Effect of the Freeze-Dried Mullein Flower Extract (Verbascum nigrum L.) Addition on Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Research Material
2.2. Reagents
2.3. Oils’ Quality Assessment
2.4. Fatty Acids Composition Determination
2.5. Preparation of Ethanolic Mullein Flowers Extracts
2.6. Methanolic Extract Preparation for Bioactive Compounds Determination
2.7. Total Phenolics Content Determination
2.8. Determination of the Total Antioxidant Capacity by the Reduction in DPPH Free Radical
2.9. Determination of Total Antioxidant Capacity by ABTS Radical Cation Reduction Method
2.10. Determination of Oxidative Stability Using the Rancimat
2.11. Determination of the Oxidation Kinetics Parameters
2.12. Statistical Analysis
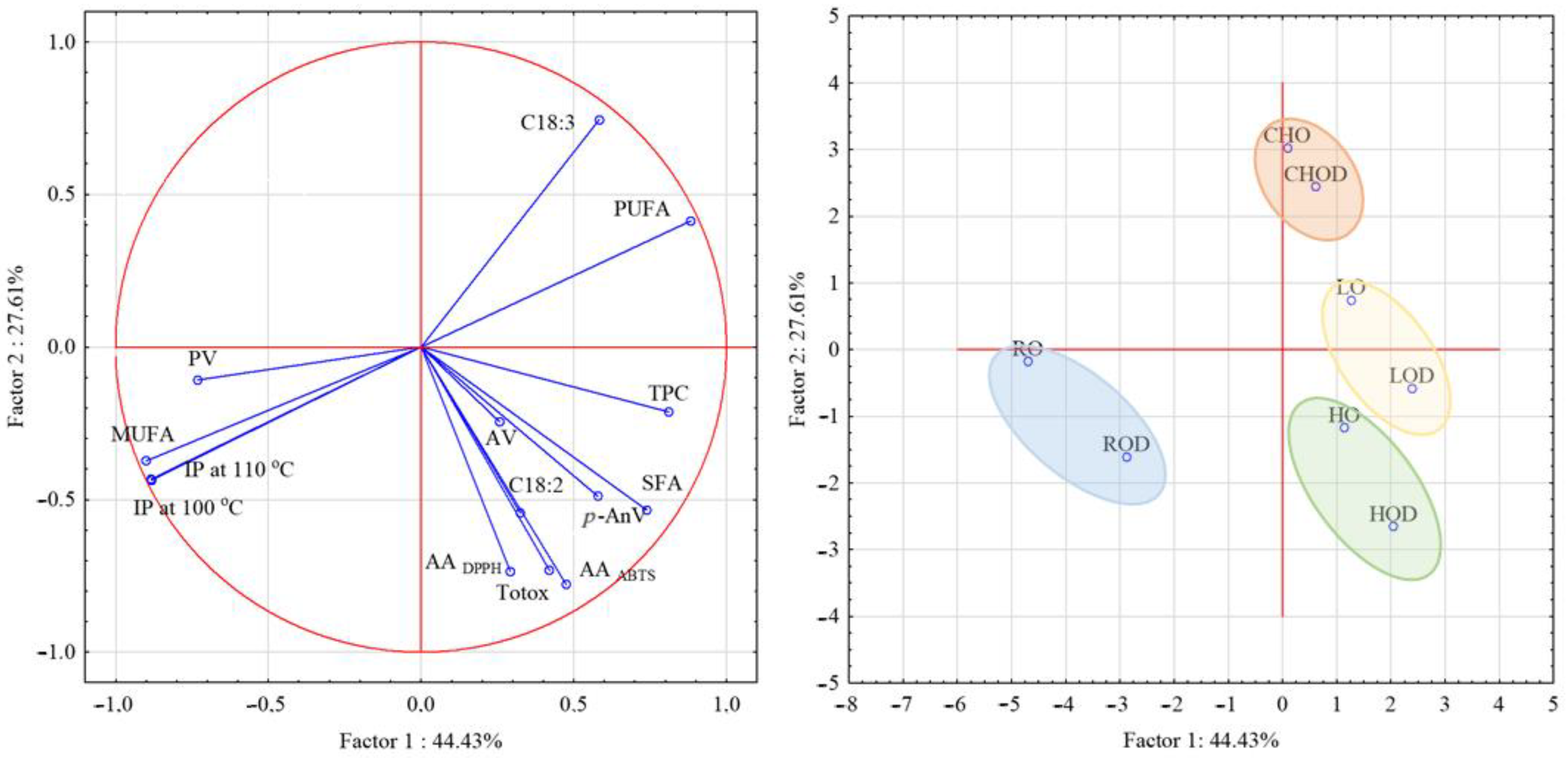
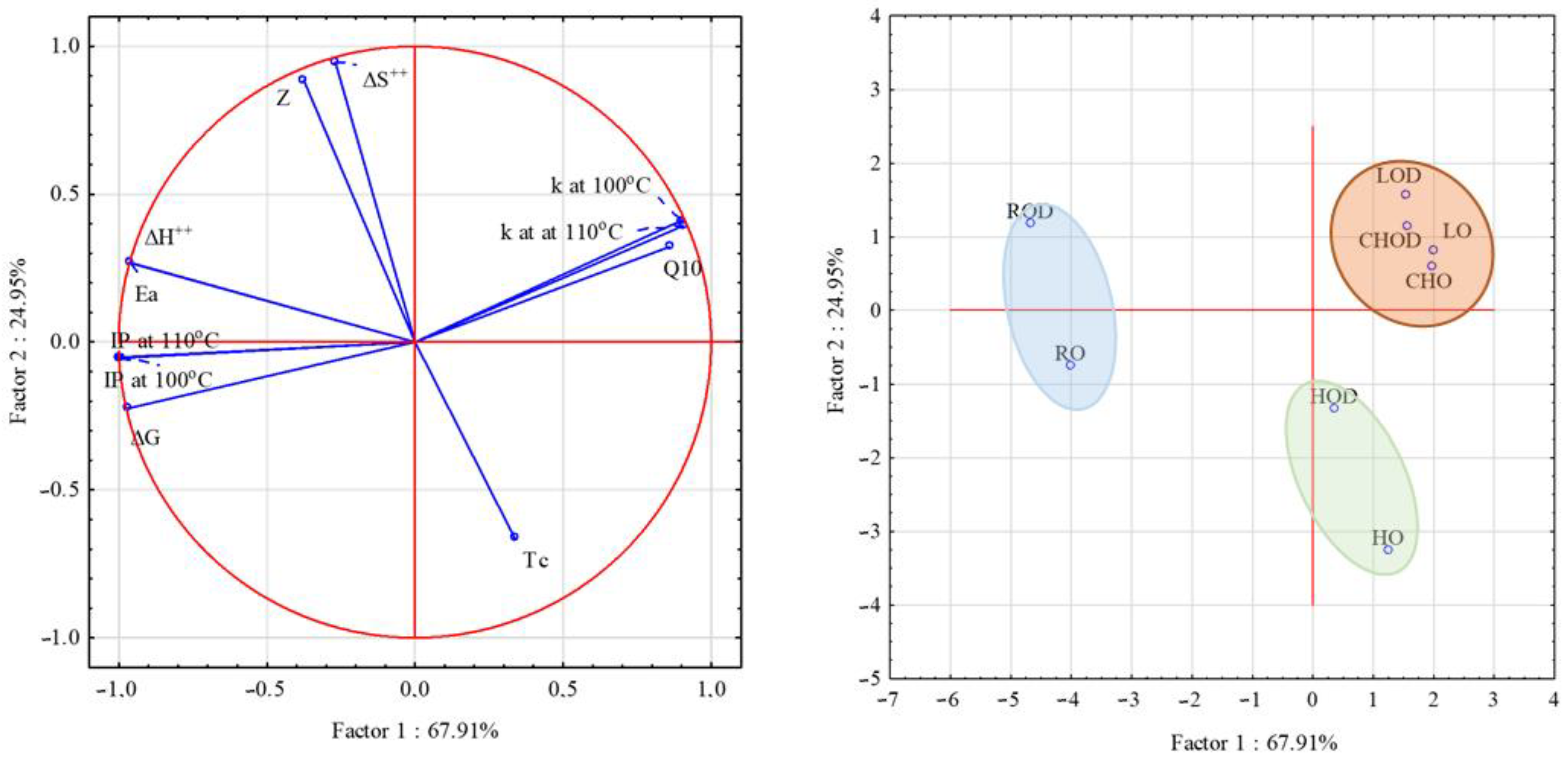
3. Results and Discussion
3.1. Effect of the Addition of Mullein Flower Extract on Oxidative Stability of the Tested Oils
3.2. Impact of the Extract Addition on the Basic Distinguishing Features of Oil Quality
3.3. Impact of the Mullein Addition on the Composition of Fatty Acids Tested Oils
3.4. Impact of the Extract on the Total Phenol Content and Antioxidant Activity
3.5. Parameters of Oxidation Kinetics of Analyzed Oils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madhujith, T.; Sivakanthan, S. Oxidative Stability of Edible Plant Oils. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Pokorný, J. Analysis of Lipid Oxidation; AOCS Press: Champaign, IL, USA, 2005. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Hu, K.; Huyan, Z.; Ding, S.; Dong, Y.; Yu, X. Investigation on Food Packaging Polymers: Effects on Vegetable Oil Oxidation. Food Chem. 2020, 315, 126299. [Google Scholar] [CrossRef] [PubMed]
- Zandi-Darehgharibi, F.; Haddadi, H.; Rafieian-Kopaei, M.; Fallah, A.A. Effects of Pistachio Green Hull Crude Extract and Its Polyphenol Fraction on Oxidative Stability of Sunflower Oil during Accelerated Storage. Biomass Convers. Biorefin. 2021, 13, 6973–6980. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Turker, A.U.; Gurel, E. Common Mullein (Verbascum thapsus L.): Recent Advances in Research. Phytother. Res. 2005, 19, 733–739. [Google Scholar] [CrossRef]
