Effects of Reactive Oxygen Levels on Chilling Injury and Storability in 21 Apricot Varieties from Different Production Areas in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition of Materials of 21 Varieties of Apricots
2.2. Apricot Cold Storage and Shelf Treatment Methods
2.3. Color, Firmness and Weight Loss Rate Determination
2.4. Determination of Soluble Sugar and Organic Acid Content, Ascorbic Acid, Total Carotenoids, Total Flavonoid Content and Total Phenolic Content
2.5. Statistics of Chilling Injury Rate and Chilling Injury Index
2.6. Determination of MDA Content and Cell Membrane Permeability
2.7. Determination of Hydrogen Peroxide, Superoxide Anion Production Ability and DPPH Free Radical Scavenging Ability
2.8. Activity of Antioxidant Enzymes
2.9. Statistical Analysis
3. Results
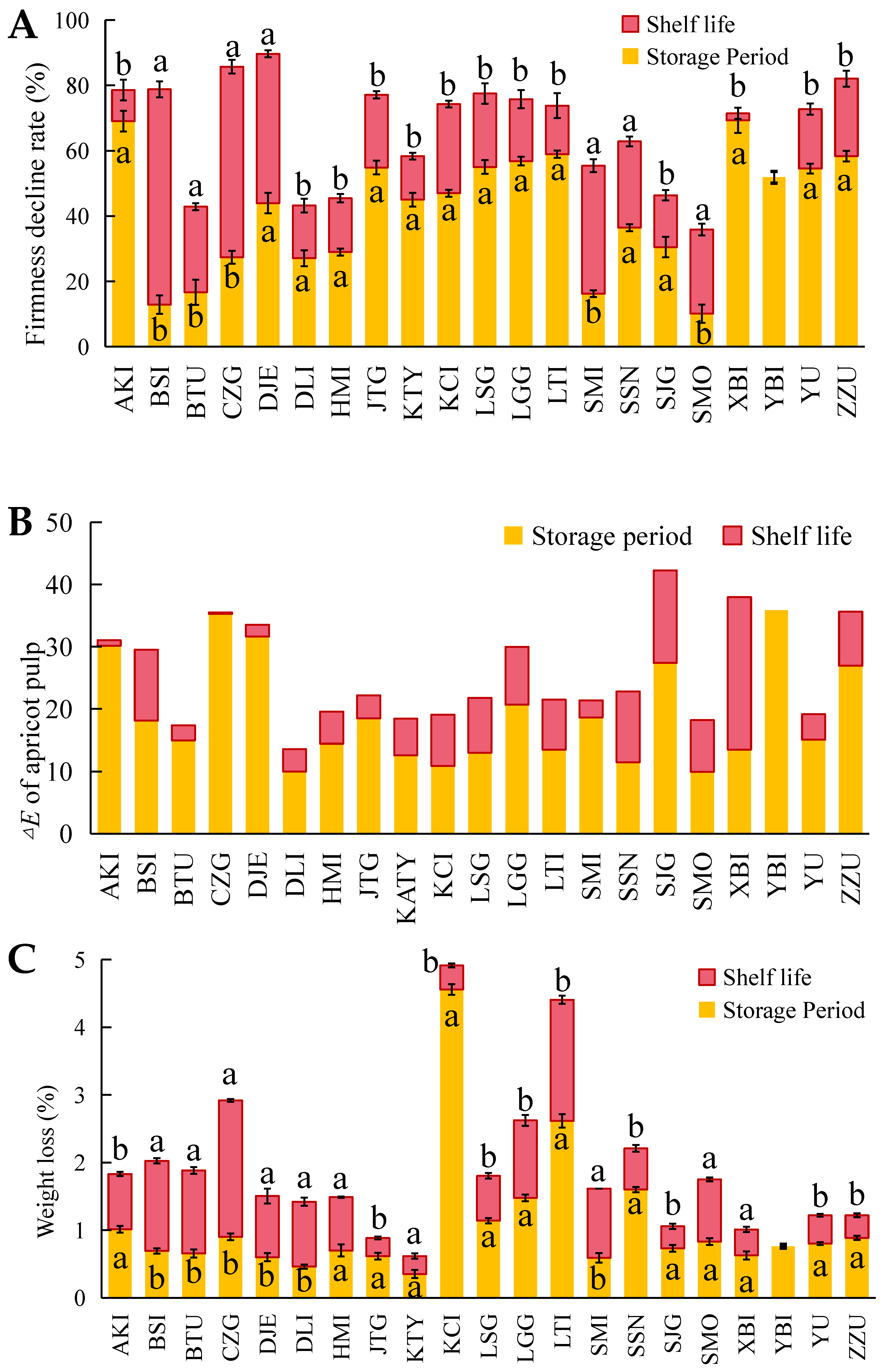
3.1. Quality Changes and Shelf-Life Ripening Performance after Cold Storage of Different Varieties of Apricots
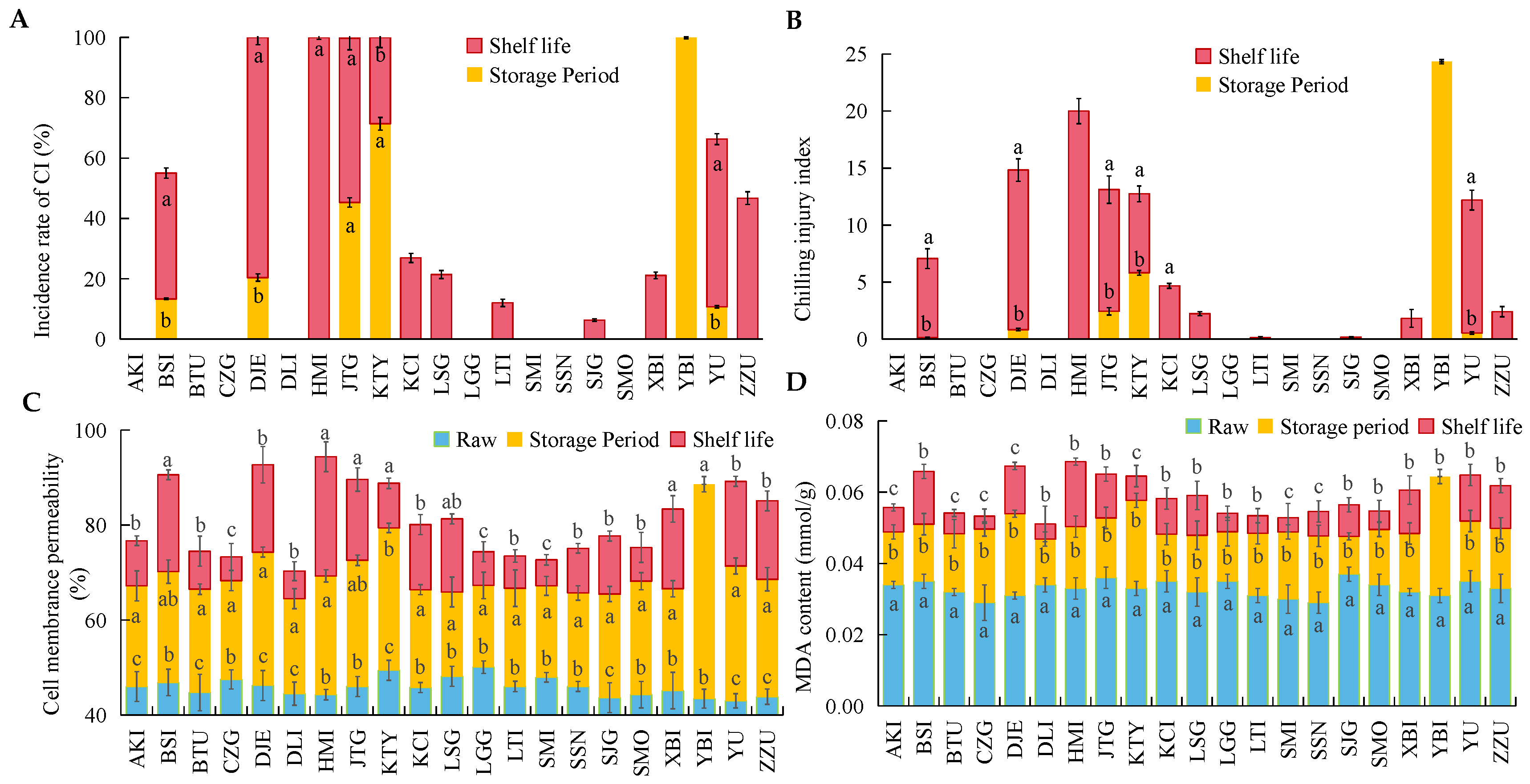
3.2. Chilling Injury in Different Apricot Varieties after Cold Storage and Shelf Life
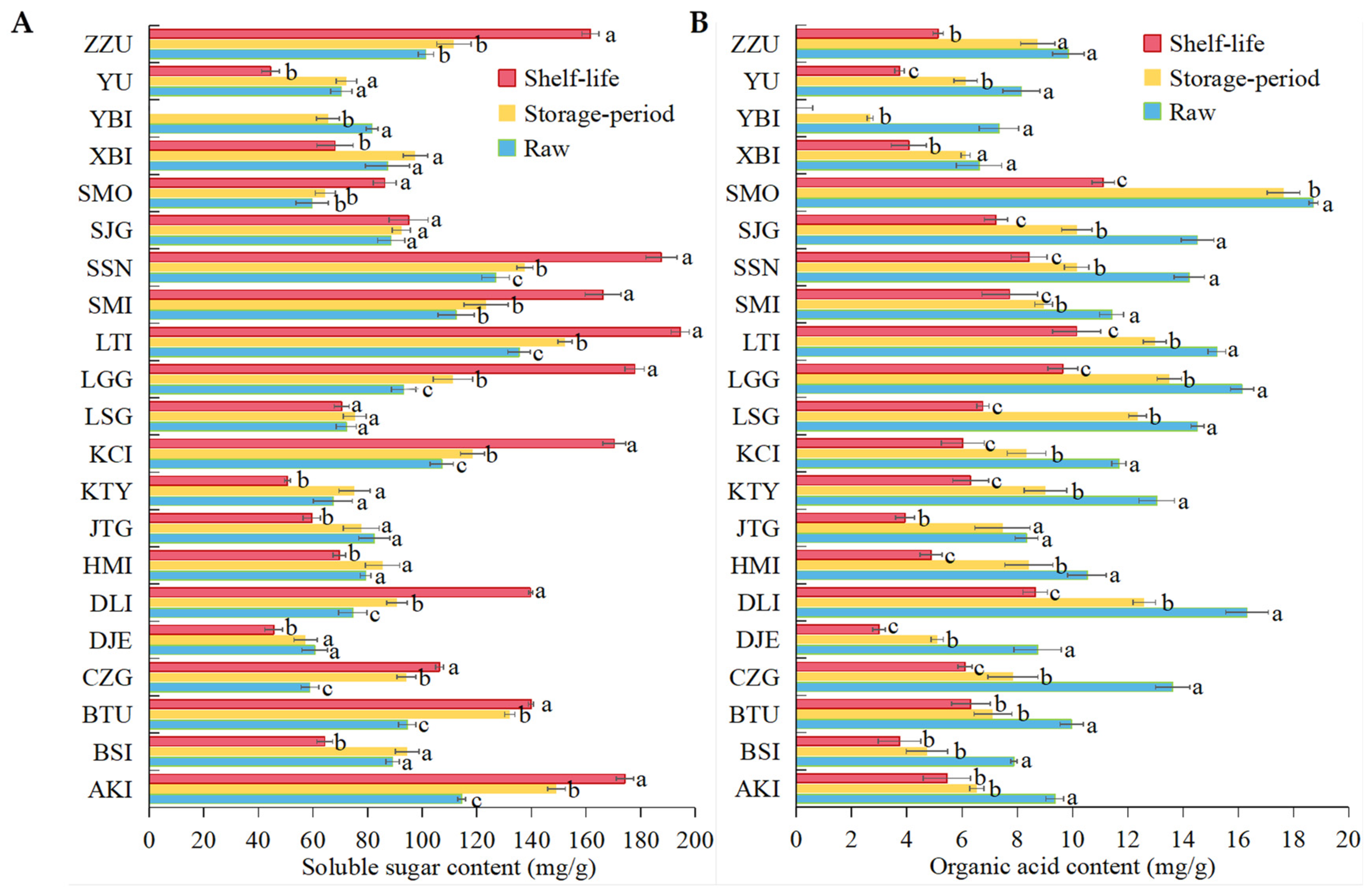
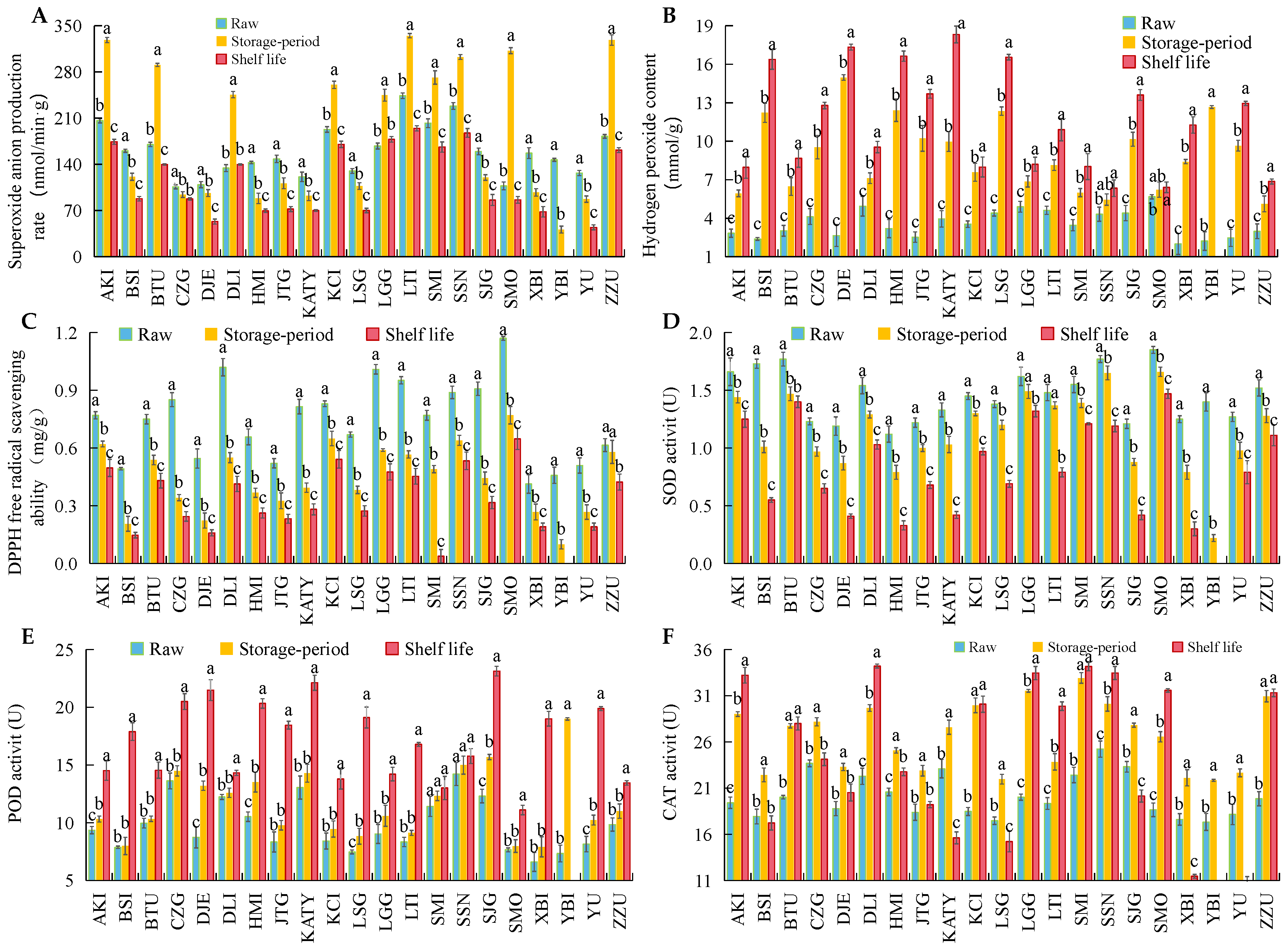
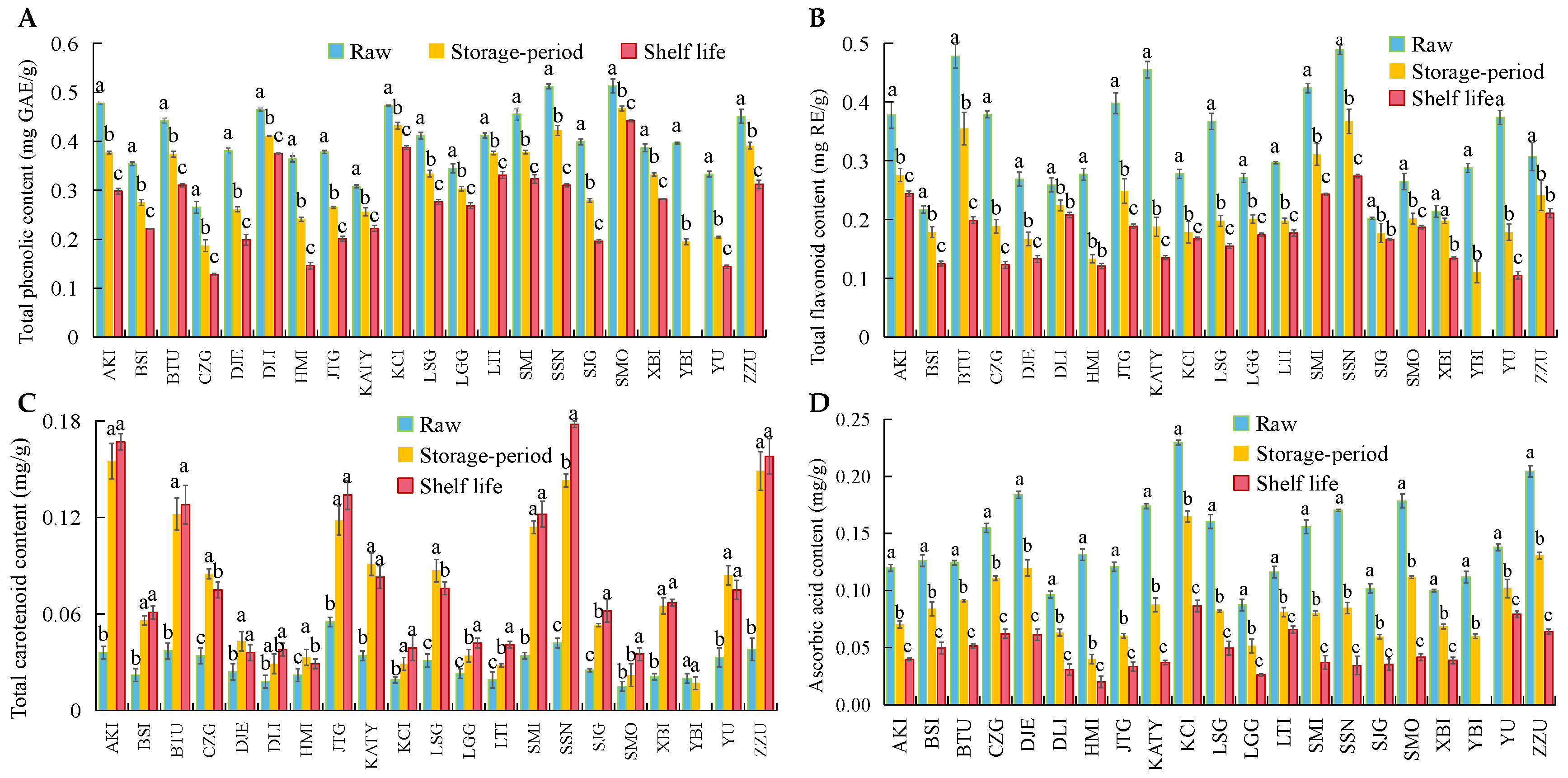
