Gastroprotective Effects of the Aqueous Extract of Finger Citron Pickled Products against Ethanol-Induced Gastric Damage: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. FCPP Aqueous Extract Preparation
2.3. Effect of FCPP Aqueous Extract on GES-1 Cell Alcohol Injury
2.4. Animal Treatment
2.5. Gastric Ulcer Evaluation
2.6. Histopathological Analysis
2.7. Determination of Serum Levels
2.8. Western Blot Analysis
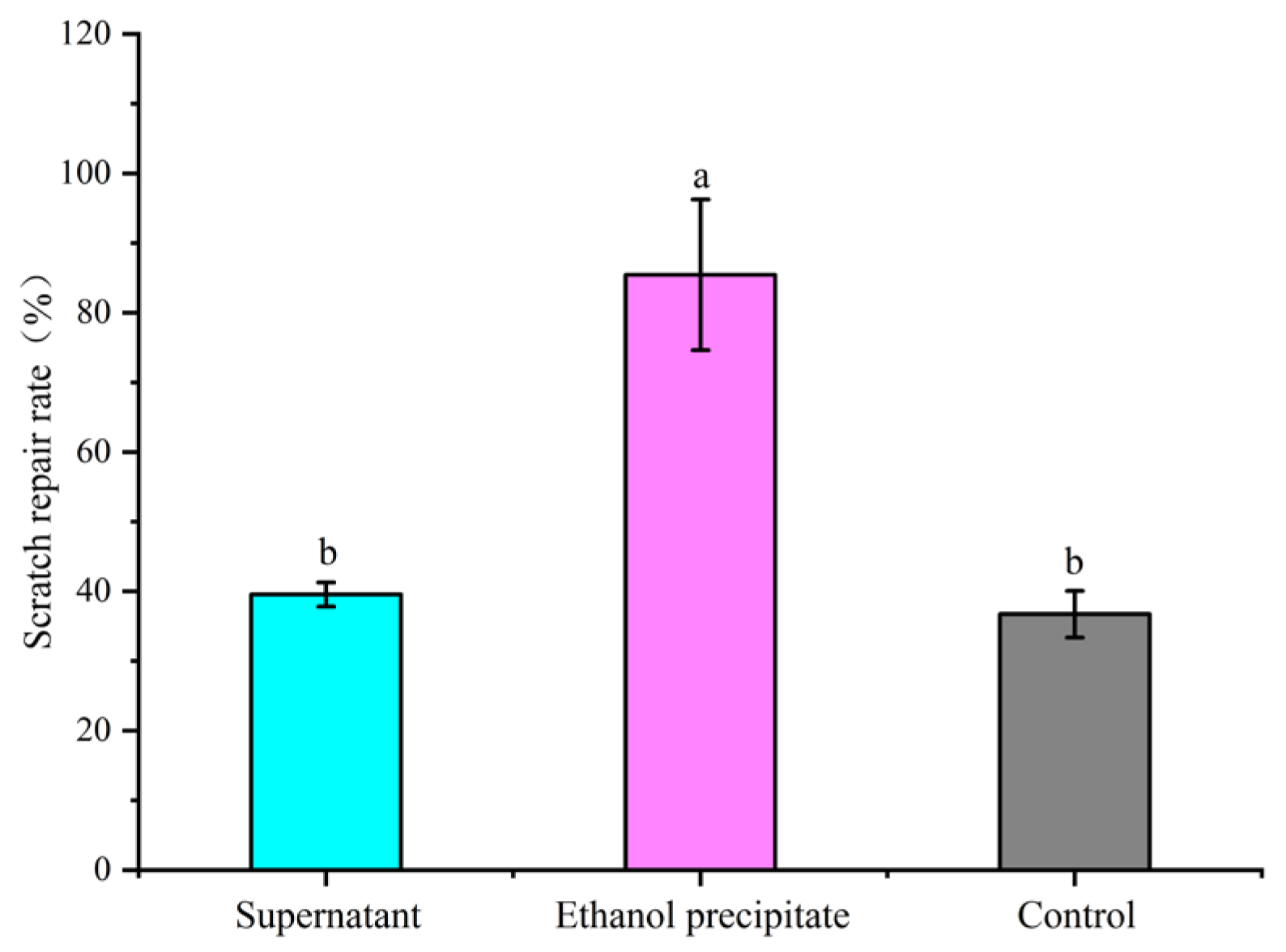
2.9. Cell Scratch Assay and Chemical Composition Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of FCPP Aqueous Extract on Alcohol Damage of GES-1 Cells
3.2. Effects of FCPP Aqueous Extract on Organ Index
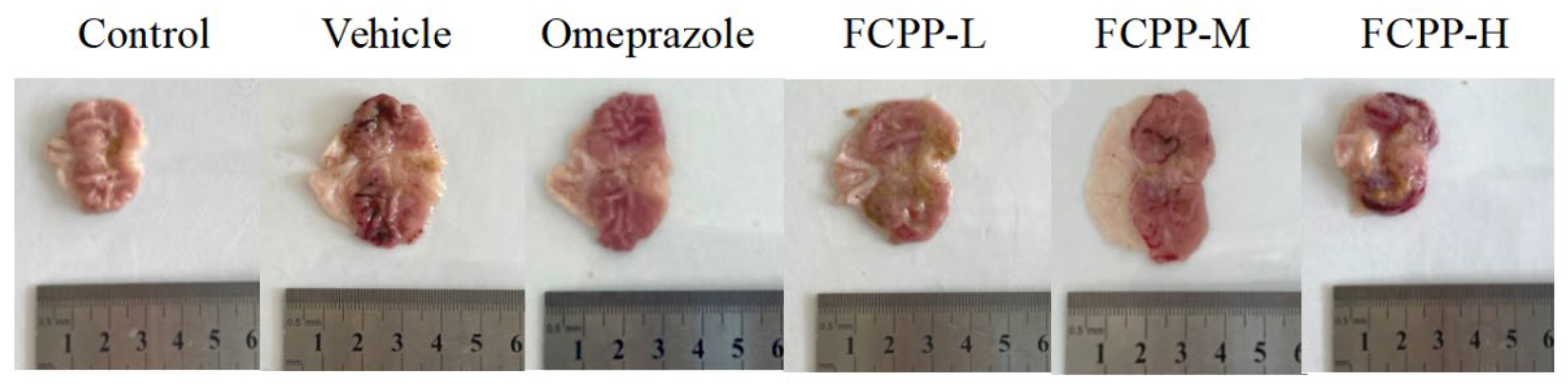
3.3. Gastric Ulcer Evaluation and Histopathological Analysis
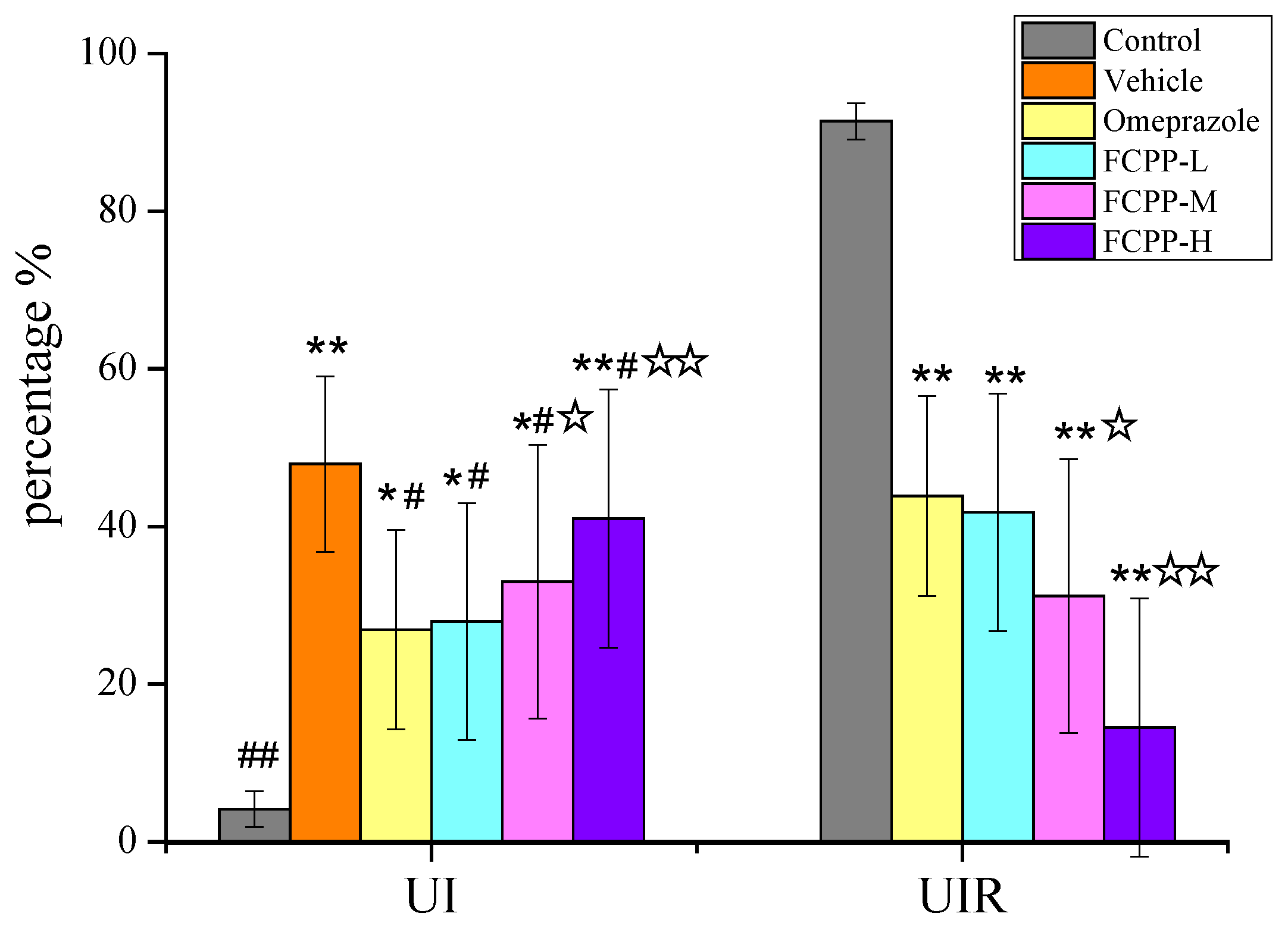
3.3.1. Effects of FCPP Aqueous Extract on UI and UIR in Rats
3.3.2. Histopathological Analysis
3.4. Effects of FCPP Aqueous Extract on the Level of SOD and MDA in Rats
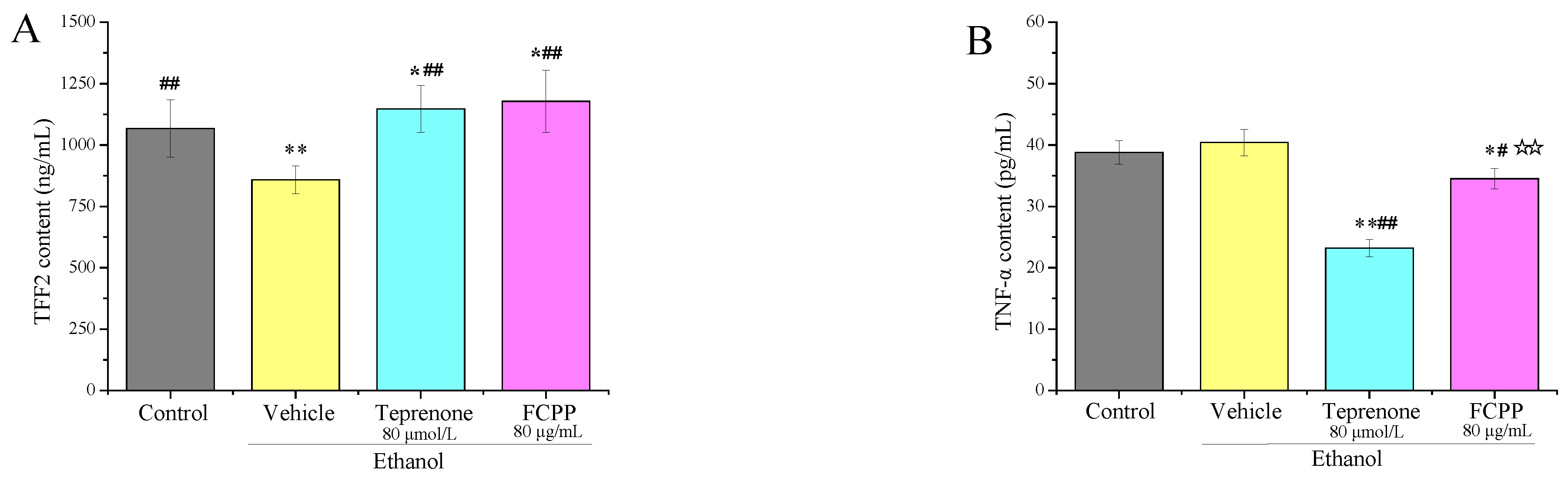
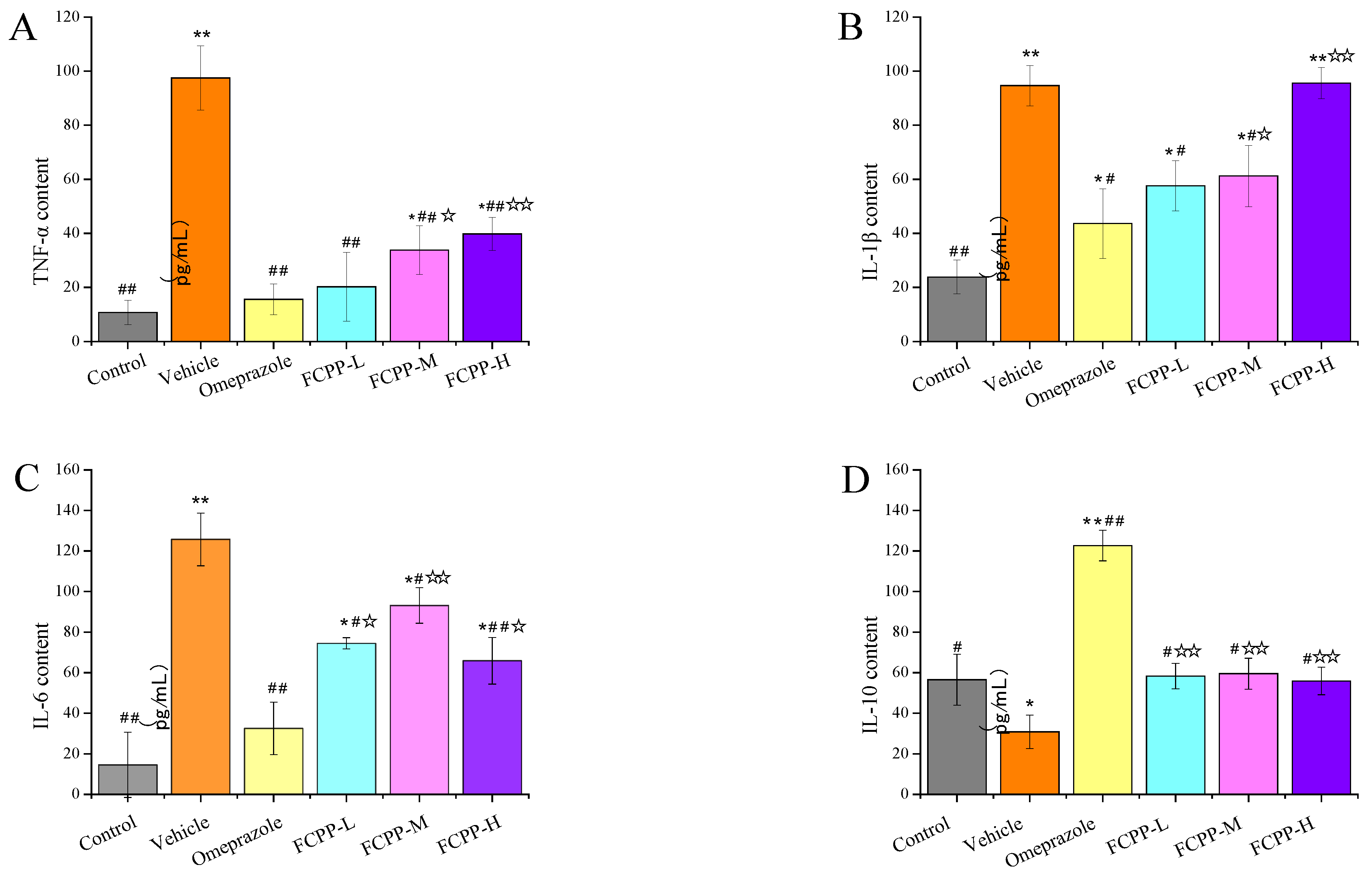
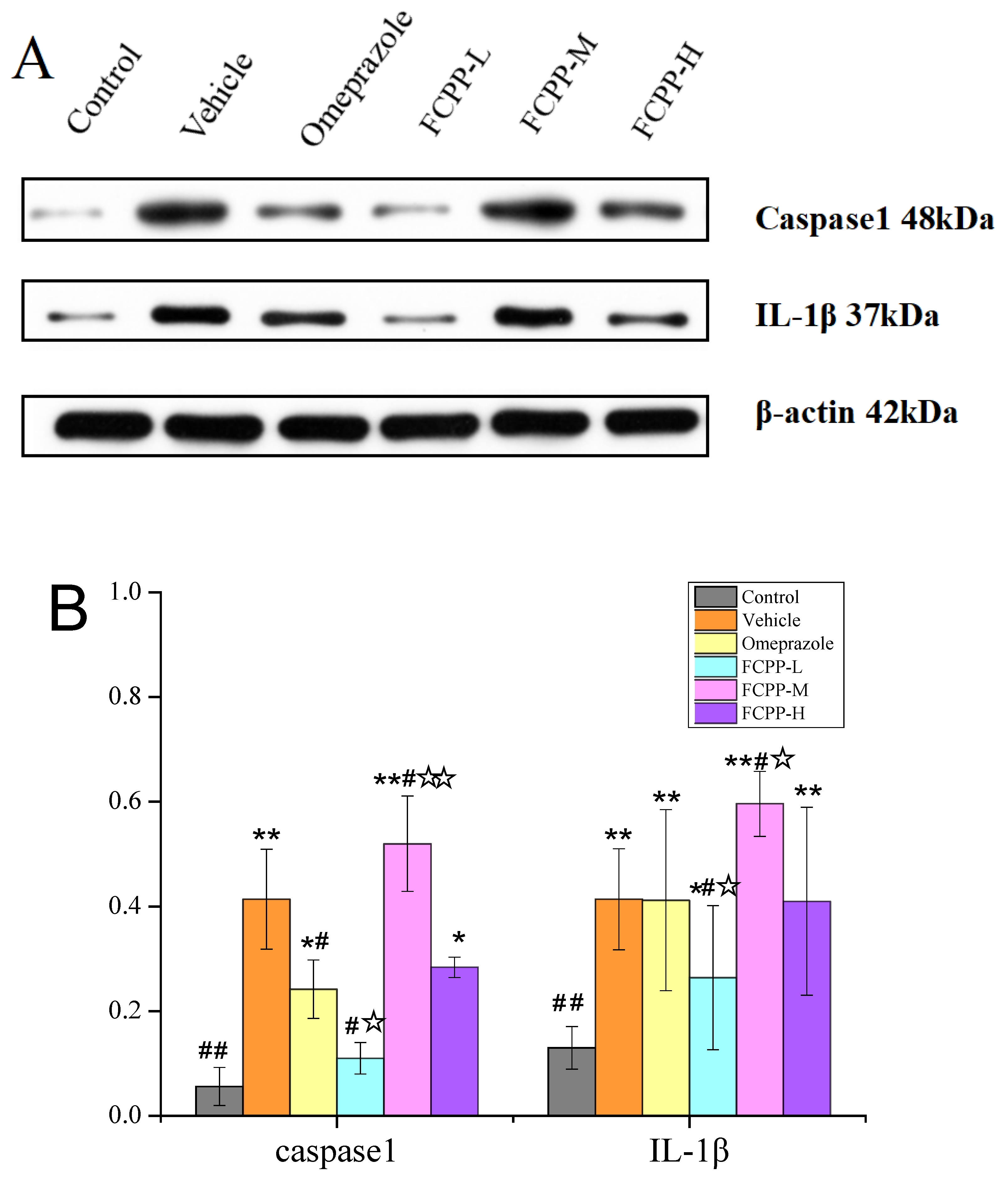
3.5. Effects of FCPP Aqueous Extract on the Expression of Pro-Inflammatory and Anti-Inflammatory Factors
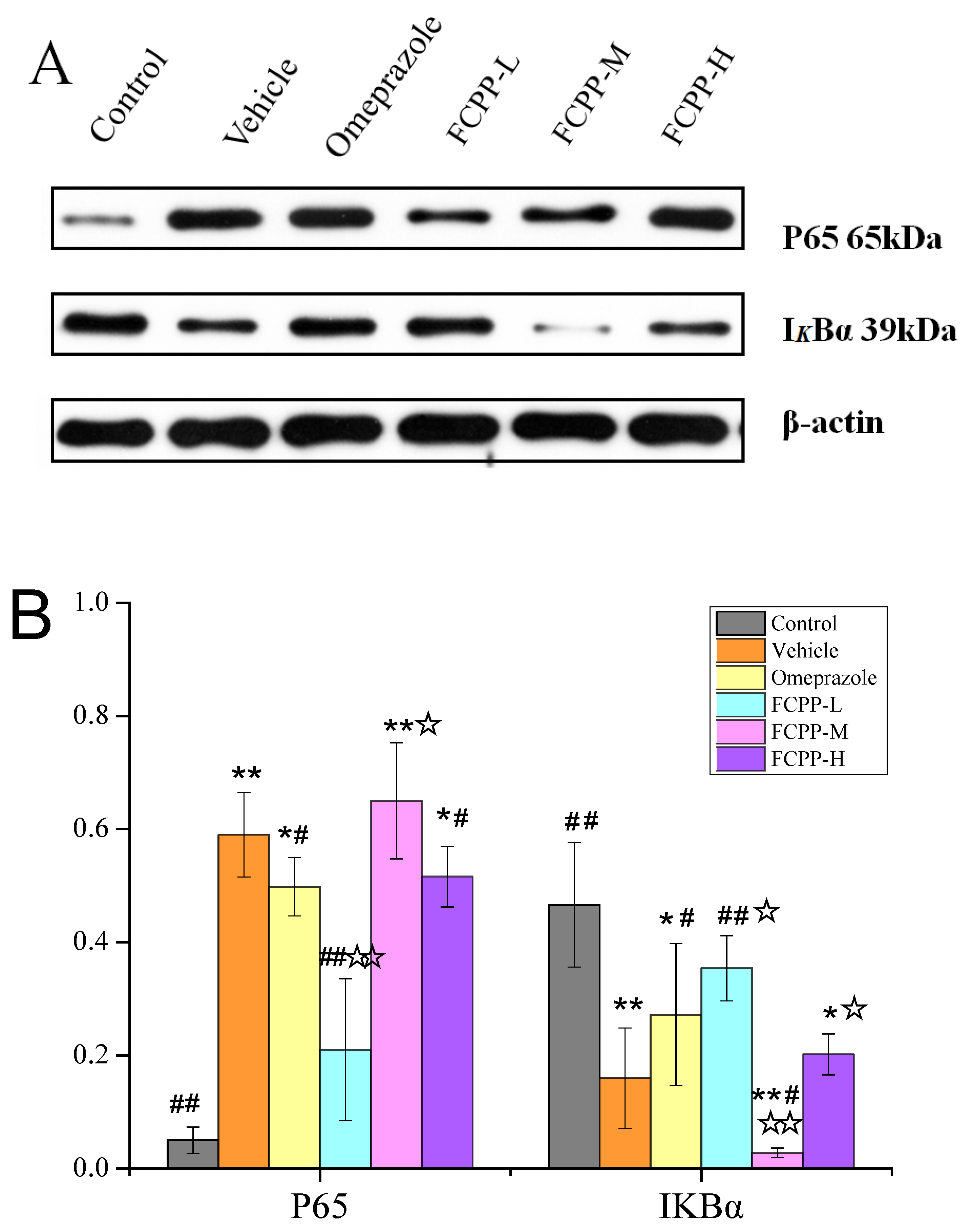
3.6. Effects of FCPP Aqueous Extract on NF-κB and NLRP3 Anti-Inflammatory Pathways after Ethanol-Induced Gastric Mucosal Injury
3.7. Analysis of the Major Active Components of FCPP Aqueous Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.Y.; Yin, J.Y.; Hu, J.L.; Nie, S.P.; Xie, M.Y. Gastroprotective polysaccharide from natural sources: Review on structure, mechanism, and structure–activity relationship. Food Front. 2022, 3, 560–591. [Google Scholar] [CrossRef]
