Effects of Shear Stress Waves on Meat Tenderness: Ultrasonoporation
Abstract
:1. Introduction
2. Background
Ultrasonoporation
3. Materials and Methods
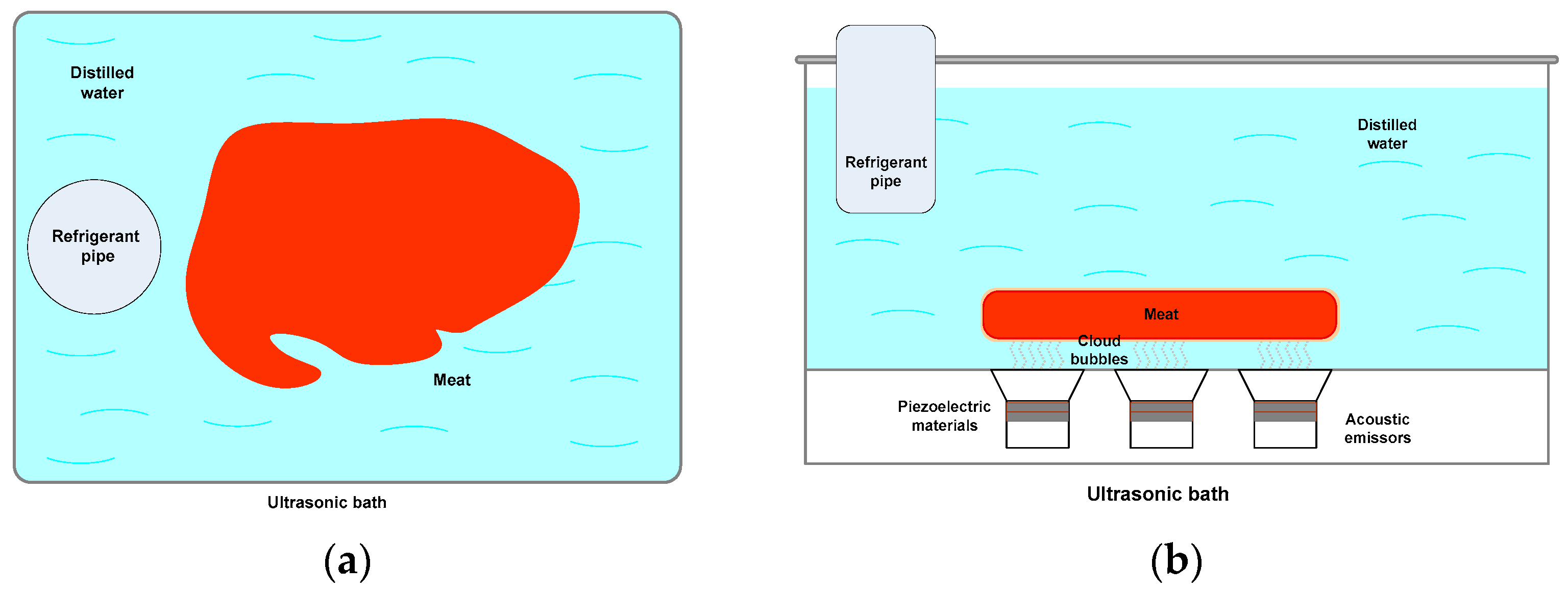
3.1. Ultrasonic Treatment
3.2. Color Measurement
3.3. pH Measurement
3.4. Shear Force Measurement
3.5. Statistical Analysis
4. Results and Discussion
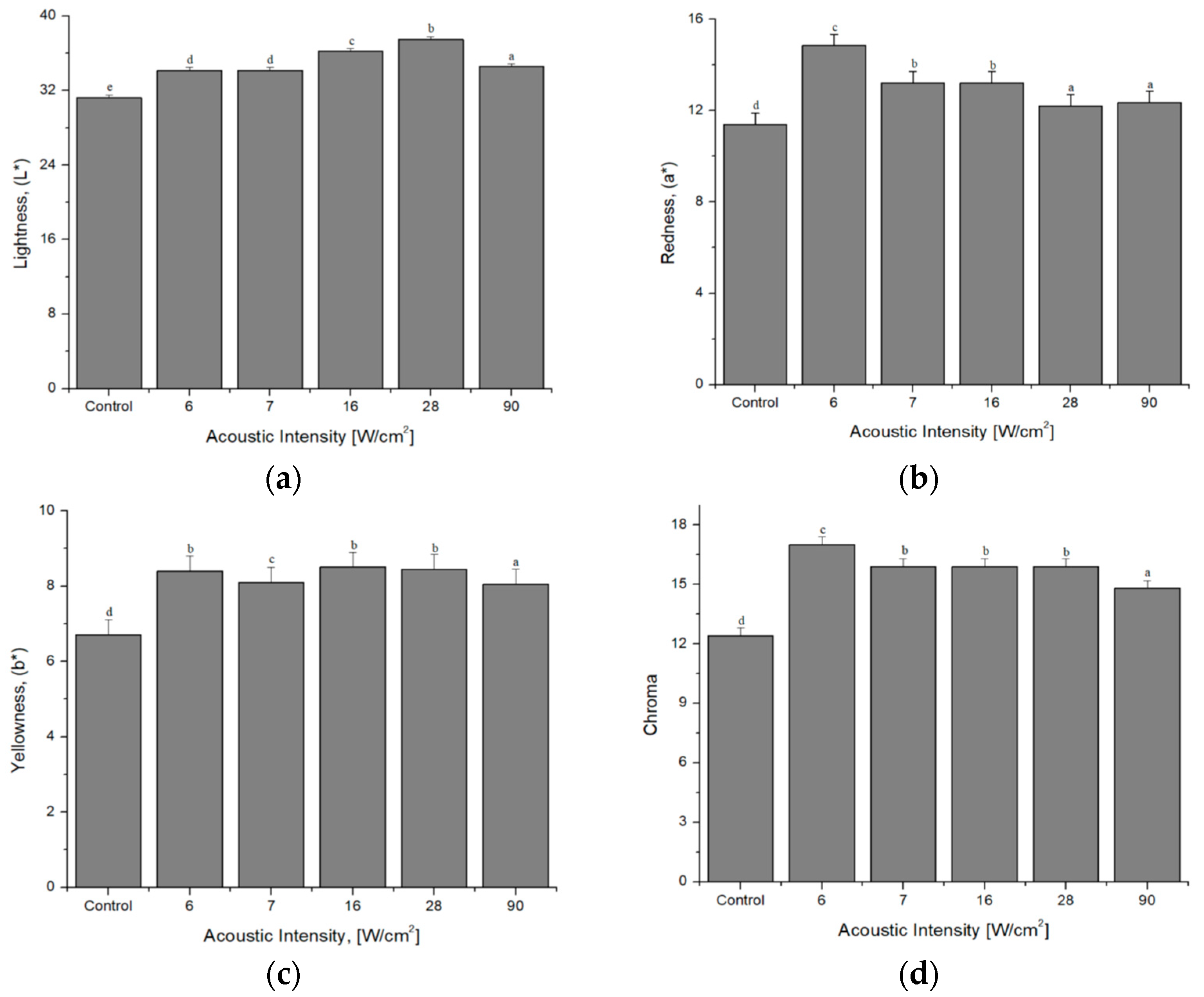
4.1. Color
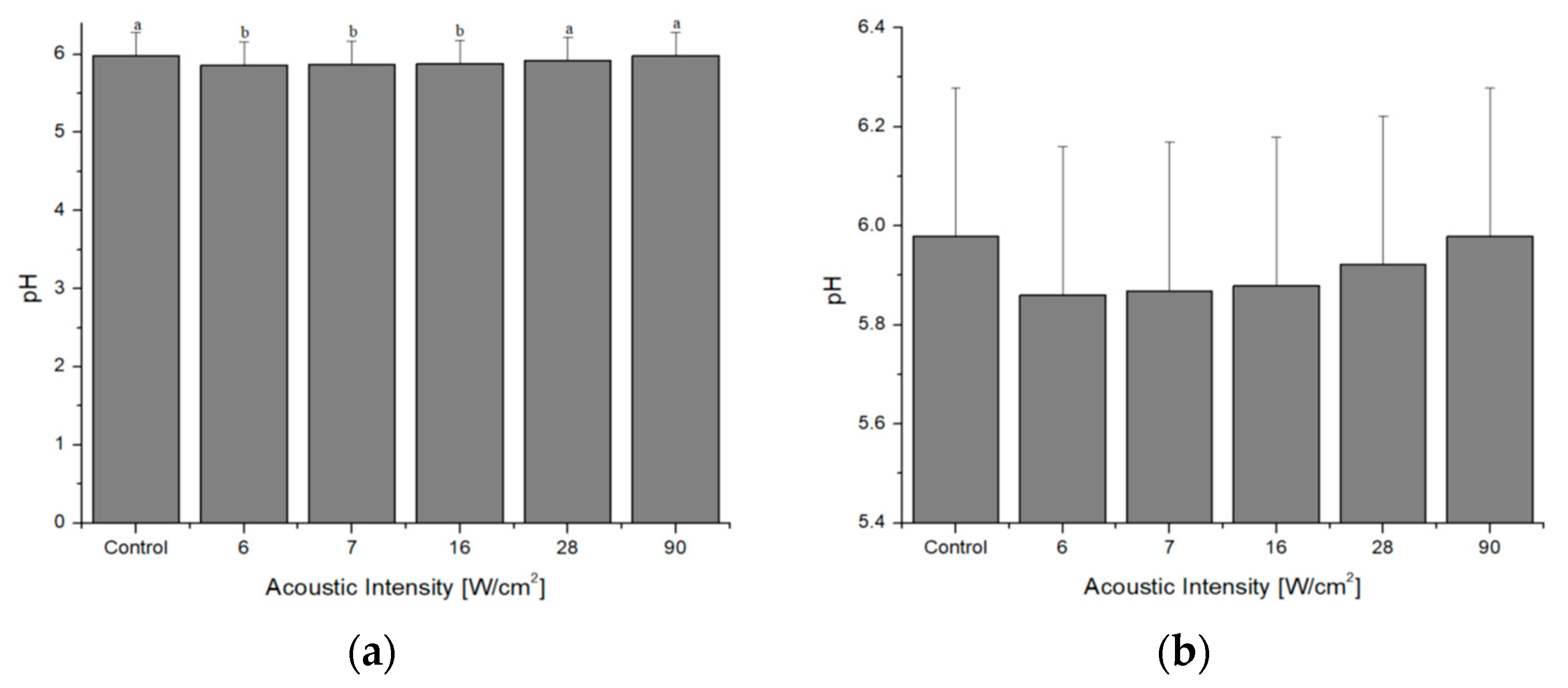
4.2. pH Measurement
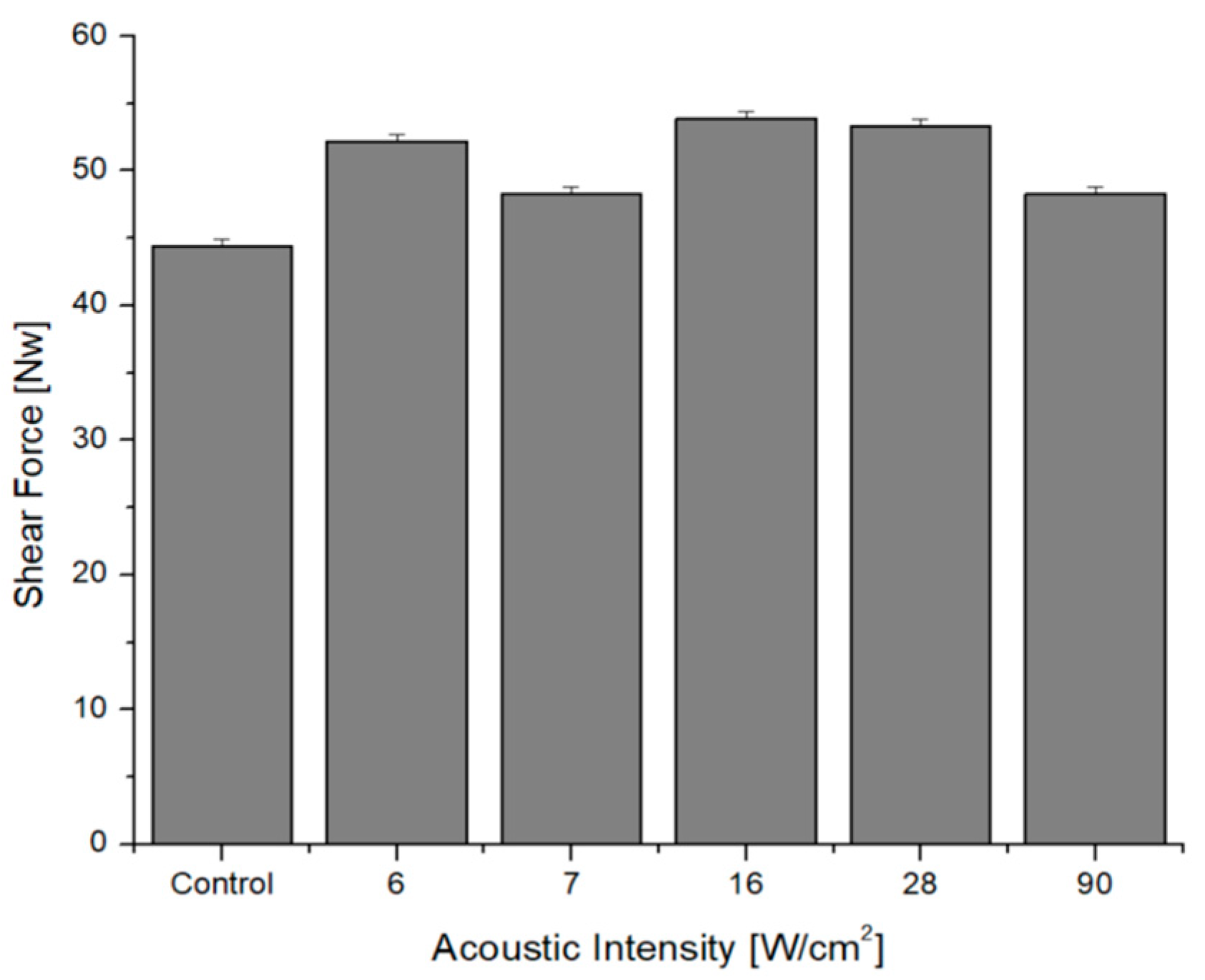
4.3. Shear Force Measurement
- The effects of ultrasound on meat tenderness are mainly examined on bovines, poultry, and, in the least cases, seafood. Muscles in beef have different internal anatomical structures. Longissimus dorsi, for instance, has almost all its myofibrils arranged collinearly, thus facilitating the internal exploration of its structure. Other muscles are organized transversally or randomly. The organization of the muscles impacts the results of the Warner-Bratzler technique and the sensory analyses. There is a variation in the mechanical stress–strain relationship of the muscles and a great influence in those that have a greater concentration of connective tissues and bone.
- The ultrasonic bath and the sonotrode-type ultrasonicator are the most used apparatus. The ultrasonic bath has up to three acoustic emitters attached to the bottom of the ultrasonic basket, and, due to its design, it has a baffle-type acoustic radiation behavior and, consequently, randomly generates clouds of filament-type microbubbles over the entire surface of the ultrasonication basket. The sonotrode-type ultrasonicator is placed directly in the fluid to be ultrasonicated. The emission generated by the ultrasonicator, depending on the acoustic intensity and the type of sonotrode, is an inverted pyramidal or half-sphere microbubble cloud, which subsequently leads to the generation of filament-shaped microbubble clouds. In both ultrasonication systems, the acoustic intensities and the frequency of the ultrasonicator vary.
- HIU has the greatest application impact on the surface of the material. When applying this technique on meat foods but theoretically ignoring the basics of ultrasonication, different hypotheses or questions will be raised, such as those concerning the application of HIU on both sides of a meat cut or/and increasing the application times. As described in Table 2, most studies similarly vary on the types of muscle, packing (packed or unpacked), or processing (marinated foods). These modifications usually are conducted unaware of the acoustic and thermal properties of the system and the environment, which notably influence the experimental results.