- Mihailović, V.; Kreft, S.; Benković, E.T.; Ivanović, N.; Stanković, M.S. Chemical Profile, Antioxidant Activity and Stability in Stimulated Gastrointestinal Tract Model System of Three Verbascum Species. Ind. Crops Prod. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Shahbaz, F.; Akhter, N.; Shahid, M.; Riaz, M.; Anjum, F.; Hussain, F. Ultrasound Assisted Extraction and Characterization of Bioactives from V Erbascum Thapsus Roots to Evaluate Their Antioxidant and Medicinal Potential. Dose Response 2022, 20, 155932582210976. [Google Scholar] [CrossRef]
- Blanco-Salas, J.; Hortigón-Vinagre, M.P.; Morales-Jadán, D.; Ruiz-Téllez, T. Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae). Biology 2021, 10, 618. [Google Scholar] [CrossRef]
- Gupta, A.; Atkinson, A.N.; Pandey, A.K.; Bishayee, A. Health-Promoting and Disease-Mitigating Potential of Verbascum thapsus L. (common mullein): A review. Phytother. Res. 2022, 36, 1507–1522. [Google Scholar] [CrossRef]
- Sharma, K.; Kaur, R.; Kumar, S.; Saini, R.K.; Sharma, S.; Pawde, S.V.; Kumar, V. Saponins: A Concise Review on Food Related Aspects, Applications and Health Implications. Food Chem. Adv. 2023, 2, 100191. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Kościński, M.; Białopiotrowicz, T.; Kaczorek, E. Verbascum nigrum L. (Mullein) Extract as a Natural Emulsifier. Food Hydrocoll. 2018, 81, 341–350. [Google Scholar] [CrossRef]
- Kalinina, S.A.; Elkina, O.V.; Kalinin, D.V.; Syropyatov, B.Y.; Dolzhenko, A.V. Diuretic Activity and Toxicity of Some Verbascum nigrum Extracts and Fractions. Pharm. Biol. 2014, 52, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Mariassyova, M. Antioxidant activity of some herbal extracts in rapeseed and sunflower oils. J. Food Nutr. Res. 2006, 45, 104–109. [Google Scholar]
- AOCS Official Method Cd 3d-63. Acid Value. In Sampling and Analysis of Commercial Fats and Oils; The American Oils Chemist’s Society: Urbana, IL, USA, 2009. [Google Scholar]
- AOCS Official Method Cd 8-53. Peroxide Value Acetic Acid (Chloroform Method). In Sampling and Analysis of Commercial Fats and Oils; The American Oils Chemist’s Society: Urbana, IL, USA, 2003. [Google Scholar]
- AOCS Official Method Cd 18-90. p-Anisidine Value. In Official Methods and Recommended Practices of the AOCS; American Oil Chemists Society Press: Champaign, IL, USA, 2011. [Google Scholar]