- Rizzato, C.; Torres, J.; Obazee, O.; Camorlinga, M.-P.; Kato, I. Variations in cag pathogenicity island genes of Helicobacter pylori from Latin American groups may influence neoplastic progression to gastric cancer. Sci. Rep. 2020, 10, 6570. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wu, Q.; Zhao, Z.; Xiong, J.; Niu, J.; Liu, C.; Liu, T.; Chai, Y.; Qu, X.; Ma, Z.; et al. Mechanisms of Dendrobium officinale polysaccharides in repairing gastric mucosal injuries based on mitogen-activated protein kinases (MAPK) signaling pathway. Bioengineered 2022, 13, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-Q.; Xue, H.; Sun, H.-J. Nervous mechanisms of restraint water-immersion stress-induced gastric mucosal lesion. World J. Gastroenterol. 2020, 26, 74–90. [Google Scholar] [CrossRef]
- Arab, H.H.; Eid, A.H.; El-Sheikh, A.A.K.; Arafa, E.A.; Ashour, A.M. Irbesartan reprofiling for the amelioration of ethanol-induced gastric mucosal injury in rats: Role of inflammation, apoptosis, and autophagy. Life Sci. 2022, 308, 120939. [Google Scholar] [CrossRef]
- Mustonen, H.; Kiviluoto, T.; Puolakkainen, P.; Kivilaakso, E. Ethanol induces volume changes and gap junction closure via intracellular Ca2+ signalling pathway in cultured rabbit gastric epithelial cells. Scand. J. Gastroenterol. 2004, 39, 104–110. [Google Scholar] [CrossRef]
- Fu, Y.; Hou, Y.; Duan, Y.; Sun, X.; Chen, S. Gastroprotective effect of an active ingredients group of Lindera reflexa Hemsl. On Ethanol-Induced gastric ulcers in Rats: Involvement of VEGFR2/ERK and TLR-2/Myd88 signaling pathway. Int. Immunopharmacol. 2022, 107, 108673. [Google Scholar] [CrossRef]
- Wang, S.-W.; Blue, S.; Zheng, F.; Lei, M.-K.; Zhang, F. Effects of fructus aurantii extracts on liver inflammation and NF-κB/NLRP3 inflammasome pathway in cCL4-induced hepatic fibrosis mice. China J. Chin. Mate Med. 2021, 46, 1474–1479. [Google Scholar]
- Sun, S.-C. The noncanonical NF-kB pathway. Immunol. Rev. 2012, 246, 125. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.-M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Duan, Z.; Yu, S.; Wang, S.; Deng, H.; Guo, L.; Yang, H.; Xie, H. Protective Effects of Piperine on Ethanol-Induced Gastric Mucosa Injury by Oxidative Stress Inhibition. Nutrients 2022, 14, 4744. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Liu, L.; Ren, J.; Huang, M.; Yuan, E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2022, 375, 131896. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.; Hwang, S.-J.; Kim, W.-J.; Youn, H.-J.; Lee, H.-J. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF-kB. Biomed. Pharmacother. 2018, 99, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Guo, S.-J.; Yang, Y.-L.; Gu, X.-X.; Li, X.-T.; Zhuang, X.-D.; Lin, Z.-Z.; Zhang, Z.-X. Production Process of Preserved Fruits Laoxianghuang. Acad. Period Prod. Process. 2014, 1, 44–45+48. [Google Scholar]
- Liu, Z.; Zhang, Z.-X.; Lai, X.; Yang, Q.-C.; Lu, Y.-T.; Huang, Q.-Z.; Zheng, Y.-Z. Analysis on HPLC Fingerprints and Index Content Determination of Lao-Xiang-Huang of Chaozhou. World Sci. Technol.-Mod. Tradit. Chin. Med. 2017, 19, 1370–1374. [Google Scholar]
- Chen, X.-A.; Chen, H.-Q.; Xiao, J.; Liu, J.-Y.; Tang, N.; Zhou, A.-M. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef]
- Guo, S.-J.; Yang, Y.-L.; Chen, H.-C.; Yang, D.-J.; Zhang, Z.-X.; Zheng, Y.-Z. Development of the Mixed Granules of Laoxianghuang and Laoyaoju. Storage Process 2016, 16, 59–65. [Google Scholar]
- Zhu, Y.; Li, M.-L.; Zhang, R.-X.; Chen, L.; Ling, K.; Cai, M.-Y.; Gu, R.-Z.; Wei, Y. Study on the Cytoprotection of Combined Oligopeptide on Ethanol-Induced GES-1 Cells Damage. Food Tech. 2020, 45, 24–30. [Google Scholar]
- Carlotto, J.; Maria-Ferreira, D.; de Souza, L.M.; Da Luz, B.B.; Dallazen, J.L.; de Paula Werner, M.F.; Cipriani, T.R. A polysaccharide fraction from “ipê-roxo” (Handroanthus heptaphyllus) leaves with gastroprotective activity. Carbohyd. Polym. 2019, 226, 115239. [Google Scholar] [CrossRef]