- Meat products can be studied as deformable materials, and their behavior might be analyzed with time-dependent models, such as Newton, Maxwell, Kelvin, Burger, and Bingham, or their combinations, stimulated via an acoustic source. Then, these results can be correlated with the results from the Warner-Bratzler technique.
- Previous knowledge of acoustic, mechanical, and thermal properties of the ultrasonication system, as well as of the meat products, would facilitate the prediction of the results and their discussion. The characteristics of acoustic physics in HIU are more than the acoustic intensity and frequency, which describe the speed that the ultrasound pressure exerts, the number of microbubbles generated, as well as the size of them. The experimental methodology should include the control of the variables that can affect this study, for example, the space where the experiments will be carried out, since it may compromise the temperature and atmospheric pressure. Additionally, the volumetric density properties of the fluid should be determined. The vibration generated from the acoustic emitters on the basis (bottom) of the ultrasonic bath and the implosions of acoustic cavitation in a stable and transitory state cause increases in temperature via convection. This influences the variation of thermal wave diffusion that finally reaches a well-defined temperature gradient point. Other studies circulated the fluid to cool it down. However, these settings make the acoustic cavitation shift to hydrodynamic cavitation and the conditions of the experiment change. In this sense, it is important to determine the acoustic field of application, either Fresnel or Fraunhofer. In the studies described in Table 2, the position of the meat with respect to the acoustic emitter is not mentioned. This detail is relevant as the effect of the acoustic radiation on the meat can be defined.
- Studies on vacuum-packed meat and meat products did not favor tenderness. This might be obvious, as there is no effect of acoustic cavitation via vacuum packaging. In acoustic physics, meat wrapped with a polymer resembles several interphase systems. The acoustic wave will travel through it; thus, the acoustic transmission effect is presented [108]. HIU applied to vacuum-packed meat has no effect on the internal structure of the meat, as the acoustic radiation generated by the shock waves does not pass through the interphase of the package; these are reflected. However, if the meat is packed with brine (or marinated), the acoustic waves have a propagation medium. So, in this case, the acoustic cavitation will influence the structure of the meat. However, the meat and the marinade should not be vacuum-packed, as there will be no effect of the ultrasound on the product.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organización Mundial de la Salud (OMS). Available online: https://www.who.int/health-topics/nutrition (accessed on 30 April 2023).
- Secretaria de Salud (SS). Available online: https://www.gob.mx/salud/articulos/el-plato-del-bien-comer-una-guia-para-una-buena-alimentacion (accessed on 30 April 2023).
- Instituto Mexicano del Seguro Social (IMSS). Available online: http://www.imss.gob.mx/salud-en-linea/platobiencomer (accessed on 30 April 2023).
- Zink, K.D.; Lieberman, D.E. Impact of meat and lower Palaeolithic food processing techniques on chewing in humans. Nature 2016, 531, 500–503. [Google Scholar] [CrossRef] [PubMed]
- American Meat Science Association. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurement of Meat, 2nd ed.; AMSA: Kearney, MO, USA, 2015. [Google Scholar]
- Barbur, S. Review: Automatitation and meat quality-global challenges. Meat Sci. 2014, 96, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kristesen, L.; Stoier, S.; Würtz, J.; Hinrischsen, L. Trends in Meat Science and Technology: The future looks bright, but the journey will be long. Meat Sci. 2014, 98, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Turantas, F.; Basyingit, G.; Kilic, B. Ultrasound in the meat industry: General applications and decontaminations efficiency. Int. J. Food Microbiol. 2015, 198, 59–69. [Google Scholar] [CrossRef]
- Zhang, W.; Maheswarapa Naveena, B.; Jo, C.; Sakata, R.; Zhou, G.; Banerjee, R.; Nishiumi, T. Technological demands of meat processing—An Asian perspective. Meat Sci. 2017, 132, 35–44. [Google Scholar] [CrossRef]
- Pogge, D.J.; Lonergan, S.M.; Hanse, S.L. Influence of supplementing vitamin C to yearling steers fed a high sulfur diet during the finishing period on meat color, tenderness and protein degradation, and fatty acid profile of the longissimus muscle. Meat Sci. 2014, 97, 419–427. [Google Scholar] [CrossRef]
- Damez, J.-L.; Clerjon, S. Quantifying and predicting meat and meat products quality attributes using electromagnetic waves: An overview. Meat Sci. 2013, 95, 879–896. [Google Scholar] [CrossRef]
- Hwang, I.H.; Thompson, J.M. The effect of time and type of electrical stimulation on the calpain system and meat tenderness in beef longissimus dorsi muscle. Meat Sci. 2001, 58, 135–144. [Google Scholar] [CrossRef]
- Clerjon, S.; Damez, J.L. Microwave sensing for meat and fish structure evaluation. Meas. Sci. Technol. 2007, 18, 1038–1045. [Google Scholar] [CrossRef]