- AOAC Official Method 996.06. Fat (Total, Saturated, and Unsaturated) in Foods; Hydrolytic Extraction Gas Chromatographic Method. In Methods and Recommended Practices of the AOCS; AOCS International: Arlington, MA, USA, 2001. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant Properties, Phenolic and Mineral Composition of Germinated Chia, Golden Flax, Evening Primrose, Phacelia and Fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Łaszewska, A. Effect of Refining Process on Antioxidant Capacity, Total Phenolics and Prooxidants Contents in Rapeseed Oils. LWT Food Sci. Technol. 2015, 64, 853–859. [Google Scholar] [CrossRef]
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic Parameter Determination of Vegetable Oil Oxidation under Rancimat Test Conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 587–592. [Google Scholar] [CrossRef]
- Cherif, M.; Rodrigues, N.; Veloso, A.C.; Zaghdoudi, K.; Pereira, J.A.; Peres, A.M. Kinetic-thermodynamic study of the oxidative stability of Arbequina olive oils flavored with lemon verbena essential oil. LWT 2021, 140, 110711. [Google Scholar] [CrossRef]
- Turan, S. Effects of Some Plant Extracts on the Oxidative Stability of Canola Oil and Its Purified Triacylglycerols: Effects of Plant Extracts on Canola Oil Oxidation. J. Food Qual. 2014, 37, 247–258. [Google Scholar] [CrossRef]
- Nikolov, N.; Generalić Mekinić, I.; Ljubenkov, I.; Šimat, V.; Skroza, D.; Anđelić, I.; Soldo, B. The Effect of Selected Herb Extracts on Oxidative Stability of Vegetable Oils. Croat. Chem. Acta 2019, 92, 331–336. [Google Scholar] [CrossRef]
- CODEX-STAN 210; Codex Alimentarius. Codex Standard for Named Vegetable Oils (Amended 2003–2005). FAO: Rome, Italy, 2013.
- Ratusz, K.; Wirkowska, M. Skutecznosc dzialania preparatu przeciwutleniajacego na rafinowane oleje roslinne i ich matryce triacyloglicerolowe. Żywność Nauka Technol. Jakość 2006, 2, 262–270. [Google Scholar]
- Krajewska, M.; Kachel, M. Changes in Selected Properties of Cold-Pressed Oils Induced by Natural Plant Additives. Appl. Sci. 2022, 12, 3646. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Rapa, M.; Ciano, S.; Ingallina, C.; Cesa, S.; Menghini, L.; Carradori, S.; Giusti, A.M.; Di Sotto, A.; et al. Commercial Hemp Seed Oils: A Multimethodological Characterization. Appl. Sci. 2020, 10, 6933. [Google Scholar] [CrossRef]
- Aladić, K. Cold Pressing and Supercritical CO2 Extraction of Hemp (Cannabis sativa) Seed Oil. Chem. Biochem. Eng. Q. 2015, 28, 481–490. [Google Scholar] [CrossRef]