3.3. Changes in ROS Metabolism and Bioactive Substances in 21 Apricot Varieties after Cold Storage
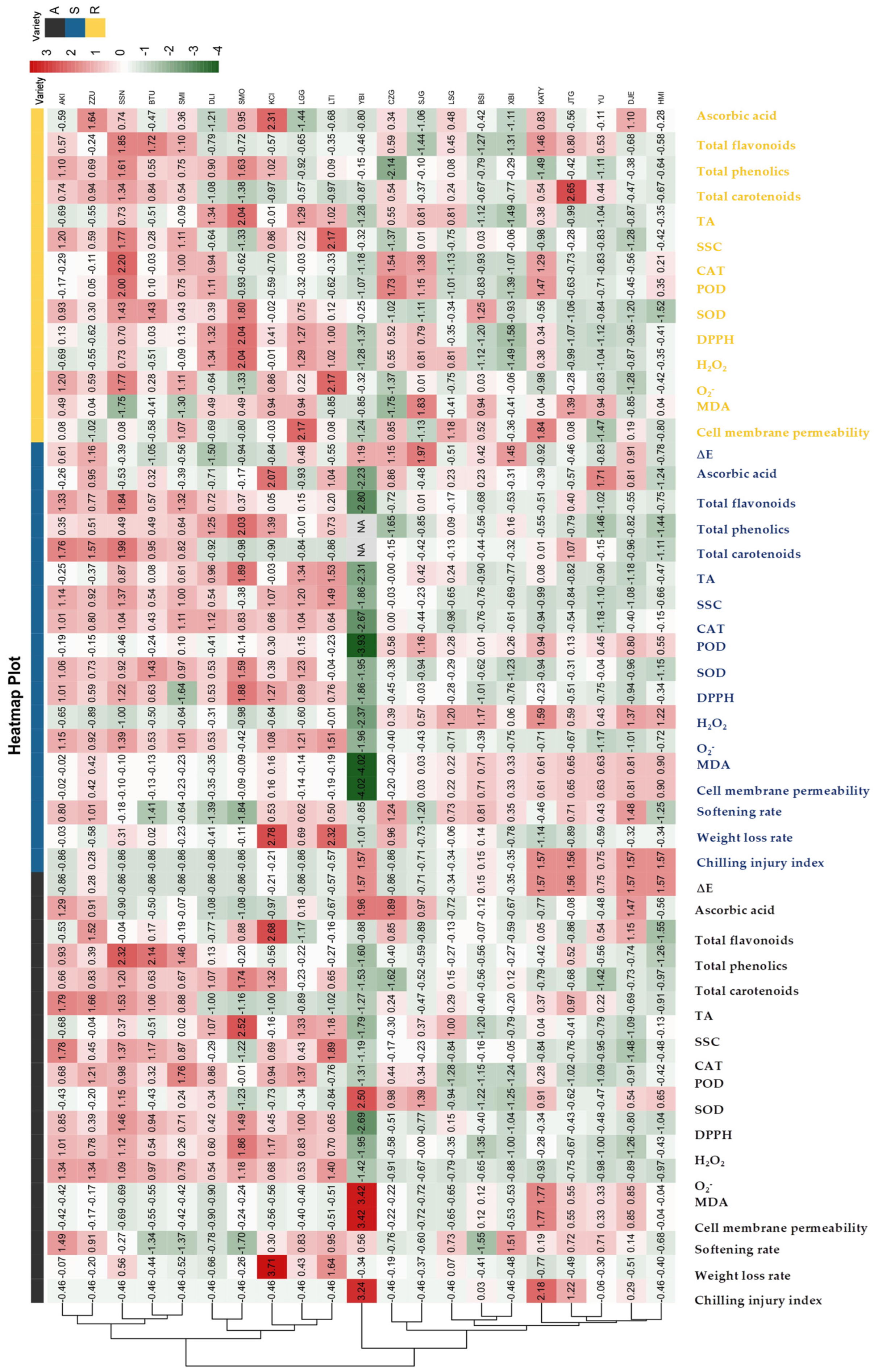
3.4. Cluster Heat Map Analysis of 21 Apricot Varieties for Storage Tolerance
4. Discussion
4.1. The Metabolic Imbalance of Reactive Oxygen Species Leads to the Occurrence of Fruit Chilling Injury
4.2. Apricots with High Initial Sugar, Acid and Other Nutrients Content Are Resistant to Low-Temperature Storage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Zhang, D.; Yu, K.; Ji, J.; Liu, N.; Zhang, Y.; Liu, W. Frequent germplasm exchanges drive the high genetic diversity of Chinese-cultivated common apricot germplasm. Hortic. Res. 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Prakash, R.; Marshall, R.; Schröder, R.; Stanley, J. Effect of harvest maturity and cold storage on correlations between fruit properties during ripening of apricot (Prunus armeniaca). Postharvest Biol. Technol. 2013, 82, 39–50. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Fan, X.; Jiao, W.; Cao, J.; Jiang, W. Near freezing point temperature storage inhibits chilling injury and enhances the shelf life quality of apricots following long-time cold storage. J. Food Process. Preserv. 2019, 43, e13958. [Google Scholar] [CrossRef]
