0.08 c 4.28 ± 0.02 b | 13.50 ± 0.25 c 8.68 ± 0.42 a | 0.004 a 0.008 b |
| Average | 74.6 | 6.33 | 4.42 | 11.09 | 0.006 |
| Range | 74.1–75.4 | 5.0–8.0 | 4.26–4.61 | 8.26–13.76 | 0.004–0.009 |
| Sorbus aria (L.) Crantz (Whitebeam) | |||||
| Site 1 Site 2 | 63.2 ± 0.34 a 65.6 ± 1.48 b | 14.33 ± 0.06 d 9.33 ± 0.06 c | 4.26 ± 0.01 b 4.07 ± 0.01 a | 10.25 ± 0.3 b 8.69 ± 1.13 a | 0.014 d 0.011 c |
| Average | 64.4 | 11.83 | 4.17 | 9.47 | 0.013 |
| Range | 62.9–67.2 | 9.0–15.0 | 4.06–4.26 | 7.71–10.58 | 0.009–0.014 |
| L* | a* | b* | RGB | |
|---|---|---|---|---|
| Crataegus monogyna Jacq. (Hawthom) | ||||
| Site 1 | 57.70 ± 0.05 b | 16.15 ± 0.02 c | 20.65 ± 0.02 b | RGB (177, 127, 103) |
| Site 2 | 51.37 ± 0.02 a | 19.33 ± 0.05 d | 15.88 ± 0.05 a | RGB (162, 110, 96) |
| Sorbus aria (L.) Crantz (Whitebeam) | ||||
| Site 1 | 71.02 ± 0.22 d | 9.06 ± 0.14 a | 29.34 ± 0.08 c | RGB (207, 167, 121) |
| Site 2 | 65.72 ± 0.07 c | 12.74 ± 0.04 b | 29.27 ± 0.04 c | RGB (198, 150, 108) |
| Q-Fast Blue BB (mg GAE/100 g dw) | Q-HBA (mg GAE/100 g dw) | Q-HCA (mg FAE/100 g dw) | Q-Flavonols (mg QE/100 g dw) | Q-TMA (mg cya-3-glu/100 g dw) | |
|---|---|---|---|---|---|
| Crataegus monogyna Jacq. (Hawthom) | |||||
| Site 1 Site 2 | 5965.2 ± 397.1 c 6391.5 ± 228.3 d | 2538.7 ± 217.3 b 3202.6 ± 87.5 c | 481.3 ± 40.2 a 739.3 ± 66.6 c | 491.9 ± 30.9 c 1050.9 ± 102.0 d | 211.6 ± 6.7 c 291.8 ± 13.2 d |
| Average | 6178.4 | 2870.6 | 610.3 | 771.4 | 251.7 |
| Range | 6636.8–5551.9 | 3298.8–2312.5 | 841.0–445.1 | 1177.6–449.6 | 304.9–204.4 |