- Mollica, F.; Lucarini, M.; Passerini, C.; Carati, C.; Pavoni, S.; Bonoldi, L.; Amorati, R. Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions. Antioxidants 2020, 9, 399. [Google Scholar] [CrossRef]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Silska, G.; Walkowiak, M. Comparative Analysis of Fatty Acid Composition in 84 Accessions of Flax (Linum usitatissimum L.). J. Pre-Clin. Clin. Res. 2019, 13, 118–129. [Google Scholar] [CrossRef]
- Berto, B.M.; Garcia, R.K.A.; Fernandes, G.D.; Barrera-Arellano, D.; Pereira, G.G. Linseed Oil: Characterization and Study of Its Oxidative Degradation. Grasas Aceites 2020, 71, 337. [Google Scholar] [CrossRef] [Green Version]
- Oteri, M.; Bartolomeo, G.; Rigano, F.; Aspromonte, J.; Trovato, E.; Purcaro, G.; Dugo, P.; Mondello, L.; Beccaria, M. Comprehensive Chemical Characterization of Chia (Salvia hispanica L.) Seed Oil with a Focus on Minor Lipid Components. Foods 2022, 12, 23. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ścibisz, I. Badanie zawartości polifenoli i aktywności przeciwutleniającej ekstraktów z roślin przyprawowych podczas ich przechowywania. Bromatol. Chem. Toksykol. 2012, 45, 358–363. [Google Scholar]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant Capacity and Total Phenolic Contents of Oregano (Origanum vulgare), Lavender (Lavandula angustifolia) and Lemon Balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef]
- Alizadeh, A. Essential Oil Composition, Phenolic Content, Antioxidant, and Antimicrobial Activity of Cultivated Satureja rechingeri Jamzad at Different Phenological Stages. Z. Naturforsch. C 2015, 70, 51–58. [Google Scholar] [CrossRef]
- Parry, J.; Hao, Z.; Luther, M.; Su, L.; Zhou, K.; Yu, L.L. Characterization of Cold-Pressed Onion, Parsley, Cardamom, Mullein, Roasted Pumpkin, and Milk Thistle Seed Oils. J. Am. Oil Chem. Soc. 2006, 83, 847–854. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-Pressed Oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Ratusz, K.; Popis, E.; Ciemniewska-Żytkiewicz, H.; Wroniak, M. Oxidative Stability of Camelina (Camelina sativa L.) Oil Using Pressure Differential Scanning Calorimetry and Rancimat Method. J. Therm. Anal. Calorim. 2016, 126, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Kozłowska, M.; Żontała, K. Stabilność oksydacyjna oleju słonecznikowego tłoczonego na zimno wzbogaconego w ekstrakty z roślin przyprawowych. Bromatol. Chem. Toksykol. 