- Kou, S.; Chen, Y.; Liu, T.; Liu, S.; Fang, Z.; Li, X.; Song, B. Crosstalk of putrescine synthetic pathway with abscisic acid signaling pathway in cold tolerance of potato. Environ. Exp. Bot. 2022, 204, 105085. [Google Scholar] [CrossRef]
- Tohá, J.; González, R.; Krarup, C. Symptoms and Sensitivity to Chilling Injury of Cantaloupe Melons during Postharvest. Chil. J. Agric. Res. 2009, 69, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, F.; Martinez, C.; Jamilena, M.; Garrido, D. Differential response of zucchini varieties to low storage temperature. Sci. Hortic. 2011, 130, 90–96. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; López-Climent, M.; Gómez-Cadenas, A.; Zacarías, L. Implication of the antioxidant system in chilling injury tolerance in the red peel of grapefruit. Postharvest Biol. Technol. 2016, 111, 214–223. [Google Scholar] [CrossRef]
- Kumari, K.; Vashistha, P.; Rai, A. Umbrella review on chilling injuries: Post-harvest issue, cause, and treatment in tomato. Sci. Hortic. 2022, 293, 110710. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Lurie, S. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Jing, G.; Huang, H.; Yang, B.; Li, J.; Zheng, X.; Jiang, Y. Effect of pyrogallol on the physiology and biochemistry of litchi fruit during storage. Chem. Cent. J. 2013, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Kang, Z.; Chen, L.; Guo, Y.; Mu, Q.; Wang, S.; Feng, C. Quality classification of kiwifruit under different storage conditions based on deep learning and hyperspectral imaging technology. J. Food Meas. Charact. 2023, 17, 289–305. [Google Scholar] [CrossRef]
- Kulbacka, J.; Saczko, J.; Chwiłkowska, A. Stres oksydacyjny w procesach uszkodzenia komórek [Oxidative stress in cells damage processes]. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2009, 27, 44–47. Available online: https://www.researchgate.net/publication/230844232_Oxidative_stress_in_cells_damage_processes_Stres_oksydacyjny_w_procesach_uszkodzenia_komorek (accessed on 15 April 2009).
- Chen, L.L.; Shan, W.; Cai, D.L.; Chen, J.Y.; Lu, W.J.; Su, X.G.; Kuang, J.F. Postharvest application of glycine betaine ameliorates chilling injury in cold-stored banana fruit by enhancing antioxidant system. Sci. Hortic. 2021, 287, 110264. [Google Scholar] [CrossRef]
- Liu, B.; Fan, X.; Shu, C.; Wang, X.; Cao, J.; Jiang, W. Effects of long-term near freezing temperature storage on shelf quality of apricot fruit. Food Sci. 2020, 41, 223–230. (In Chinese) [Google Scholar] [CrossRef]