| Sorbus aria (L.) Crantz (Whitebeam) | |||||
| Site 1 Site 2 | 4056.8 ± 160.3 b 1831.1 ± 115.2 a | 894.0 ± 61.3 a 990.7 ± 36.1 a | 615.0 ± 42.4 b 501.1 ± 43.8 a | 242.1 ± 17.9 a 392.5 ± 34.9 b | 15.5 ± 1.1 a 51.9 ± 2.2 b |
| Average | 2943.9 | 942.3 | 558.1 | 317.3 | 33.7 |
| Range | 4234.0–1701.7 | 1022.9–839.3 | 653.1–432.1 | 440.8–205.2 | 53.9–14.6 |
| Q-DPPH (mg TE/100 g dw) | Q-Folin–Ciocalteu (mg GAE/100 g dw) | Q-FRAP (mg TE/100 g dw) | |
|---|---|---|---|
| Crataegus monogyna Jacq. (Hawthom) | |||
| Site 1 Site 2 | 801.1 ± 30.3 a 947.2 ± 42.6 c | 3036.6 ± 112.1 b 4443.2 ± 157.7 c | 13781.1 ± 639.7 c 15,350.7 ± 825.8 d |
| Average | 874.2 | 3739.9 | 13781.1 |
| Range | 730.1–1008.3 | 2863.0–4648.7 | 12,800.6–16,538.6 |
| Sorbus aria (L.) Crantz (Whitebeam) | |||
| Site 1 Site 2 | 876.9 ± 37.9 b 869.8 ± 36.6 b | 897.5 ± 27.9 a 822.5 ± 22.0 a | 6823.2 ± 120.9 b 5348.2 ± 335.6 a |
| Average | 873.4 | 860.0 | 6085.7 |
| Range | 812.8–921.8 | 779.9–944.6 | 5016–7022.1 |
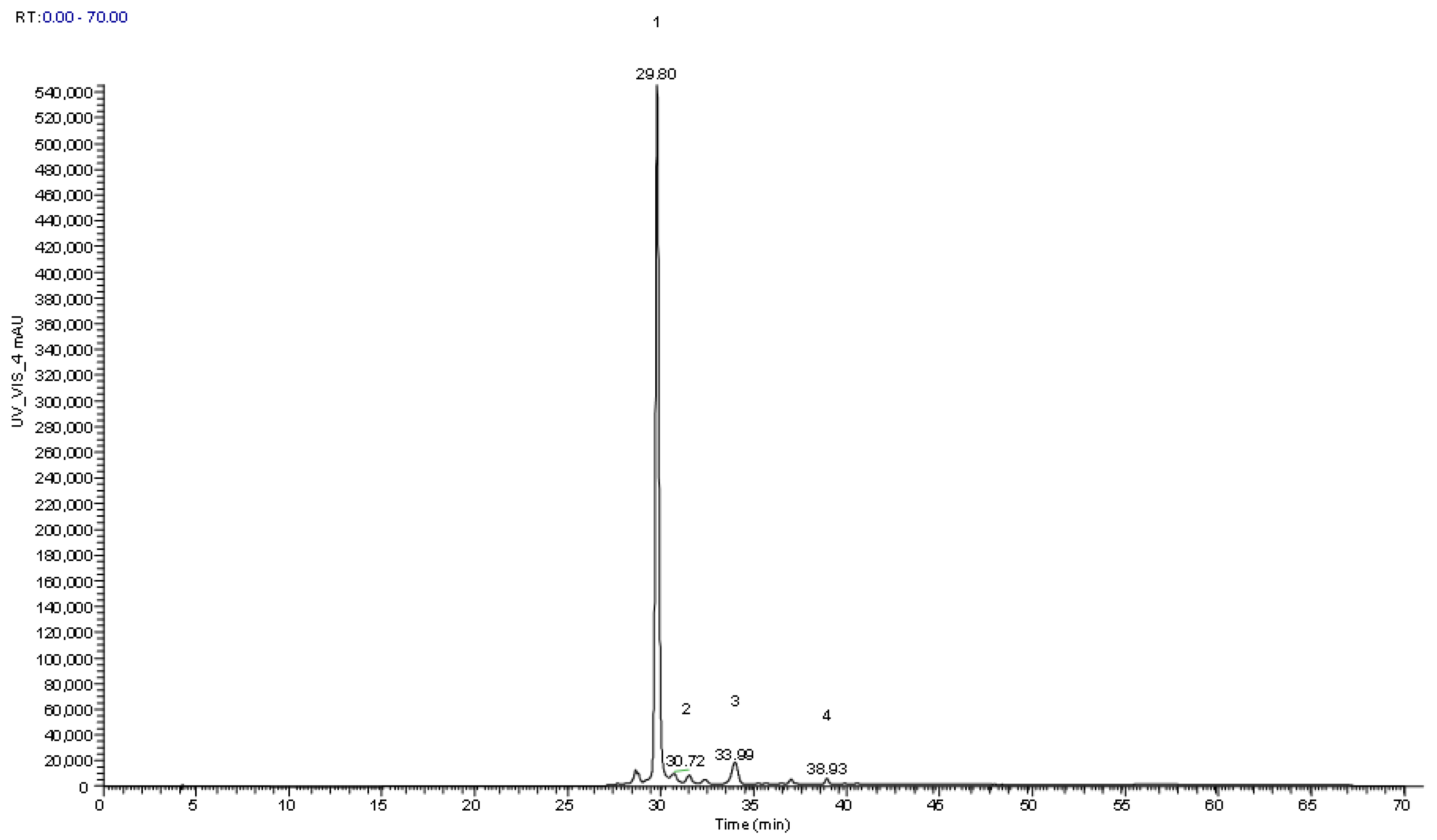
| Peak | Rt (min) | λmáx (nm) | [M]+ (m/z) | MS2(m/z) | Tentative Identification | Quantification (mg/g dw) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | |||||||
| 1cm | 29.8 | 516 | 449 | 287 (100) | Cyanidin-O-hexoxide | 6.63 ± 0.01 | 9.876 ± 0.005 | <0.001 |
| 2cm | 30.8 | 517 | 449 | 287 (100) | Cyanidin-3-O-glucoside | 0.20 ± 0.01 | 0.393 ± 0.005 | <0.001 |
| 3cm | 34.0 | 516 | 463 | 301 (100) | Peonidin-O-hexoxide | 0.355 ± 0.005 | 0.294 ± 0.002 | <0.001 |