- Solomon, M.B.; Long, J.B.; Eastridge, J.S. The hydrodyne: A new process to improve beef tenderness. J. Anim. Sci. 1997, 75, 1534–1537. [Google Scholar] [CrossRef]
- Solomon, M. Hydrodyne Exploding meat terderness. AG Res. Mag. 1998, 46, 8–10. [Google Scholar]
- Solomon, M. The Hydrodyne process for tenderizing meat. In Proceedings of the 51st Annual Reciprocal Meat Conference, University of Connecticut, Storrs, CT, USA, 28 June–1 July 1998; pp. 171–176. [Google Scholar]
- Zuckerman, H.; Solomon, M.B. Ultrastructural changes in bovine longissimus muscle casused by the hydrodyne process. J. Muscle Foods 1998, 9, 419–426. [Google Scholar] [CrossRef]
- Boye, J.I.; Arcand, I. (Eds.) Green Technologies in Food Production and Processing; Springer: Echo Bay, ON, Canada, 2012. [Google Scholar]
- Sun, D.-W. (Ed.) Emerging Technologies for Food Processing; Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Mohareb, E.; Piyasena, P. Applications of ultrasound in the food industry: A review. NutraCos 2002, 1, 36–40. [Google Scholar]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Application and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Lens, P.; Virkutyte, J. Low-frequency ultrasound in biotechnology: State of the art. Trends Biotechnol. 2009, 27, 298–306. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Chemat, F.; Humma, Z.-E.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P.; Paniwnyk, L.; Pollet, B. Ultrasound starts quiet revolution in material science. Mater. World 1999, 7, 69–71. [Google Scholar]
- Gómez-Díaz, J.; López-Malo, A. Aplicaciones del ultrasonido en el tratamiento de alimentos. Temas. Sel. Ing. Aliment. 2009, 3, 59–73. [Google Scholar]
- Delgado, J.O. Aplicación del ultrasonido en la industria de los alimentos. Rev. Espec. Ing. Procesos Aliment. Y Biomater. 2012, 6, 141–152. [Google Scholar] [CrossRef]
- Robles-Ozuna, L.E.; Ochoa-Martínez, L.A. Ultrasonido y sus aplicaciones en el proceso de alimentos. Rev. Iber. Tecnol. Postcosecha 2012, 13, 109–122. [Google Scholar]
- Reza Kasaai, M. Input power-mechanism relationship for ultrasonic irradiation: Food and polymer applications. Nat. Sci. 2013, 5, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Sango, D.M.; Abela, D.; McElhatton, A.; Valdramis, V.P. Assisted ultrasound applications for the production of safe foods. J. Appl. Microbiol. 2014, 116, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Sahin Ercan, S.; Soysal, C. Use of ultrasound in food preservation. Nat. Sci. 2013, 5, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F. Impact of ultrasound on structure, physicochemical properties, modification, and applications of starch. Trends Food Sci. Technol. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Singh Sharanagat, V. Advances in applications of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 1205293. [Google Scholar] [CrossRef]
- Feng, H.; Yang, W.; Hielscher, T. Power Ultrasound. Food Sci. Technol. Int. 2008, 14, 433–436. [Google Scholar] [CrossRef]
- Gallego-Juarez, J.A. High power ultrasonic processing: Recent developments and prospective advanvces. Phys. Procedia 2010, 3, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Leighton, T.G. The Acoustic Bubble, 1st ed.; Elsevier Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-323-14413-1. [Google Scholar]
- Caraveo, O.; Alarcon-Rojo, A.D.; Renteria, A.; Santellano, E.; Paniwnyk, L. Physicochemical and microbiological characteristics of beef treated with high-intensity ultrasound and stored at 4 °C. J. Sci. Food Agric. 2015, 95, 2487–2493. [Google Scholar] [CrossRef]
- Zhang, M.; Haili, N.; Chen, Q.; Xia, X.; Kong, B. Influence of ultrasound-assisted immersion freezing on the freezing rate quality of porcine longissimus muscles. Meat Sci. 2018, 136, 1–8. [Google Scholar] [CrossRef]
- Blasco, A.; Nagy, I.; Hernández, P. Genetics of growth, carcass and meat quality in rabbits. Meat Sci. 2018, 145, 178–185. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Burgess, C.; Kerry, J.; Tiwari, B.K. Influence of extrinsic operational parameters on salt diffusion during ultrasound assisted meat curing. Ultrasonics 2018, 83, 164–170. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.L.; Mason, S.; Bekhit, A.E.-D. Role of calpain system in meat ternerness: A review. Food Sci. Hum. Wellness 2018, 7, 196–204. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Jayasooriya, S.D.; Bhandari, B.R.; Torley, P.; D’Arcy, B.R. Effect of high power ultrasound wave son properties of meat: A review. Int. J. Food Prop. 2004, 7, 301–319. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, Z.; Liu, Q.; Chen, Q.; Kong, B. Changes in microstructure, quality and water distribution of porcine longissimus muscles subjected to ultrasound-assisted immersion freezing during frozen storage. Meat Sci. 2019, 151, 24–32. [Google Scholar] [CrossRef]
- de Lima Alves, L.; da Silva, M.S.; Martins Flores, D.R.; Rodrigues Athayde, D.; Roggia Ruviaro, A.; da Silva Brum, D.; Fagundes Batista, V.S.; de Oliveira Mello, R.; de Menezes, C.R.; Bastianello Campagnol, P.C.; et al. Effect of ultrasound on the physicochemical and microbiological characteristics of Italian salami. Food Res. Int. 2017, 106, 363–373. [Google Scholar] [CrossRef]