- Ma, Q.; Suo, J.; Huber, D.J.; Dong, X.; Han, Y.; Zhang, Z.; Rao, J. Effect of hot water treatments on chilling injury and expression of a new C-repeat binding factor (CBF) in ‘Hongyang’ kiwifruit during low temperature storage. Postharvest Biol. Technol. 2014, 97, 102–110. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Arena, S.; Rocco, M.; Verrillo, F.; Novi, G.; Viscosi, V.; Marra, M.; Scaloni, A. Proteomic analysis of apricot fruit during ripening. J. Proteom. 2013, 78, 39–57. [Google Scholar] [CrossRef] [Green Version]
- Barba, A.I.O.; Hurtado, M.C.; Mata, M.C.S.; Ruiz, V.F.; de Tejada, M.L.S. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, W.; Wang, X.; Cao, J.; Jiang, W. Polyphenol composition and antioxidant capacity in pulp and peel of apricot fruits of various varieties and maturity stages at harvest. Int. J. Food Sci. Technol. 2018, 53, 327–336. [Google Scholar] [CrossRef]
- Ma, L. Effects of chito-oligosaccharide combined with other preservative on storage quality of apricot fruit. China Agric. Univ. 2015, 8–12. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1015582626.nh&DbName=CDFD2015 (accessed on 1 September 2015). (In Chinese).