2011, 44, 877–882. [Google Scholar]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the Oxidative Stability of Linseed (Linum usitatissimum L.) Oil by Pressure Differential Scanning Calorimetry and Rancimat Measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, É.S.; Borsato, D.; Silva, L.R.C.; Chendynski, L.T.; Angilelli, K.G.; Canesin, E.A. Kinetic Parameters of the Oxidation Reaction of Commercial Biodiesel with Natural Antioxidant Additives. Ind. Crops Prod. 2018, 125, 59–64. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E. Comparison of the Oxidative Stability of Soybean and Sunflower Oils Enriched with Herbal Plant Extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, S.M.B.; Brewer, M.S.; Safari, J.; Nowroozi, M.; Abadi Sherahi, M.H.; Sadeghi, B.; Ghafoori, M. Antioxidant Activity, Reaction Mechanisms, and Kinetics of Matricaria recutita Extract in Commercial Blended Oil Oxidation. Int. J. Food Prop. 2016, 19, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Farhoosh, R.; Hoseini-Yazdi, S.-Z. Evolution of Oxidative Values during Kinetic Studies on Olive Oil Oxidation in the Rancimat Test. J. Am. Oil Chem. Soc. 2014, 91, 281–293. [Google Scholar] [CrossRef]
- Mahdavianmehr, H.; Farhoosh, R.; Sharif, A. Thermal Antioxidative Kinetics of Hydroxytyrosol in Selected Lipid Systems of Different Unsaturation Degree. J. Am. Oil Chem. Soc. 2016, 93, 1655–1661. [Google Scholar] [CrossRef]

| Extract Concentration (mg/kg oil) | Induction Time (h) | Protection Factor (PF) | ||||||
|---|---|---|---|---|---|---|---|---|
| ROM | HOM | LOM | CHOM | ROM | HOM | LOM | CHOM | |
| 0 | 9.81 a ± 0.16 | 12.11 a ± 0.11 | 6.70 a ± 0.04 | 6.44 a ± 0.14 | - | - | - | - |
| 2 | 9.82 ab ± 0.11 | 13.78 b ± 0.34 | 6.71 a ± 0.04 | 6.55 ab ± 0.04 | 1.00 | 1.14 | 1.00 | 1.02 |
| 5 | 9.98 ab ± 0.05 | 13.84 b ± 0.15 | 6.72 ab ± 0.17 | 6.65 ab ± 0.10 | 1.02 | 1.14 | 1.00 | 1.03 |
| 10 | 10.01 bcd ± 0.08 | 13.91 b ± 0.18 | 6.72 ab ± 0.10 | 6.82 ab ± 0.05 | 1.02 | 1.15 | 1.00 | 1.06 |
| 15 | 10.02 abc ± 0.06 | 14.05 b ± 0.41 | 6.75 ab ± 0.19 | 6.80 ab ± 0.14 | 1.02 | 1.16 | 1.01 | 1.06 |
| 20 | 10.32 abcd ± 0.11 | 13.74 b ± 0.50 | 7.17 b ± 0.03 | 6.96 b ± 0.20 | 1.05 | 1.13 | 1.07 | 1.08 |
| 30 | 10.35 abcd ± 0.05 | 13.75 b ± 0.54 | 7.19 b ± 0.06 | 6.73 ab ± 0.26 | 1.05 | 1.14 | 1.07 | 1.04 |
| 40 | 10.42 abcd ± 0.03 | 13.57 ab ± 0.17 | 7.20 b ± 0.06 | 6.82 ab ± 0.04 | 1.06 | 1.12 | 1.08 | 1.06 |
| 60 | 10.79 d ± 0.37 | 13.65 b ± 0.44 | 7.25 b ± 0.25 | 6.87 ab ± 0.03 | 1.10 | 1.13 | 1.08 | 1.07 |