| 4cm | 39.0 | 522 | 557 | 395 (100), 287 (10) | Cyanidin derivative | 0.087 ± 0.001 | 0.088 ± 0.001 | 0.001 |
| Total anthocyanins | 7.27 ± 0.02 | 10.652 ± 0.002 | <0.001 | |||||
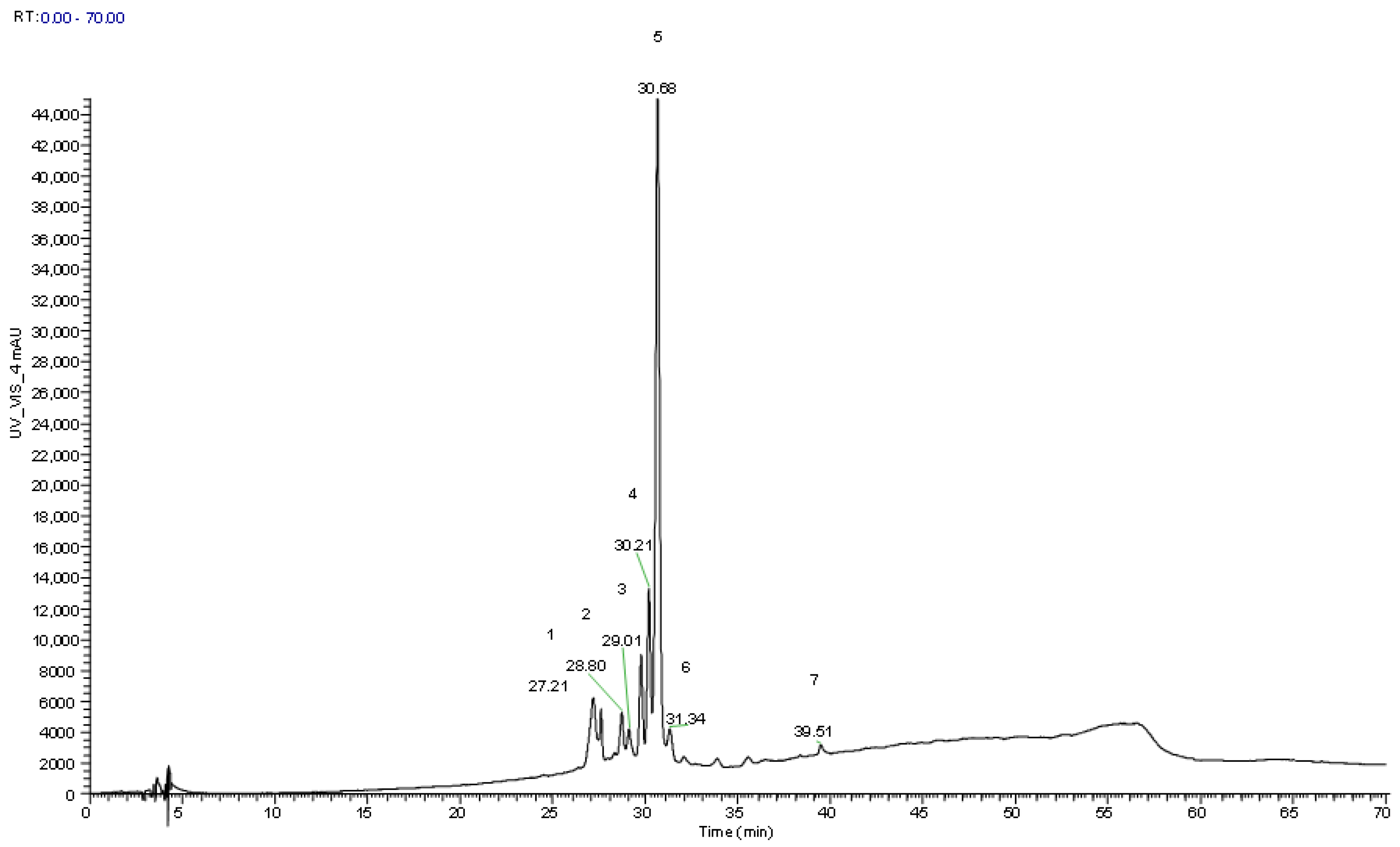
| Peak | Rt (min) | λmáx (nm) | [M]+ (m/z) | MS2(m/z) | Tentative Identification | Quantification (mg/g dw) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | |||||||
| 1sa | 27.2 | 513 | 611 | 449 (98), 287 (100) | Cyanidin-O-hexoyl-O-hexoxide | 0.15 ± 0.00 | Not detected | - |
| 2sa | 28.8 | 519 | 611 | 449 (12), 287 (100) | Cyanidin-O-hexosyl-O-hexoxide | 0.08 ± 0.00 | 0.32 ± 0.00 | <0.001 |
| 3sa | 29.1 | 515 | 611 | 449 (11), 287 (100) | Cyanidin-O-hexosyl-O-hexox ide | 0.06 ± 0.00 | 0.25 ± 0.01 | <0.001 |
| 4sa | 30.2 | 515 | 581 | 287 (100) | Cyanidin-O-hexosyl-O-pentoxide | 0.15 ± 0.00 | Not detected | - |
| 5sa | 30.7 | 515 | 449 | 287 (100) | Cyanidin-3-O-glucoside | 0.65 ± 0.00 | 5.13 ± 0.02 | <0.001 |
| 6sa | 31.3 | 515 | 565 | 287 (100) | Cyanidin-O-deoxyhexosyl-pentoxide | 0.08 ± 0.00 | 0.43 ± 0.00 | <0.001 |
| 7sa | 39.5 | 523 | 557 | 395 (100), 287 (5) | Cyanidin derivate | 0.06 ± 0.00 | 0.31 ± 0.00 | <0.001 |
| Total anthocyanins | 1.24 ± 0.01 | 6.43 ± 0.01 | <0.001 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamayo-Vives, C.; García-Herrera, P.; Sánchez-Mata, M.C.; Cámara-Hurtado, R.M.; Pérez-Rodríguez, M.L.; Aceituno, L.; Pardo-de-Santayana, M.; Días, M.I.; Barros, L.; Morales, P. Wild Fruits of Crataegus monogyna Jacq. and Sorbus aria (L.) Crantz: From Traditional Foods to Innovative Sources of Pigments and Antioxidant Ingredients for Food Products. Foods 2023, 12, 2427. https://doi.org/10.3390/foods12122427