- Amiri, A.; Sharifian, P.; Soltanizadeh, N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018, 111, 139–147. [Google Scholar] [CrossRef]
- Kang, D.; Zou, Y.; Chen, Y.; Xing, L.; Zhou, G.; Zhang, W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016, 33, 47–53. [Google Scholar] [CrossRef]
- Ozuna, C.; Cárcel, J.A.; Walde, P.M.; Garcia-Perez, J.V. Low-temperature drying of salted cod (Gadus morhu) assited by high power ultrasound: Kinetics and physical properties. Innov. Food Sci. Emerg. Technol. 2014, 23, 146–155. [Google Scholar] [CrossRef]
- Kang, D.; Wang, A.; Zhou, G.; Zhang, W.; Xu, S.; Guo, G. Power ultrasonic on mass transport of beef: Effects of ultrasound intensity and NaCl concentration. Innov. Food Sci. Emerg. Technol. 2016, 35, 36–44. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, L.; Luna-Rodriguez, L.; Carrillo-Lopez, L.M.; Alarcon-Rojo, A.D.; Garcia-Galicia, I.; Reyes-Villagrana, R. Ultrasound as an alternative to conventional marination: Acceptability and mass transfer. J. Food Qual. 2017, 2017, 8675720. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Kang, Z.-L.; Zhao, Y.-Y.; Xu, X.-L.; Zhou, G.-H. Use of high-intensity ultrasound to improve functional properties of batter suspensions prepared from pse-like chicken breast meat. Food Bioprocess Technol. 2014, 7, 3466–3477. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, R. Effect of ultrasonication on secondary structure and heat induced gelation of chicken myofibrils. J. Food Sci. Technol. 2016, 53, 3340–3348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, C.K.; Allen, P.; Morin, C.; Lyng, J.G. The effect of ultrasonic salting on protein and water–protein interactions in meat. Food Chem. 2014, 147, 245–251. [Google Scholar] [CrossRef]
- Gambuteanu Filimon, C.; Alexe, P. Effects of ultrasound on technological properties of meat a review. Annals Food Sci. Technol. 2013, 14, 176–182. [Google Scholar]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.C.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jimenez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Hui, Y.H. (Ed.) Handbook of Meat and Meat Processing, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Badui Dergal, S. La Ciencia de Los Alimentos en la Práctica, 2nd ed.; Pearson Educación De México: Ciudad de México, México, 2015; p. 246. [Google Scholar]
- Vilgis, T.A. Soft matter food physics—The physics of food and cooking. Rep. Prog. Phys. 2015, 78, 124602. [Google Scholar] [CrossRef]
- Toldrá, F. (Ed.) Handbook of Meat Processing, 1st ed.; Wiley-Blackwell: Singapore, 2010. [Google Scholar]
- Bourne, M.C. Food Texture and Viscosity, Concept and Measurement, 2nd ed.; Academic Press: London, UK, 2002. [Google Scholar]
- Warner, D.; McDonell, C.; Bekhit, A.E.D.; Claus, J.; Vaskoska, R.; Sikes, A.; Dunshea, F.R.; Ha, M. Systematic review of emerging and innovative technologies for meat tenderization. Meat Sci. 2017, 132, 72–89. [Google Scholar] [CrossRef]
- Cummings, E.J.; Lyng, J.G. (Eds.) Emerging Technologies in Meat Processing Production, Processing and Technology, 1st ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Nishihara, T.; Doty, P. The sonic fragmentation of collagen macromolecules. Proc. Natl. Acad. Sci. USA 1958, 44, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, J.B.; Anderson, D.B.; Schmidt, G.R.; Theno, D.M.; Siegel, D.G. Effects of ultrasonic treatment on binding strength in cured ham rolls. J. Food Sci. 1978, 43, 866–869. [Google Scholar] [CrossRef]
- Smith, N.B.; Cannnon, J.J.; Novakofski, E.E.; McKeith, F.K.; O’Brien, W.D., Jr. Tenderization of Semitendenious Muscle Using High Intensity Ultrasound. In Proceedings of the IEEE 1991 Ultrasonics Symposium, Orlando, FL, USA, 8–11 December 1991; Volume 2, pp. 1371–1374. [Google Scholar] [CrossRef]
- Pohlman, F.W.; Dikeman, M.E.; Kropf, D.H. Effects of high intensity ultrasound treatment storage time and cooking method on shear, sensory, instrumental color and cooking properties of packaged and unpackaged beef pectoralis muscle. Meat Sci. 1997, 46, 89–100. [Google Scholar] [CrossRef]
- Pohlman, F.W.; Dikeman, M.E.; Zayas, J.F. The effect of low-intensity ultrasound treatment on shear properties, color stability and shelf-life of vacuum-packaged beef semitendinosus and biceps femoris muscles. Meat Sci. 1997, 45, 329–337. [Google Scholar] [CrossRef]
- Lyng, J.G.; Allen, P.; McKenna, B.M. The influence of high intensity ultrasound baths on aspects of beef tenderness. J. Muscle Foods 1997, 8, 237–249. [Google Scholar] [CrossRef]
- Got, F.; Culioli, J.; Berge, P.; Vignon, X.; Astruc, T.; Quideau, J.M.; Lethiecq, M. Effects of high-intensity high-frequency ultrasound on ageing rate, ultrastructure and some physic-chemical properties of beef. Meat Sci. 1999, 51, 35–42. [Google Scholar] [CrossRef]
- Jayasooriya, S.D.; Torley, P.J.; D’Arcy, B.R.; Bhandari, B.R. Effects of high power ultrasound and ageing on the physical properties of bovine semitendinosus and longissimus muscles. Meat Sci. 2007, 75, 628–639. [Google Scholar] [CrossRef]