| 100 | 10.44 bcd ± 0.23 | 13.51 b ± 0.21 | 6.95 ab ± 0.16 | 6.85 ab ± 0.03 | 1.06 | 1.12 | 1.04 | 1.06 |
| 200 | 10.62 cd ± 0.11 | 13.71 b ± 0.03 | 6.99 ab ± 0.23 | 6.86 ab ± 0.11 | 1.08 | 1.13 | 1.04 | 1.06 |
| Oil | AV (mg KOH/g) | PV (mEq O2/kg) | p-AnV | Totox (2PV + p-AnV) |
|---|---|---|---|---|
| RO | 1.22 bc ± 0.01 | 1.74 b ± 0.06 | 0.37 a ± 0.14 | 3.85 b ± 0.03 |
| HO | 1.01 a ± 0.01 | 6.30 c ± 0.14 | 0.74 ab ± 0.30 | 13.34 d ± 0.59 |
| LO | 1.67 d ± 0.02 | 1.98 b ± 0.03 | 0.62 ab ± 0.09 | 4.58 b ± 0.15 |
| CHO | 1.11 ab ± 0.02 | 0.77 a ± 0.04 | 0.64 ab ± 0.07 | 2.18 a ± 0.15 |
| ROM (60 mg/kg) | 1.62 d ± 0.02 | 1.74 b ± 0.01 | 0.61 ab ± 0.13 | 4.09 b ± 0.10 |
| HOM (15 mg/kg) | 1.33 c ± 0.01 | 10.50 d ± 0.21 | 1.20 bc ± 0.20 | 22.20 e ± 0.75 |
| LOM (60 mg/kg) | 2.23 e ± 0.08 | 2.00 b ± 0.10 | 1.71 c ± 0.08 | 5.71 c ± 0.20 |
| CHOM (20 mg/kg) | 1.19 b ± 0.01 | 0.77 a ± 0.01 | 0.19 a ± 0.02 | 1.73 a ± 0.01 |
| Fatty Acids (%) | Oils | |||||||
|---|---|---|---|---|---|---|---|---|
| RO | ROM (60 mg/kg) | HO | HOM (15 mg/kg) | LO | LOM (60 mg/kg) | CHO | CHOM [20 mg/kg] | |
| C16:0 | 2.54 a | 2.36 a | 2.86 ab | 3.09 ab | 3.02 ab | 3.42 b | 3.06 ab | 3.18 ab |
| C18:0 | 2.20 a | 1.73 a | 2.88 ab | 2.93 b | 4.35 c | 4.24 c | 2.57 ab | 2.38 ab |
| C18:1 | 68.15 d | 69.14 d | 14.40 b | 13.85 b | 15.28 c | 14.94 bc | 7.47 a | 7.40 a |
| C18:2 | 16.21 b | 17.06 b | 57.86 c | 55.77 c | 11.64 a | 11.15 a | 18.32 b | 17.98 b |
| α-C18:3 | 7.16 a | 6.83 a | 16.06 b | 16.80 b | 65.28 c | 65.16 c | 68.34 c | 68.72 c |
| Ɣ-C18:3 | - | - | 3.43 a | 3.47 a | - | - | - | - |
| C20:0 | 1.26 b | 0.86 a | 0.85 a | 1.41 b | - | - | - | - |
| C20:1 | 1.96 b | 1.59 b | 0.42 a | 0.63 a | - | - | - | - |
| C22:0 | - | - | 0.69 a | 1.16 b | - | - | - | - |
| Other | 0.52 | 0.43 | 0.55 | 0.89 | 0.43 | 1.09 | 0.24 | 0.34 |
| ⅀SFA | 6.00 | 5.38 | 7.83 | 9.48 | 7.80 | 8.75 | 5.87 | 5.90 |
| ⅀MUFA | 70.11 | 70.73 | 14.82 | 14.48 | 15.28 | 14.94 | 7.47 | 7.40 |
| ⅀PUFA | 23.37 | 23.89 | 77.35 | 76.04 | 76.92 | 76.31 | 86.66 | 86.70 |
| Extract Concentration [mg/kg oil] | Total Phenolic Compounds Content (mg GAE/100 g) | |||
|---|---|---|---|---|
| ROM | HOM | LOM | CHOM | |
| 0 | 235.42 a ± 1.32 | 385.70 ab ± 1.36 | 374.64 ab ± 0.54 | 313.70 a ± 0.78 |
| 2 | 246.25 b ± 1.87 | 386.67 ab ± 1.51 | 374.12 a ± 0.38 | 355.69 b ± 1.22 |
| 5 | 257.56 c ± 1.42 | 385.46 ab ± 1.32 | 374.50 ab ± 0.56 | 381.79 c ± 1.43 |