- Nabil, M.; Raey, M.; Abdo, W.; Abdelfattah, M.-A.; El-Shazly, A.-M.; Sobeh, M.; Mahmoud, M.-F. Gastro-Protective Effects of Albizia anthelmintica Leaf Extract on Indomethacin-Induced Gastric Ulcer in Wistar Rats: In Silico and In Vivo Studies. Antioxidants 2021, 10, 176. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Huang, H. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chem. 2022, 388, 133000. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Chen, Z. Sulfated modification of the polysaccharides obtained from defatted rice bran and their antitumor activities. Int. J. Biol. Macromol. 2009, 44, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, Z.; Meng, H.; Shi, X.; Xie, J. Immunomodulation effect of polysaccharides from liquid fermentation of Monascus purpureus 40269 via membrane TLR-4 to activate the MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2022, 201, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Rtibi, K.; Grami, D.; Wannes, D.; Selmi, S.; Amri, M.; Sebai, H.; Marzouki, L. Ficus carica aqueous extract alleviates delayed gastric emptying and recovers ulcerative colitis-enhanced acute functional gastrointestinal disorders in rats. J. Ethnopharmacol. 2018, 224, 242–249. [Google Scholar] [CrossRef]
- Ortizmasia, D.; Hernandez, C.; Quintana, E.; Velazquez, M.; Cebrian, S.; Riano, A.; Calatayud, S.; Esplugues, J.-V.; Barrachina, M.-D. iNOS-derived nitric oxide mediates the increase in TFF2 expression associated with gastric damage: Role of HIF-1. FASEB J. 2010, 24, 136. [Google Scholar] [CrossRef]
- Mazzon, E.; Cuzzocrea, S. Role of TNF-α in lung tight junction alteration in mouse model of acute lung inflammation. Respir. Res. 2007, 8, 75. [Google Scholar] [CrossRef]
- Eman, A.; El-Ghffar, A.; Al-Sayed, E.; Safia, M.; Shehata, O. The protective role of Ocimum basilicum L. (Basil) against aspirin-induced gastric ulcer in mice: Impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct. 2018, 9, 4457–4468. [Google Scholar]
- Sebai, H.; Jabri, M.; Souli, A.; Hosni, K.; Selmi, S.; Tounsi, H.; Tebourbi, O.; Boubaker, S.; El-Benna, J.; Sakly, M. Protective effect of Artemisia campestris extract against aspirin-induced gastric lesions and oxidative stress in rat. RSC Adv. 2014, 4, 49831–49841. [Google Scholar] [CrossRef]