- Stadnik, J.; Dolatowski, Z.J.; Baranowska, H.M. Effect of ultrasound treatment on water holding properties and microstructure of beef (m. semimembranosus) during ageing. LWT-Food Sci. Technol. 2008, 41, 2151–2158. [Google Scholar] [CrossRef]
- Sikes, A.L.; Mawson, R.; Stark, J.; Warner, R. Quality properties of pre- and post-rigor beef muscle after interventions with high frequency ultrasound. Ultrasound Sonoichem. 2014, 21, 2138–2143. [Google Scholar] [CrossRef]
- Chen, L.; Feng, X.-C.; Zhang, Y.-Y.; Liu, X.-B.; Zhang, W.-G.; Li, C.-B.; Ullah, N.; Xu, X.-L.; Zhou, G.-H. Effects of ultrasonics processing on caspase-3, calpain expression and myofibrillar structure of chicken during post-mortem ageing. Food Chem. 2015, 177, 280–287. [Google Scholar] [CrossRef]
- Chang, H.-J.; Wang, Q.; Tang, C.-H.; Zhou, G.-H. Effects of ultrasound treatment on connective tissue collagen and meat quality of beef semitendinosus. J. Food Qual. 2015, 38, 256–267. [Google Scholar] [CrossRef]
- Hu, J.; Ge, S.; Huang, C.; Cheung, P.C.K.; Lin, L.; Zhang, Y.; Zheng, B.; Lin, S.; Huang, X. Tenderization effect of whelk meat using ultrasonic treatment. Food Sci. Nutr. 2018, 6, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Cichoski, A.J.; Silva, M.S.; Vaz Leaes, Y.S.; Bauermann Brasil, C.C.; de Menezes, C.R.R.; Smanioto, J.; Wagner, R.P.; Bastianello Campagnol, C. Ultrasound: A promising technology to improve the technological quality of meat emulsions. Meat Sci. 2019, 148, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Basso Pinton, M.; Pereira Correa, L.; Facchi, M.M.X.; Heck, R.T.; Vaz Leaes, Y.S.; Cichoski, A.J.; Lorenzo, J.M.; dos Santos, M.; Rodriguez Pollonio, M.A.; Bastianello Campagnol, P.C. Ultrasound: A new approach to reduce phosphate content of meat emulsions. Meat Sci. 2019, 152, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Shi, H.; Xu, P.; Jiang, D.; Zhang, X.; Xu, W.; Wang, D. Combined effect of ultrasound and sodium bicarbonate marination on chicken breast tenderness and its molecular mechanism. Ultrason. Sonochem. 2019, 59, 104735. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, L.; Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Garcia-Galicia, I.A.; Huerta-Jimenez, M.; Paniwnyk, L. Does ultrasound equally improve the quality of beef? An insight into longissimus lumborum, infraspinatus and cleidooccipitalis. Meat Sci. 2020, 160, 107963. [Google Scholar] [CrossRef]
- Kang, D.; Gao, X.; Ge, Q.; Zhou, G.; Zhang, W. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017, 38, 317–325. [Google Scholar] [CrossRef]
- Barekat, S.; Soltanizadeh, N. Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 223–229. [Google Scholar] [CrossRef]
- Pena-Gonzalez, E.M.; Alarcon-Rojo, A.D.; Renteria, A.; Garcia, I.; Santellano, E.; Quintero, A.; Luna, L. Quality and sensory profile of ultrasound-treated beef. Ital. J. Food Sci. 2017, 29, 463–475. [Google Scholar] [CrossRef]
- Wang, A.; Kang, D.; Zhang, W.; Zhang, C.; Zou, Y.; Zhou, G. Changes in calpain activity, protein degradation and microstructure of beef M. semitendinosus by the application of ultrasound. Food Chem. 2018, 245, 724–730. [Google Scholar] [CrossRef]
- Barekat, S.; Soltanizadeh, N. Effects of ultrasound on microstructure and enzyme penetration in beef longissimus lumborum muscle. Food Bioprocess Technol. 2018, 11, 680–693. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, W.; Kang, D.; Zhou, G. Improvement of tenderness and water holding capacity of spiced beef by the application of ultrasound during cooking. Int. J. Food Sci. Technol. 2018, 53, 828–836. [Google Scholar] [CrossRef]
- Li, K.; Kang, Z.-L.; Zou, Y.-F.; Xu, X.-L.; Zhou, G.-H. Effect of ultrasound treatment on functional properties of reduced-salt chicken meat batter. J. Food Sci. Technol. 2015, 52, 2622–2633. [Google Scholar] [CrossRef]
- Ojha, K.S.; Keenan, D.F.; Bright, A.; Kerry, J.P.; Tiwari, B.K. Ultrasound assisted diffusion of sodium salt replacer and effect on physicochemical properties of pork meat. Int. J. Food Sci. Technol. 2016, 51, 37–45. [Google Scholar] [CrossRef]
- Filomena-Ambrosio, A.; Quintanilla-Carvajal, M.X.; Puig, A.; Hernando, I.; Hernandez-Carrion, M.; Sotelo-Diaz, I. Changes of the water-holding capacity and microstructure of panga and tilapia surimi gels using different stabilizers and processing methods. Food Sci. Technol. Int. 2016, 22, 68–78. [Google Scholar] [CrossRef]
- Fan, D.; Huang, L.; Li, B.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Acoustic intensity in ultrasound field and ultrasound-assisted gelling of surimi. LWT—Food Sci. Technol. 2017, 75, 497–504. [Google Scholar] [CrossRef]
- Gomez-Salazar, J.A.; Ochoa-Montes, D.A.; Ceron-Garcia, A.; Ozuna, C.; Sosa-Morales, M.E. Effect of Acid marination assisted by power ultrasound on the quality of rabbit meat. J. Food Qual. 2018, 2018, 5754930. [Google Scholar] [CrossRef]
- García-Galicia, I.A.; Huerta-Jimenez, M.; Morales-Piñon, C.; Diaz-Almanza, S.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Estepp, C.; Alarcon-Rojo, A.D. The impact of ultrasound and vacuum pack on quality properties of beef after modified atmosphere on display. J. Food Process Eng. 2019, 43, e13044. [Google Scholar] [CrossRef]