| 10 | 298.16 d ± 1.07 | 385.96 ab ± 1.98 | 387.50 c ± 1.54 | 391.58 d ± 1.00 |
| 15 | 340.75 e ± 1.45 | 399.50 c ± 2.01 | 384.97 b ± 0.93 | 394.76 e ± 0.75 |
| 20 | 351.95 f ± 1.10 | 405.93 d ± 1.56 | 383.42 b ± 1.23 | 401.24 f ± 0.87 |
| 30 | 354.28 g ± 0.98 | 407.36 e ± 1.32 | 393.25 d ± 1.05 | 405.99 g ± 1.43 |
| 40 | 358.72 h ± 1.65 | 410.62 h ± 1.76 | 391.03 c ± 0.54 | 419.48 h ± 1.08 |
| 60 | 363.25 i ± 0.54 | 408.50 i ± 1.07 | 397.24 d ± 0.97 | 423.33 i ± 1.54 |
| 100 | 364.97 j ± 0.43 | 411.38 h ± 1.26 | 418.63 e ± 1.23 | 428.24 j ± 1.01 |
| 200 | 372.18 k ± 0.87 | 433.78 j ± 1.46 | 476.96 f ± 1.54 | 444.10 k ± 1.23 |
| Flowers extract | 1678.0 ± 2.23 | |||
| Oil | ABTS | DPPH | ||
|---|---|---|---|---|
| AA (µM Trolox/kg) | % Reducing ABTS | AA (µM Trolox/kg) | % Reducing DPPH | |
| RO | 489.0 c ± 2.1 | 54.7 c | 36.9 a ± 5.9 | 15.8 b |
| HO | 804.2 e ± 5.2 | 71.7 f | 147.8 d ± 5.1 | 20.6 e |
| LO | 751.0 f ± 8.1 | 68.7 d | 135.5 c ± 5.5 | 20.4 d |
| CHO | 171.1 a ± 4.1 | 37.6 a | 25.6 a ± 5.2 | 16.0 a |
| ROM (60 mg/kg) | 525.0 d ± 8.5 | 56.6 e | 221.7 g ± 6.4 | 24.3 h |
| HOM (15 mg/kg) | 886.1 g ± 9.2 | 76.0 g | 184.9 f ± 7.3 | 22.3 g |
| LOM (60 mg/kg) | 888.8 g ± 10.1 | 76.1 g | 155.3 e ± 7.1 | 20.9 f |
| CHOM (20 mg/kg) | 324.9 b ± 7.2 | 46.8 b | 102.8 b ± 4.6 | 18.6 c |
| Flowers extract | 5234.2 ± 11.2 | - | 1060.0 ± 14.3 | - |
| Oil | Induction Time (h) | |||||
|---|---|---|---|---|---|---|
| 80 °C | 90 °C | 100 °C | 110 °C | 120 °C | 130 °C | |
| RO | - | - | 19.24 d | 9.81 e | 5.14 a | 2.64 a |
| HO | 23.98 d | 12.11 d | 6.30 b | 3.13 c | - | - |
| LO | 13.48 b | 6.70 b | 3.41 a | 1.72 b | - | - |
| CHO | 13.10 a | 6.44 a | 3.31 a | 1.68 a | - | - |
| ROM (60 mg/kg) | - | - | 21.32 e | 10.79 f | 5.50 b | 2.83 b |
| HOM (15 mg/kg) | 28.01 e | 14.05 e | 6.80 c | 3.60 d | - | - |
| LOM (60 mg/kg) | 14.31 c | 7.25 b | 3.44 a | 1.81 b | - | - |
| CHOM (20 mg/kg) | 13.89 b | 6.96 c | 3.32 a | 1.78 b | - | - |
| Oil | Oxidation Kinetics Parameters | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | Tc (K−1) | Z (h−1) | k (h−1) at | ΔH++ (kJ/mol) | ΔS++ (J/mol K) | Q10 | ΔG at 100 °C (kJ/mol) | ΔG at 110 °C (kJ/mol) | IP25 °C (days) | IP4 °C (days) | ||||||
| 80 °C | 90 °C | 100 °C | 110 °C | 120 °C | 130 °C | |||||||||||
| RO | 82.48 ab | 6.88 × 102 | 1.85 × 1013 | - | - | 0.0520 aA | 0.1019 aB | 0.1946 aC | 0.3788 aD | 79.3 ab | −127.0 ab | 1.81 | 126.67 b | 127.94 b | 40 | 137 |