- Ma, X. Study on the formation mechanism of apricot fruit flocculation during cold storage. XinJiang Agric. Univ. 2015, 15–23. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1015645897.nh&DbName=CMFD2016 (accessed on 20 September 2015). (In Chinese).
- Cao, J.; Jiang, W.; Zhao, Y. Experimental Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef]
- Chance, B. Assay of catalase and peroxidase. Meth. Enzymol. 1995, 2, 764–775. [Google Scholar]
- Xin, Q.; Sun, J.; Fan, X.; Li, X.; Jiang, L.; Hao, G.; Zhou, X.; Liu, B. Heat Shock Treatment Promoted Callus Formation on Postharvest Sweet Potato by Adjusting Active Oxygen and Phenylpropanoid Metabolism. Agriculture 2022, 12, 1351. [Google Scholar] [CrossRef]
- Xin, Q.; Sun, J.; Feng, X.; Zhao, Z.; Liu, B.; Jiang, L.; Hao, G.; Liu, B. Heat Shock Treatment Promotion Wound Curing and Metabolic Mechanism of Sweet Potato. Food Sci. 2022, 43, 228–238. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, J.; Li, M.; Suo, J.; Zhao Ha Yang, Q. Chilling Tolerance Difference Among Three Kiwifruit Cultivars. Acta Hortic. Sin. 2013, 40, 341–349. (In Chinese) [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. Catalase activity assays. Curr. Protoc. Toxicol. 2006, 27, 7. [Google Scholar]
- Peláez-Vico, M.Á.; Fichman, Y.; Zandalinas, S.I.; Van Breusegem, F.; Karpiński, S.M.; Mittler, R. ROS and redox regulation of cell-to-cell and systemic signaling in plants during stress. Free Radic. Biol. Med. 2022, 193, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, P.; Gill, R.; Nehra, A.; Sharma, K.K.; Hasanuzzaman, M.; Prasad, R.; Gill, S.S. Chapter 15-Reactive oxygen species (ROS) management in engineered plants for abiotic stress tolerance. In Advancement in Crop Improvement Techniques; Tuteja, N., Tuteja, R., Passricha, N., Saifi, S.K., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 241–262. [Google Scholar] [CrossRef]
- Fürtauer, L.; Weiszmann, J.; Weckwerth, W.; Nägele, T. Dynamics of Plant Metabolism during Cold Acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Li, J.; Wang, L.; Li, X.; Liu, Y.; Wang, X.; Ma, Y. Integrated transcriptomic and metabolomics analysis reveals abscisic acid signal transduction and sugar metabolism pathways as defense responses to cold stress in Argyranthemum frutescens. Environ. Exp. Bot. 2023, 205, 105115. [Google Scholar] [CrossRef]
- Xi, Y.; Fan, X.; Zhao, H.; Li, X.; Cao, J.; Jiang, W. Postharvest fruit quality and antioxidants of nectarine fruit as influenced by chlorogenic acid. LWT 2017, 75, 537–544. [Google Scholar] [CrossRef]