- Diaz-Almanza, S.; Reyes-Villagrana, R.; Alarcon-Rojo, A.D.; Huerta-Jimenez, M.; Carrillo-Lopez, L.M.; Estepp, C.; Urbina-Perez, J.; García-Galicia, I.A. Time matters when ultrasonicating beef: The best time for tenderness is not the best for reducing microbial counts. J. Food Process Eng. 2019, 42, e13210. [Google Scholar] [CrossRef]
- Reyes-Villagrana, R.A.; Huerta-Jimenez, M.; Salas-Carrazco, J.L.; Carrillo-Lopez, L.M.; Alarcon-Rojo, A.D.; Sanchez-Vega, R.; Garcia-Galicia, I.A. High-intensity ultrasonication of rabbit carcases: A first glance into a small-scale model to improve meat quality traits. Ital. J. Anim. Sci. 2020, 195, 44–550. [Google Scholar] [CrossRef]
- García-Galicia, I.A.; Gonzalez-Vacame, V.G.; Huerta-Jimenez, M.; Carrillo-Lopez, L.M.; Tirado-Gallegos, J.M.; Reyes-Villagrana, R.A.; Alarcon-Rojo, A.D. Ultrasound versus traditional ageing: Physicochemical properties in beef longissimus lumborum. CYTA—J. Food 2020, 18, 675–682. [Google Scholar] [CrossRef]
- Chávez-Martínez, A.; Reyes-Villagrana, R.A.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Tirado-Gallegos, J.M.; Bolivar-Jacobo, N.A. Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiology Quality. Foods 2020, 9, 1688–1727. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Monterrubio, A.L.; Chávez-Martínez, A.; Juárez-Moya, J.; Sánchez-Vega, R.; Tirado-Gallegos, J.M.; Reyes-Villagrana, R.A. High-Intensity Ultrasound and Its Interaction with Foodstuff and Nanomaterials. In Trends and Innovation in Food Science, 1st ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80356-066-3. [Google Scholar] [CrossRef]
- Delalande, A.; Kotopoulis, S.; Postema, M.; Midoux, P.; Pichon, C. Sonoporation: Mechanistic insights and ongoing challenges for gene transfer. Gene 2013, 525, 191–199. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.D., Jr. Ultrasound—Biophysics mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, E.G. Fourier Acoustics, Sound Radiation; Academic Press: New York, NY, USA, 1999. [Google Scholar]
- Wiszecki, G.; Stiles, W.S. Color Science, Concepts and Methods, Quantitative Data, and Formulae, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Lindahl, G.; Lundström, K.; Tornberg, E. Contribution of pigment content, myoglobin forms and internal reflectance to the colour of pork loin and ham from pure breed pigs. Meat Sci. 2001, 59, 141–151. [Google Scholar] [CrossRef]
- O’Sullivan, M.G.; Byrne, D.V.; Martens, M. Evaluation of pork colour: Sensory colour assessment using trained and untrained sensory panellist. Meat Sci. 2003, 63, 119–129. [Google Scholar] [CrossRef]
- Nowak, K.W.; Ropelewska, E.; Bekhit, A.E.D.; Markowski, M. Ultrasound Applications in the Meat Industry; Bekhit, A.E.D., Ed.; Advances in Meat Processing; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxfordshire, UK, 2017. [Google Scholar]
- Macdougall, D.B. Changes in the colour and opacity of meat. Food Chem. 1982, 9, 75–82. [Google Scholar] [CrossRef]
- Killinger, K.M.; Calkins, C.R.; Umberger, W.J.; Feuz, D.M.; Eskridge, K.M. Consumer visual preference and valour for beef steaks differing in marbling level and color. J. Anim. Sci. 2004, 82, 3288–3293. [Google Scholar] [CrossRef]
- Madrigal-Melchor, J.; Enciso-Muñoz, A.; Contreras-Solorio, D.A.; Saldaña-Saldaña, X.; Reyes-Villagrana, R.A. A new alternative method for the generation of acoustic filters, modulating acoustic impedance: Theorical model. Open J. Acoust. 2017, 7, 39–51. [Google Scholar] [CrossRef] [Green Version]
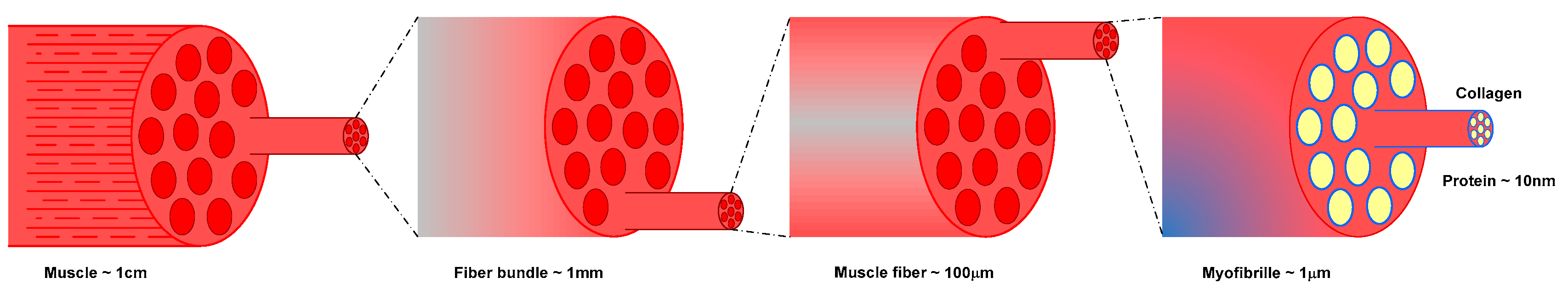
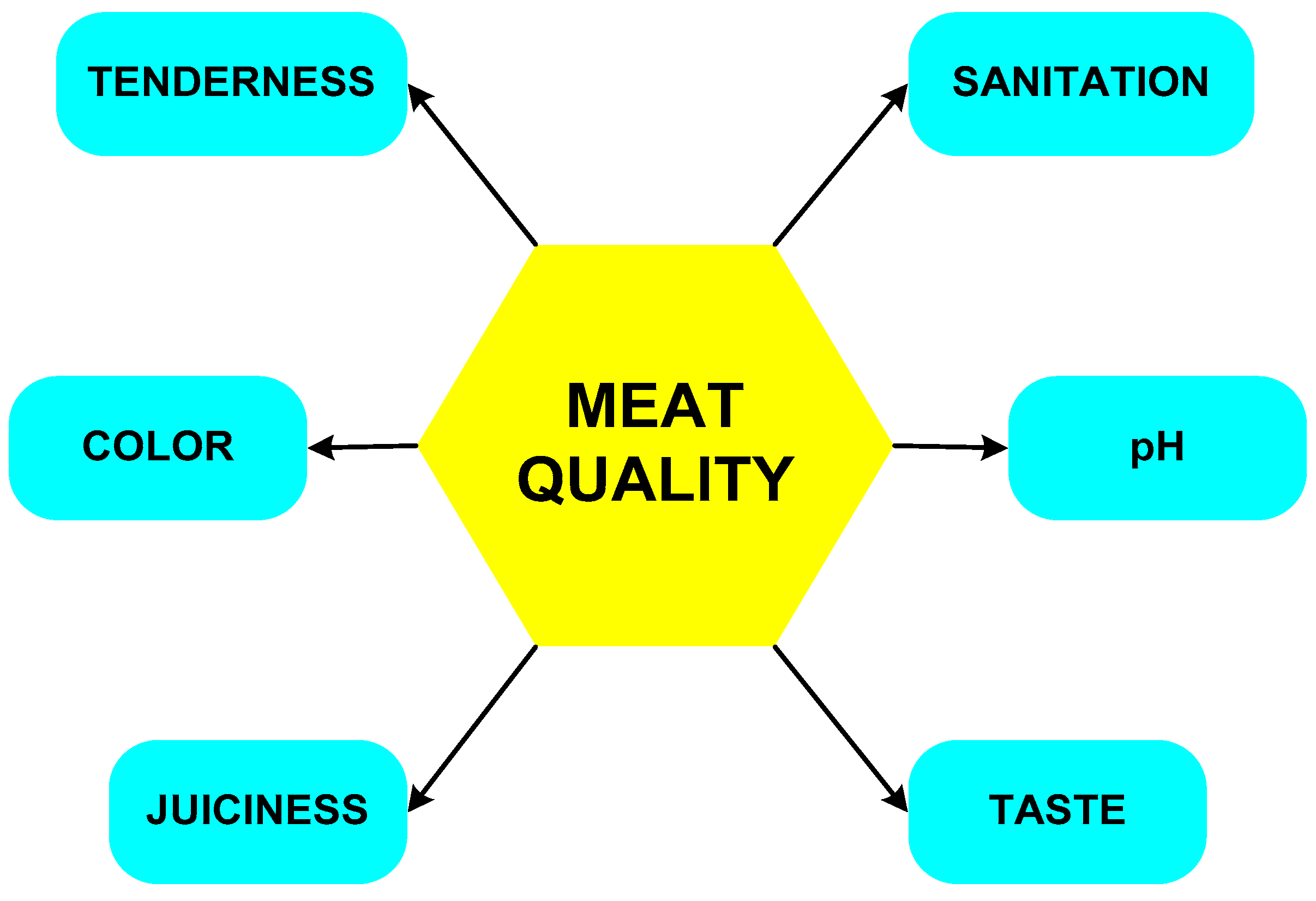
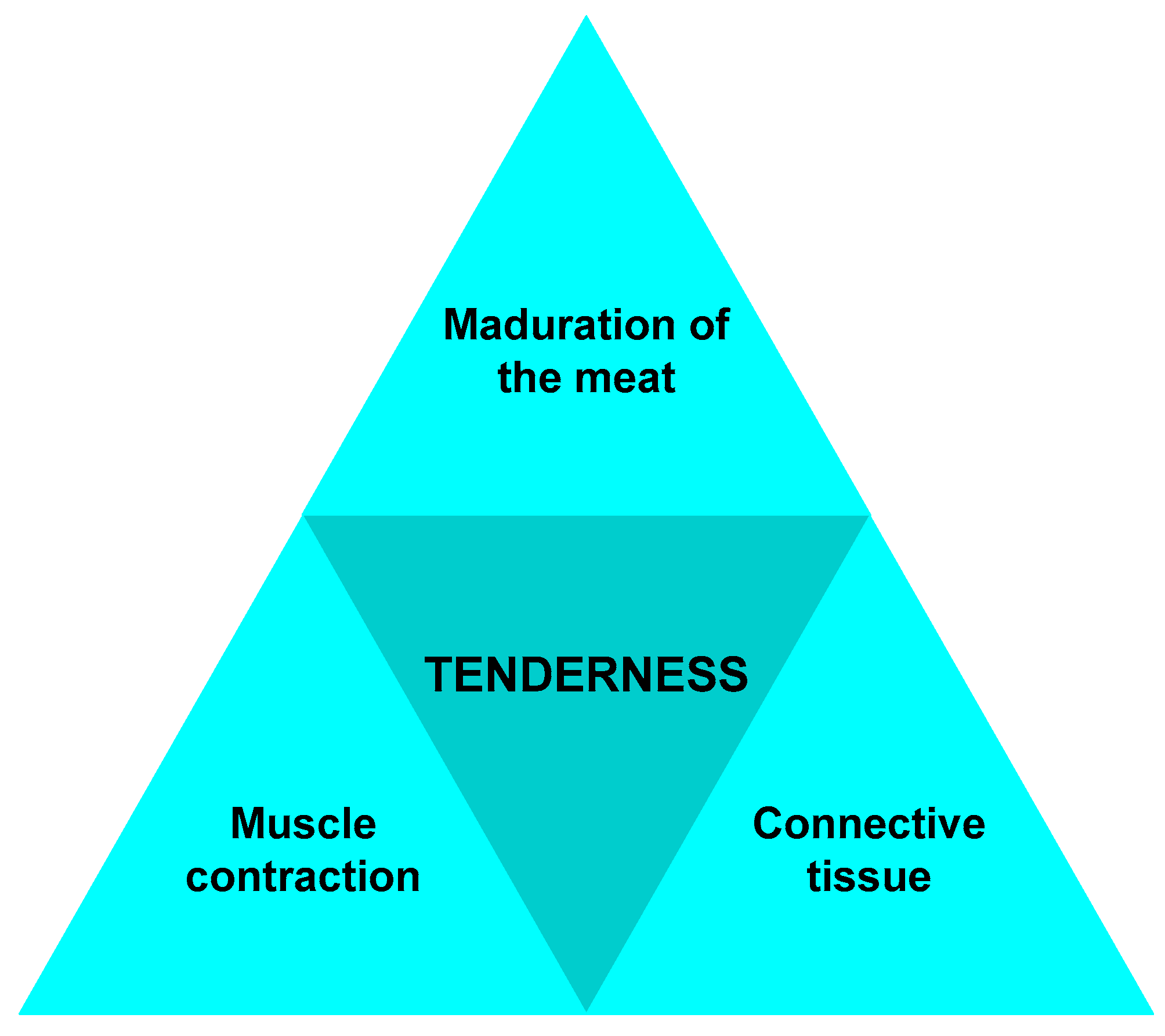
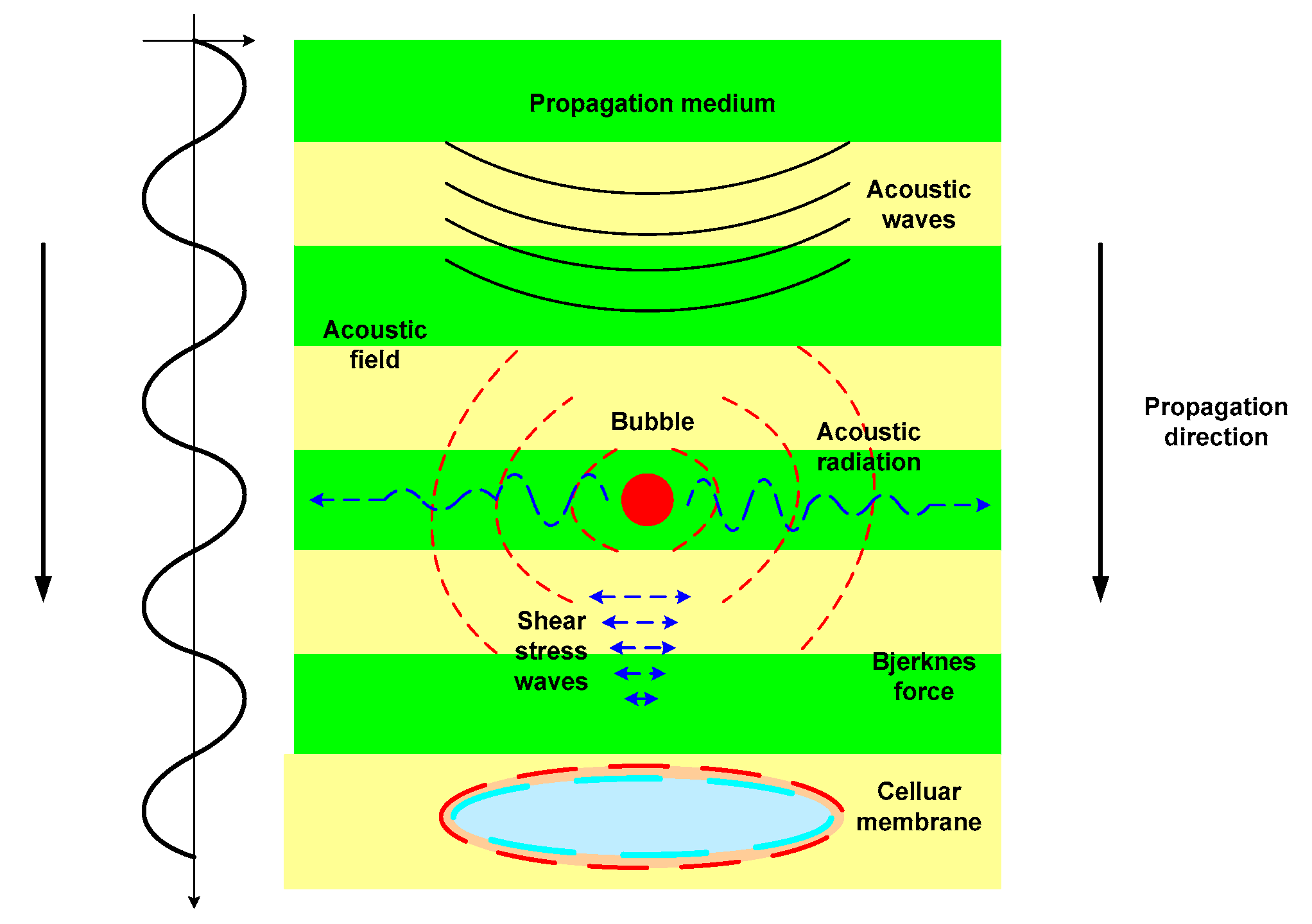
| Component (%) | Meat Type | ||||||
|---|---|---|---|---|---|---|---|
| Beef | Pork | Mutton | Chicken | Turkey | Tuna | Mojarra | |
| Water | 71 | 68 | 60 | 66 | 70 | 69 | 79 |
| Protein | 21 | 13 | 19 | 18 | 21 | 24 | 17 |
| Lipids | 7 | 18 | 20 | 15 | 8 | 6 | 3 |
| Minerals | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sample Description | Ultrasonic Parameters (Intensity, Frequency, Time, Temperature) | Texture—Tenderness 1 | Analyses to Demonstrate Changes 2 | Observations | Ref. |
|---|---|---|---|---|---|
| Soluble beef collagen | 50 W, 9 kHz, 10 to 440 min; 8–11 °C. | - | SA ACP | Molecular weights and hydrodynamic conditions were reviewed Viscosity reduction | [64] |
| Pork Cured ham Cylindric samples 25 long × 3.5 cm diameter | 40 kHz; 15, 30, 60, and 120 min. | Instron universal testing machine | SA MI | Effects of muscle microstructure, breaking force, cooking performance, and protein stability | [65] |
| Beef m. Semitendinosus 10 × 5 × 2.5 cm sample size | 1000 W, 25.9 kHz, 0, 2, 4, and 8 min (longitudinal cuts); 0, 2, 4 8 and 16 min (cross sections). | WB | SA | Increase in tenderness in 2 and 4 min | [66] |
| Beef m. Biceps pectoralis | 22 W/cm2; 20 kHz; 0, 5, and 10 min | WB | SA | Low effect on tenderness | [67] |
| Beef Vacuum packed m. Semitendinosus 2.5 × 6.4 × 10.2 cm 2.5 × 5.1 × 10.2 cm m. Biceps femoris 1.3 × 7.6 × 10.2 cm | 1.55 W/cm2; 20 KHz; 8, 16, and 24 min. 1.55 W/cm2; 2 kHz; 30 min (15 min/side). | WB | SA | No effect on tenderness | [68] |