- Chen, W.; Wu, D.; Jin, Y.; Li, Q.; Yang, Y. Pre-protective effect of polysaccharides purified from Hericium erinaceus against ethanol-induced gastric mucosal injury in rats. Int. J. Biol. Macromol. 2020, 159, 948–956. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, B.; Lv, L.; Wang, Z.; He, J.; Chen, Z.; Wen, X.; Zhang, Y.; Sun, W.; Li, Y. Gastroprotective effect of aucubin against ethanol-induced gastric mucosal injury in mice. Life Sci. 2017, 189, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.-F.; Zlotnik, A.; Mosmann, T.-R.; Howard, M.; O’Garra, A. Pillars Article: IL-10 Inhibits Cytokine Production by Activated Macrophages. J. Immunol. 2016, 197, 1539. [Google Scholar] [PubMed]
- Lecoeur, H.; Prina, E.; Rosazza, T.; Kokou, K.; Spth, G.-F. Targeting Macrophage Histone H3 Modification as a Leishmania Strategy to Dampen the NF-κB/NLRP3-Mediated Inflammatory Response. Cell Rep. 2020, 30, 1870–1882.e4. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-N.; Liang, Y.-N.; Zhang, D.-B.; Ren, L.-L.; Li, L.-H.; Yu, J.-G.; Zhang, Z.; Tang, Z.-S.; Wang, Z. Study on the protective effect of cinnamaldehyde DSS induced ulcerative colitis in mice. Nat. Prod. Res. Dev. 2021, 33, 8. [Google Scholar]
- Cao, S.-D.; Xu, W.; Sun, W.-F.; Li, J.; Shi, W.-H. Effect of Qingrejiebi Prescription on gouty arthritis. West Chin. Med. 2019, 32, 79–81. [Google Scholar]
- Liu, Y.; Liu, J.; Liu, Y.-T.; Miao, M.-J.; Yuan, H.-X.; Yu, M.-M. Effect of Xuanfu Daizhe Tang on NLRP3/Caspase-1 Pathway of RE Model Rats. Chin. J. Exper. Trad. Med. Formulae 2019, 25, 13–18. [Google Scholar]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.-J.; Choi, Y.-H.; Chung, K.-T.; Choi, B.-T. Effects of mycelial culture of Phellinus linteus on ethanol-induced gastric ulcer in rats. Phytother. Res. 2006, 20, 396–402. [Google Scholar] [CrossRef]