- Khaliq, G.; Ghazali, H.M.; Ding, P.; Ali, A.; Muda Mohamed, M.T. Influence of gum arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biol. Technol. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Mohamed, M.; Rahmat, A.; Fry, J.; Abu Bakar, M.F. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, Y.; Wu, J.; Stevanato, P.; Sha, S.; Geng, G.; Wang, Y. Low night temperature-induced feedback inhibition of photosynthesis through sucrose accumulation in sugar beet (Beta vulgaris L.) leaves. Environ. Exp. Bot. 2022, 204, 105083. [Google Scholar] [CrossRef]
- Ruoyi, K.; Zhaoxin, L.; Zhifang, Y. Effect of coating and intermittent warming on enzymes, soluble pectin substances and ascorbic acid of Prunus persica (Cv. Zhonghuashoutao) during refrigerated storage. Food Res. Int. 2005, 38, 331–336. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Y.; Yan, L.; Xie, J. Effect of edible coatings on enzymes, cell-membrane integrity, and cell-wall constituents in relation to brittleness and firmness of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) during storage. Food Chem. 2011, 124, 569–575. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, H.; Wang, X.; Cao, J.; Jiang, W. Sugar and organic acid composition of apricot and their contribution to sensory quality and consumer satisfaction. Sci. Hortic. 2017, 225, 553–560. [Google Scholar] [CrossRef]
- Yang, S.; Tang, X.F.; Ma, N.N.; Wang, L.Y.; Meng, Q.W. Heterology expression of the sweet pepper CBF3 gene confers elevated tolerance to chilling stress in transgenic tobacco. J. Plant Physiol. 2011, 168, 1804–1812. [Google Scholar] [CrossRef]
- Zhou, W.; Niu, Y.; Ding, X.; Zhao, S.; Li, Y.; Fan, G.; Liao, K. Analysis of carotenoid content and diversity in apricots (Prunus armeniaca L.) grown in China. Food Chem. 2020, 330, 127223. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cui, K.; Yang, L.; Shu, C.; Liu, J.; Zhu, Z.; Yang, Z.; Jiang, W. Near freezing temperature storage alleviates cell wall polysaccharide degradation and softening of apricot (Prunus armeniaca L.) fruit after simulated transport vibration. Sci. Hortic. 2021, 288, 110296. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhang, Z.; He, H.; Shi, L.; Zhu, X.; Cui, K. Near-freezing temperature storage improves shelf-life and suppresses chilling injury in postharvest apricot fruit (Prunus armeniaca L.) by regulating cell wall metabolism. Food Chem. 2022, 387, 132921. [Google Scholar] [CrossRef] [PubMed]
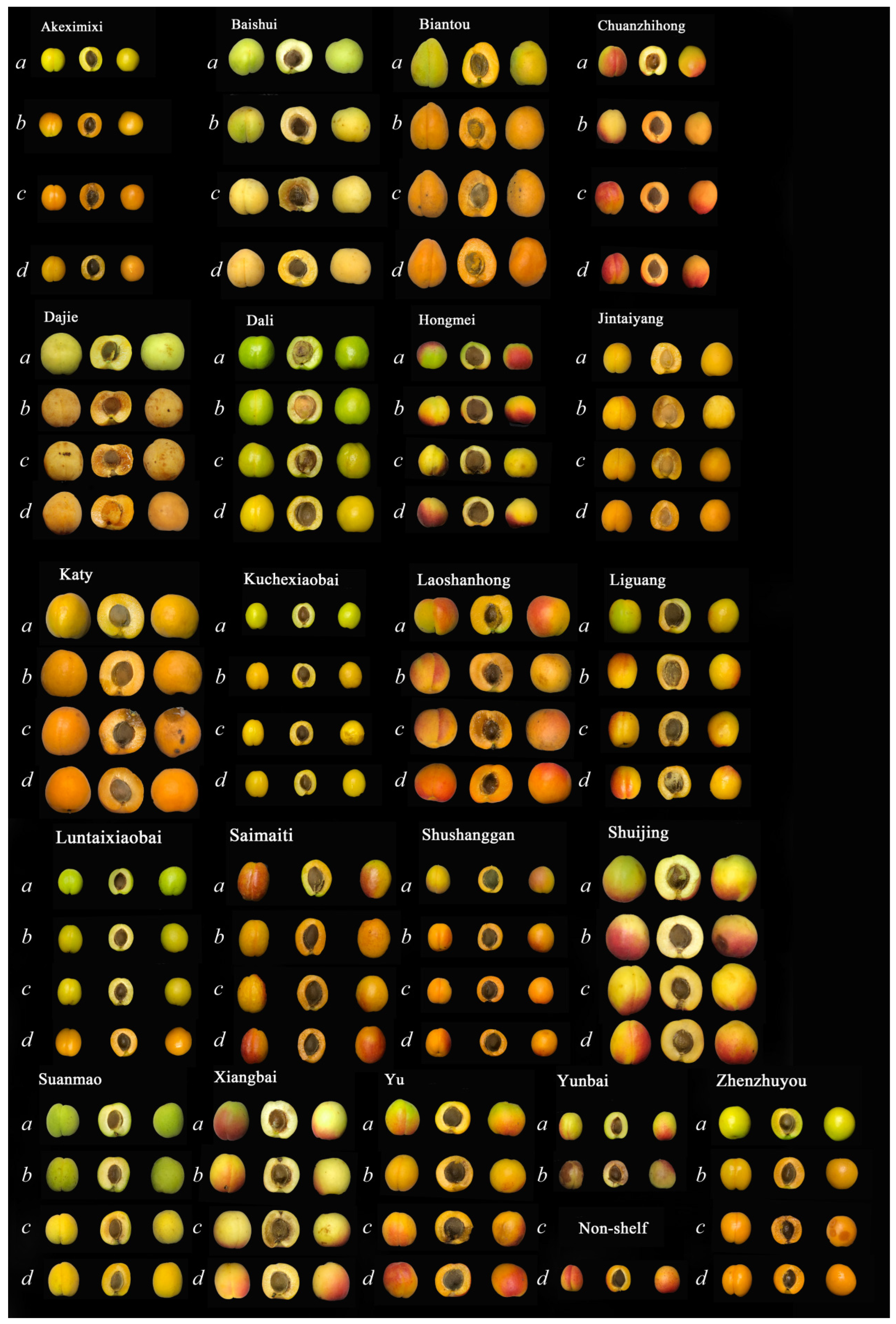