| HO | 76.06 a | 7.34 × 102 | 7.35 × 1012 | 0.0417 abA | 0.0818 aB | 0.1587 bC | 0.3195 cD | - | - | 73.0 a | −134.2 b | 1.84 | 123.06 ab | 124.40 ab | 16 | 58 |
| LO | 76.99 ab | 6.85 × 102 | 1.81 × 1013 | 0.0741 abA | 0.1493 aB | 0.2933 cC | 0.5814 deD | - | - | 74.0 ab | −126.6 ab | 1.86 | 121.22 ab | 122.49 ab | 10 | 35 |
| CHO | 76.73 a | 6.83 × 102 | 1.71 × 1013 | 0.0763 bA | 0.1553 bB | 0.3021 cC | 0.5952 eD | - | - | 73.7 a | −127.1 ab | 1.85 | 121.11 ab | 122.38 ab | 9 | 33 |
| ROM (60 mg/kg) | 84.11 b | 6.73 × 102 | 2.79 × 1013 | - | - | 0.0469 aA | 0.0927 aB | 0.1818 aC | 0.3534 aD | 80.9 b | −123.5 ab | 1.83 | 126.97 b | 128.20 b | 47 | 167 |
| HOM (15 mg/kg) | 77.34 ab | 6.77 × 102 | 9.83 × 1012 | 0.0357 aA | 0.0712 aB | 0.1471 bC | 0.2778 bD | - | - | 74.5 ab | −131.8 b | 1.85 | 123.66 ab | 124.98 ab | 19 | 71 |
| LOM (60 mg/kg) | 78.09 ab | 6.95 × 102 | 2.47 × 1013 | 0.0699 abA | 0.1379 aB | 0.2907 cC | 0.5525 dD | - | - | 75.0 ab | −124.1 ab | 1.86 | 121.29 ab | 122.53 ab | 10 | 37 |
| CHOM (20 mg/kg) | 77.61 ab | 6.90 × 102 | 2.17 ×1013 | 0.0720 abA | 0.1437 bB | 0.3012 cC | 0.5618 deD | - | - | 74.6 ab | −125.2 ab | 1.85 | 121.30 ab | 122.55 ab | 10 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symoniuk, E.; Marczak, Z.; Brzezińska, R.; Janowicz, M.; Ksibi, N. Effect of the Freeze-Dried Mullein Flower Extract (Verbascum nigrum L.) Addition on Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils. Foods 2023, 12, 2391. https://doi.org/10.3390/foods12122391
Symoniuk E, Marczak Z, Brzezińska R, Janowicz M, Ksibi N. Effect of the Freeze-Dried Mullein Flower Extract (Verbascum nigrum L.) Addition on Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils. Foods. 2023; 12(12):2391. https://doi.org/10.3390/foods12122391
Chicago/Turabian StyleSymoniuk, Edyta, Zuzanna Marczak, Rita Brzezińska, Monika Janowicz, and Nour Ksibi. 2023. "Effect of the Freeze-Dried Mullein Flower Extract (Verbascum nigrum L.) Addition on Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils" Foods 12, no. 12: 2391. https://doi.org/10.3390/foods12122391
APA StyleSymoniuk, E., Marczak, Z., Brzezińska, R., Janowicz, M., & Ksibi, N. (2023). Effect of the Freeze-Dried Mullein Flower Extract (Verbascum nigrum L.) Addition on Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils. Foods, 12(12), 2391. https://doi.org/10.3390/foods12122391