- Choi, H.-S.; Lim, J.; Chun, H.-J.; Lee, M.; Sun, E.; Kim, B.; Keum, Y.-S.; Seo, Y.-T.; Jeen, S.-H.; Um, H.-S.; et al. The effect of polaprezinc on gastric mucosal protection in rats with ethanol-induced gastric mucosal damage: Comparison study with rebamipide. Life Sci. 2013, 93, 69–77. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, Y.; Wang, L.; Mao, X.; Xi, P.; Peng, Y.; Castro, M.-G. Recombinant Adenovirus-Mediated Intestinal Trefoil Factor Gene Therapy for Burn-Induced Intestinal Mucosal Injury. PLoS ONE 2013, 8, 62429. [Google Scholar] [CrossRef]
- Yong, Z.; Yu, G.; Xiang, Y.; Wu, J.; Ping, J.; Lee, W.; Zhang, Y. Bm-TFF2, a toad trefoil factor, promotes cell migration, survival and wound healing. Biochem. Biophys. Res. Commun. 2010, 398, 559–564. [Google Scholar]
- Huang, C.; Chen, Y.; Wang, D.-C.; Chao, C.-C.; Lin, W.-T.; Huang, C.-Y.; Mei-Chic, H. Cytoprotective Effect of American Ginseng in a Rat Ethanol Gastric Ulcer Model. Molecules 2014, 19, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Steven, O.-O.; Uwadiegwu, A.-P.; Chinonyelum, A.-N. Preliminary studies on the anti-ulcer potentials of Vitex doniana crude extracts on experimental rat model of ethanol induced gastric ulcer. Asian Pac. J. Trop. Dis. 2016, 6, 736–740. [Google Scholar] [CrossRef]
- Ben, B.-Z.; Tlili, M.; Alimi, H.; Miled, B.; Rhouma, K.-B.; Sakly, M.; Ksouri, R.; Schneider, Y.-J.; Tebourbi, O. Protective effects of edible Rhus tripartita (Ucria) stem extract against ethanol-induced gastric ulcer in rats. J. Funct. Foods 2017, 30, 260–269. [Google Scholar]
- Gomaa, A.-M.-S.; El- Motalleb, N.A.A.; Aamer, H.-A. Antioxidant and anti-inflammatory activities of alpha lipoic acid protect against indomethacin-induced gastric ulcer in rats. Biomed. Pharmacother. 2018, 101, 188–194. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Ko, W.-C.; Chou, P.-H.; Chen, Y.-H.; Lin, H.-J.; Liu, Y.-H.; Tsai, H.-W.; Lee, J.-C.; Jane, P. Proton-Pump Inhibitor Exposure Aggravates Clostridium difficile-Associated Colitis: Evidence From a Mouse Model. J. Infect. Dis. 2015, 212, 654–663. [Google Scholar] [CrossRef]