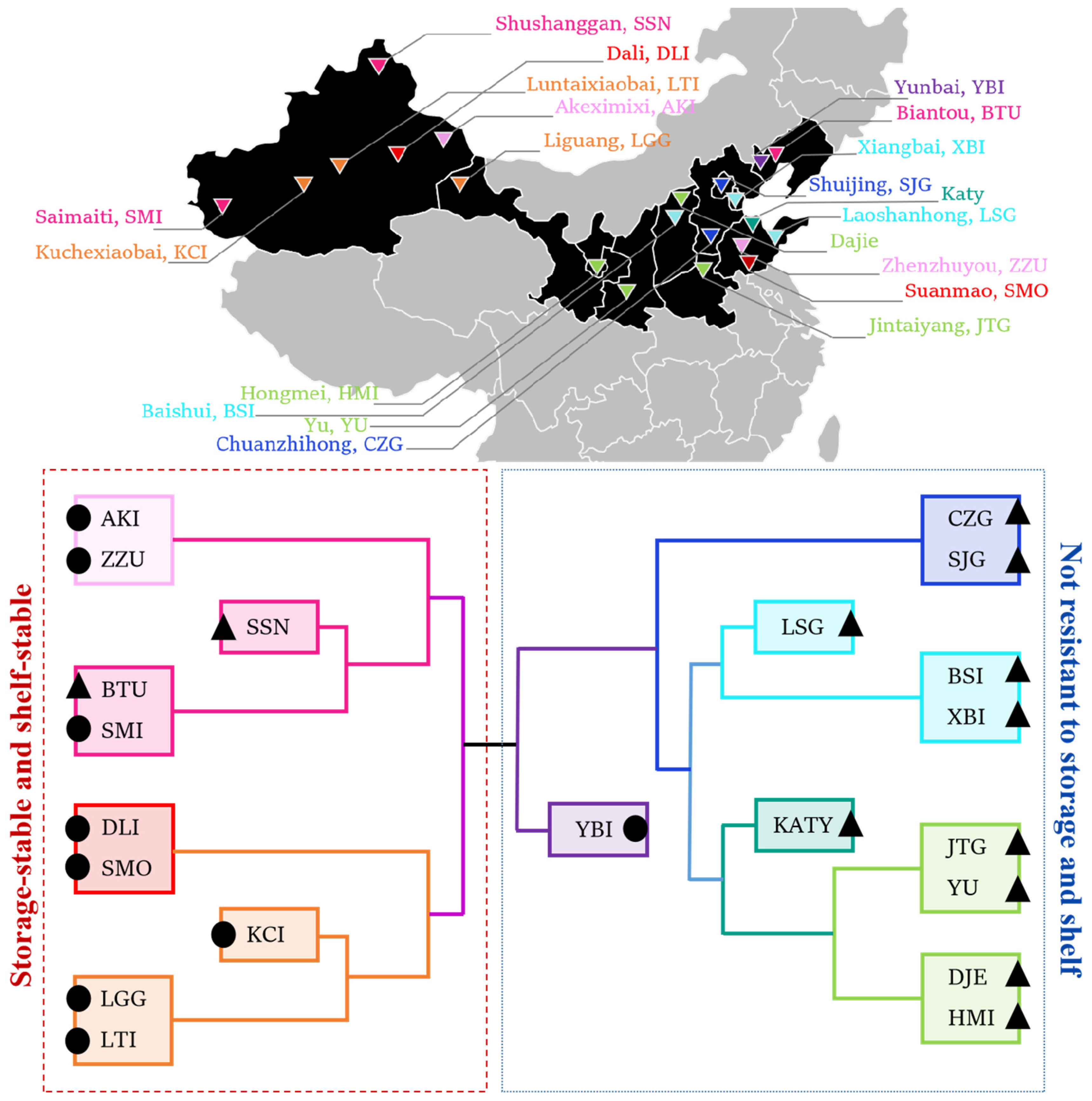
| Apricot | Abbreviation | Harvest Time | Surface | Producing Area | Apricot | Abbreviation | Harvest Time | Surface | Producing Area |
|---|---|---|---|---|---|---|---|---|---|
| Akeximixi | AKI | 28th May | Hairless | Xinjiang | Baishui | BSI | 19th May | Hairy | Shanxi |
| Biantou | BTU | 15th June | Hairy | Liaoning | Chuanzhihong | CZG | 18th June | Hairy | Hebei |
| Dajie | DJE | 22nd May | Hairy | Shanxi | Dali | DLI | 2nd July | Hairless | Xinjiang |
| Hongmei | HMI | 17th June | Hairy | Ningxia | Jintaiyang | JTG | 15th June | Hairy | Henan |
| Katy | KATY | 20th May | Hairy | Shandong | Kuchexiaobai | KCI | 6th June | Hairless | Xinjiang |
| Laoshanhong | LSG | 25th May | Hairy | Shandong | Liguang | LGG | 10th June | Hairless | Gansu |
| Luntaibai | LTI | 4th June | Hairless | Xinjiang | Saimaiti | SMI | 20th June | Hairless | Xinjiang |
| Shushanggan | SSN | 18th June | Hairy | Xinjiang | Shuijing | SJG | 12th June | Hairy | Beijing |
| Suanmao | SMO | 18th June | Hairy | Shandong | Xiangbai | XBI | 20th June | Hairy | Tianjin |
| Yunbai | YBI | 5th July | Hairless | Liaoning | Yu | YU | 5th June | Hairy | Shaanxi |
| Zhenzhuyou | ZZU | 10th June | Hairless | Shandong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Q.; Zhou, X.; Jiang, W.; Zhang, M.; Sun, J.; Cui, K.; Liu, Y.; Jiao, W.; Zhao, H.; Liu, B. Effects of Reactive Oxygen Levels on Chilling Injury and Storability in 21 Apricot Varieties from Different Production Areas in China. Foods 2023, 12, 2378. https://doi.org/10.3390/foods12122378
Xin Q, Zhou X, Jiang W, Zhang M, Sun J, Cui K, Liu Y, Jiao W, Zhao H, Liu B. Effects of Reactive Oxygen Levels on Chilling Injury and Storability in 21 Apricot Varieties from Different Production Areas in China. Foods. 2023; 12(12):2378. https://doi.org/10.3390/foods12122378
Chicago/Turabian StyleXin, Qi, Xinqun Zhou, Weibo Jiang, Min Zhang, Jing Sun, Kuanbo Cui, Yu Liu, Wenxiao Jiao, Handong Zhao, and Bangdi Liu. 2023. "Effects of Reactive Oxygen Levels on Chilling Injury and Storability in 21 Apricot Varieties from Different Production Areas in China" Foods 12, no. 12: 2378. https://doi.org/10.3390/foods12122378
APA StyleXin, Q., Zhou, X., Jiang, W., Zhang, M., Sun, J., Cui, K., Liu, Y., Jiao, W., Zhao, H., & Liu, B. (2023). Effects of Reactive Oxygen Levels on Chilling Injury and Storability in 21 Apricot Varieties from Different Production Areas in China. Foods, 12(12), 2378. https://doi.org/10.3390/foods12122378