| Beef Vacuum packed m. L. thoracis lumborum, Semimembranosus, Biceps femoris | Hilsonic: 0.29 W/cm2; 47 kHz. Kerry: 0.39 W/cm2; 34–42 kHz. Ultrawave: 0.62 W/cm2; 30–40 kHz. | Bite force trendometer | SA | No major effects on tenderness | [69] |
| Beef m. Semimembranosus | 10 W/cm2; 2.5 MHz; 2, 6, and 15 s. | Universal testing machine | SA MI | Unfavorable effects on tenderness | [70] |
| Beef m. Semitendinosus and Longissimus 60 mm × 40 cm × 20 mm | 12 W/cm2; 24 kHz; 4 min. | WB | SA | Beneficial effects on tenderness, including maturity time of 3 and 7 days | [71] |
| Beef m. Semimembranosus 70 × 70 × 80 mm | 2 W/cm2; 4 kHz; 2 min. | Nuclear magnetic resonance | SA MI | Favorable and unfavorable results | [72] |
| Beef m. L. lumborum pre- and post-rigor | 48 kPa–65 kPa at 600 kHz; 48 kPa at MHz. | Tenderometer | SA | Unfavorable effects | [73] |
| Chicken Breast 2.5 × 5.5 × 1.0 cm | 1500 W; 40 kHz, 30 or 60 min | Transmission electron microscopy | ACP SA MI | Favorable effects | [74] |
| Beef Vacuum packed m. Semitendinosus 2.5 × 5.0 × 5.0 cm | 1500 W; 40 kHz; 10, 20, 30, 40, 50, or 60 min; 20 °C. | WB Optical microscopy SEM | SA MI | Favorable effects | [75] |
| Molluscs 2 × 2 × 2 cm | 100–250 W; 45 kHz; 2–16 min; 10–60 °C. | WB SEM | SA MI Sensory evaluative | Favorable effects | [76] |
| Beef Lean (100 g) | 1000 W; 25 kHz; 60% amplitude 5.5 min, 10 °C. | TPA | SA ACP | Favorable effects | [77] |
| Beef Lean (100 g) | 230 W; 25 kHz; 60% amplitude 0, 9, and 18 min. | TPA | SA Sensory evaluative | Unfavorable effects | [78] |
| Chicken Breast 4 × 4 × 2 cm | 350 W; 20 kHz; 5 min. | TPA SEM | SA MI | Favorable effects | [79] |
| Beef m. L. lumborum 13 × 9 × 2.5 cm Infraspinatus, Cleidooccipitalis 6 × 7 × 2.5 | 11 W/cm2; 40 kHz; 0, 40, 60, and 80 min. | WB SEM | SA MI | Favorable effects for some muscles and not for others | [80] |
| Beef m. L. dorsi 20 × 50 × 10 mm | 150 and 300 W, 20 kHz; 30 and 120 min. | WB Electronic microscopy via transmission | SA MI | Increase tenderness | [81] |
| Beef m. L. lumborum 3 × 3 × 3 cm | 100 and 300 W, 20 kHz; 10, 20, and 30 min, 11–17 °C. | WB Optical microscopy | SA MI | Favorable effects | [82] |
| Beef m. L. dorsi | 11 W/cm2, 40 kHz; 60 min. | WB | SA Sensory evaluative | Benefits tenderness | [83] |
| Beef Vacuum packed m. Semitendinosus 80 × 70 × 25 mm | 25 W/cm2, 20 kHz; 20 or 40 min. | WB Transmission electron microscopy | SA IEM | Benefits tenderness | [84] |
| Beef m. L. lumborum 3 × 3 × 3 cm | 100 and 300 W, 20 kHz; 10, 20, and 30 min. Pulse train | Light microscopy SEM | SA IEM | Benefits tenderness combined with papain | [85] |
| Beef Flanks 8 × 8 × 8 cm | 0, 400, 600, 800, and 1000 W, 20 kHz; 80, 100, and 120 min. | TPA Nuclear magnetic resonance Transmission electron microscopy | SA IEM | Benefits tenderness with powers > 800 W and 120 min | [86] |
| Chicken Breast | 300 W, 40 kHz; 0, 10, 20, 30, and 40 min. | TPA SEM Nuclear magnetic resonance | SA IEM | There were no beneficial effects | [87] |
| Pork m. Semitendinosus 60 × 100 × 20 mm | 90 and 54.9 W/cm2, 20 kHz; 120 min. | WB Nuclear magnetic resonance | SA | Benefits tenderness, with intensity 54.9 W/cm2 | [88] |
| Fish fillets Pangasius hypothalamus and Oreochromis niloticus 10 × 10 × 10 mm | 150 W, 40 kHz; 15 min. | TPA SEM | SA IEM | Unfavorable effects | [89] |
| Seafood Silver carp Surimi gel | 300 W; 25, 45, 80, and 130 kHz. | Acoustic intensity measurements | SA | As the acoustic intensity increases, the force increases | [90] |
| Rabbit | 110 W; 40 kHz; 0 and 120 min; 4 °C. | TPA | SA | Increased toughness | [91] |
| Beef Vacuum packed m. L. lumborum, Semitendinosus 10 × 89 × 2.5 cm | 16 W/cm2, 28 W/cm2; 37 kHz; 40 min (20 min/side), 5 °C. | WB | SA | Unfavorable effects | [92] |
| Beef m. L. lumborum 10 × 5 × 2.5 cm | 90 W/cm2; 37 kHz; 0, 10, 20, or 40 min; 4 °C. | WB | SA | Tenderness benefits in times of 40 min | [93] |
| Rabbit Vacuum packed m. L. dorsi, Semimembranosus, Semitendinosus | 12 W/cm2; 24 kHz; 15 min; 5 °C. | WB | SA | Favorable effects on tenderness | [94] |
| Beef Vacuum packed m. L. lumborum 2.5 cm thick | 90 W/cm2; 37 kHz, 40 min/side, 4 °C. | WB | SA | Unfavorable effects on tenderness | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Villagrana, R.A.; Madrigal-Melchor, J.; Chávez-Martínez, A.; Juárez-Moya, J.; Rentería-Monterrubio, A.L. Effects of Shear Stress Waves on Meat Tenderness: Ultrasonoporation. Foods 2023, 12, 2390. https://doi.org/10.3390/foods12122390
Reyes-Villagrana RA, Madrigal-Melchor J, Chávez-Martínez A, Juárez-Moya J, Rentería-Monterrubio AL. Effects of Shear Stress Waves on Meat Tenderness: Ultrasonoporation. Foods. 2023; 12(12):2390. https://doi.org/10.3390/foods12122390
Chicago/Turabian StyleReyes-Villagrana, Raúl Alberto, Jesús Madrigal-Melchor, América Chávez-Martínez, Juliana Juárez-Moya, and Ana Luis Rentería-Monterrubio. 2023. "Effects of Shear Stress Waves on Meat Tenderness: Ultrasonoporation" Foods 12, no. 12: 2390. https://doi.org/10.3390/foods12122390
APA StyleReyes-Villagrana, R. A., Madrigal-Melchor, J., Chávez-Martínez, A., Juárez-Moya, J., & Rentería-Monterrubio, A. L. (2023). Effects of Shear Stress Waves on Meat Tenderness: Ultrasonoporation. Foods, 12(12), 2390. https://doi.org/10.3390/foods12122390