- Zeng, Q.; Ko, C.-H.; Siu, W.-S.; Li, L.F.; Han, X.-Q.; Yang, L.; Lau, C.-B.-S.; Hu, J.-M.; Leung, P.-C. Polysaccharides of Dendrobium officinale Kimura & Migo protect gastric mucosal cell against oxidative damage-induced apoptosis in vitro and in vivo. J. Ethnopharmacol. 2017, 208, 214–224. [Google Scholar] [PubMed]
- Goldminz, A.-M.; Au, S.-C.; Kim, N.; Gottlieb, A.-B.; Lizzul, P.-F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiong, B.; Liu, H.; Chen, Z.; Yang, J. Koumine Suppresses IL-1β Secretion and Attenuates Inflammation Associated with Blocking ROS/NF-κB/NLRP3 Axis in Macrophages. Front. Pharmacol. 2021, 11, 622074. [Google Scholar] [CrossRef]
- Xu, T.; Liu, R.; Lu, X.; Wu, X.; Petr, H.; Mao, Y.; Jiang, Q.; Juan, L.; Yang, Z. Lycium barbarum polysaccharides alleviate LPS-induced inflammatory responses through PPARγ/MAPK/NF-κB pathway in bovine mammary epithelial cells. J. Anim. Sci. 2022, 100, 345. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Fang, C.; Ding, S.; Liu, X.; Hu, J.; Xu, J.; Mei, Q. PAP-1 ameliorates DSS-induced colitis with involvement of NLRP3 inflammasome pathway. Int. Immunopharmacol. 2019, 75, 105776. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Wang, Y.; Zhao, M. NLRP3 inflammasome activation regulated by NF-κB and DAPK contributed to paraquat-induced acute kidney injury. Immunol. Res. 2017, 65, 687–698. [Google Scholar] [CrossRef] [PubMed]


| Experimental Groups | Number of Animals | Dose of Administration | Modeling |
|---|---|---|---|
| Control | 10 | normal saline (5 mL·kg−1·d−1) | normal saline (5 mL·kg−1) |
| Vehicle | 10 | normal saline (5 mL·kg−1·d−1) | anhydrous ethanol (5 mL·kg−1) |
| FCPP-H | 10 | FCPP aqueous extract (800 mg·kg−1·d−1, 5 mL·kg−1·d−1) | anhydrous ethanol (5 mL·kg−1) |
| FCPP-M | 10 | FCPP aqueous extract (400 mg·kg−1·d−1, 5 mL·kg−1·d−1) | anhydrous ethanol (5 mL·kg−1) |