Tamayo-Vives C, García-Herrera P, Sánchez-Mata MC, Cámara-Hurtado RM, Pérez-Rodríguez ML, Aceituno L, Pardo-de-Santayana M, Días MI, Barros L, Morales P. Wild Fruits of Crataegus monogyna Jacq. and Sorbus aria (L.) Crantz: From Traditional Foods to Innovative Sources of Pigments and Antioxidant Ingredients for Food Products. Foods. 2023; 12(12):2427. https://doi.org/10.3390/foods12122427
Chicago/Turabian StyleTamayo-Vives, Cristina, Patricia García-Herrera, María Cortes Sánchez-Mata, Rosa M. Cámara-Hurtado, María Luisa Pérez-Rodríguez, Laura Aceituno, Manuel Pardo-de-Santayana, María Inês Días, Lillian Barros, and Patricia Morales. 2023. "Wild Fruits of Crataegus monogyna Jacq. and Sorbus aria (L.) Crantz: From Traditional Foods to Innovative Sources of Pigments and Antioxidant Ingredients for Food Products" Foods 12, no. 12: 2427. https://doi.org/10.3390/foods12122427
APA StyleTamayo-Vives, C., García-Herrera, P., Sánchez-Mata, M. C., Cámara-Hurtado, R. M., Pérez-Rodríguez, M. L., Aceituno, L., Pardo-de-Santayana, M., Días, M. I., Barros, L., & Morales, P. (2023). Wild Fruits of Crataegus monogyna Jacq. and Sorbus aria (L.) Crantz: From Traditional Foods to Innovative Sources of Pigments and Antioxidant Ingredients for Food Products. Foods, 12(12), 2427. https://doi.org/10.3390/foods12122427