| FCPP-L | 10 | FCPP aqueous extract (100 mg·kg−1·d−1, 5 mL·kg−1·d−1) | anhydrous ethanol (5 mL·kg−1) |
| Omeprazole | 10 | Omeprazole (50 mg·kg−1·d−1, 5 mL·kg−1·d−1) | anhydrous ethanol (5 mL·kg−1) |
| Organ Index (mg/g) | ||||
|---|---|---|---|---|
| Group | Thymus | Spleen | Kidney | Liver |
| Control | 2.39 ± 0.3 | 3.23 ± 0.49 | 8.13 ± 0.65 | 31.03 ± 3.02 |
| Vehicle | 2.48 ± 0.52 | 3.06 ± 0.28 | 8.17 ± 0.76 | 33.52 ± 4.28 |
| Omeprazole | 2.43 ± 0.26 | 3.1 ± 0.37 | 8.13 ± 0.38 | 33.83 ± 1.82 |
| FCPP-L 100 mg/kg | 2.6 ± 0.25 * | 3.14 ± 0.29 | 8.19 ± 0.6 | 33.48 ± 2.48 |
| FCPP-M 400 mg/kg | 2.37 ± 0.34 | 3.17 ± 0.4 | 8.23 ± 0.5 | 34.24 ± 1.64 * |
| FCPP-H 800 mg/kg | 2.28 ± 0.28 # | 3.02 ± 0.46 | 8.07 ± 0.42 | 32.58 ± 1.53 |
| Sugar Content (%) | Protein Content (%) | TPC a (%) | UAC b (%) |
|---|---|---|---|
| 70 ± 0.001 | 0.420 ± 0.086 | 0.04 ± 0.01 | 40.90 ± 1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, D.; Wang, Q.; Zhou, A. Gastroprotective Effects of the Aqueous Extract of Finger Citron Pickled Products against Ethanol-Induced Gastric Damage: In Vitro and In Vivo Studies. Foods 2023, 12, 2355. https://doi.org/10.3390/foods12122355
Chen X, Yang D, Wang Q, Zhou A. Gastroprotective Effects of the Aqueous Extract of Finger Citron Pickled Products against Ethanol-Induced Gastric Damage: In Vitro and In Vivo Studies. Foods. 2023; 12(12):2355. https://doi.org/10.3390/foods12122355
Chicago/Turabian StyleChen, Xiaoai, Dan Yang, Qun Wang, and Aimei Zhou. 2023. "Gastroprotective Effects of the Aqueous Extract of Finger Citron Pickled Products against Ethanol-Induced Gastric Damage: In Vitro and In Vivo Studies" Foods 12, no. 12: 2355. https://doi.org/10.3390/foods12122355
APA StyleChen, X., Yang, D., Wang, Q., & Zhou, A. (2023). Gastroprotective Effects of the Aqueous Extract of Finger Citron Pickled Products against Ethanol-Induced Gastric Damage: In Vitro and In Vivo Studies. Foods, 12(12), 2355. https://doi.org/10.3390/foods12122355






