Development of Smart Films of a Chitosan Base and Robusta Coffee Peel Extract for Monitoring the Fermentation Process of Pickles
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Anthocyanins Extracted from RCP
2.3. UV-Vis Spectra of Anthocyanin Solution from RCP
2.4. Film Preparation
2.5. Film Characterization
2.5.1. Color
2.5.2. Thickness
2.5.3. Water Content (WC)
2.5.4. Swelling Property (SP)
2.5.5. Water Vapor Permeability (WVP)
2.5.6. Mechanical Properties (MP)
2.5.7. Water Contact Angle (WCA)
2.5.8. UV-Vis Light Barrier Property
2.5.9. Microstructure
2.5.10. Fourier Transform Infrared (FTIR) Analysis
2.5.11. Color Change at Different pH Values
2.6. Monitoring the Fermentation Process of Pickle
2.7. Statistical Analysis
3. Results and Discussion
3.1. Color Changes of RCP Extract
3.2. Color of Film
3.3. Physicochemical Characterization
3.4. Mechanical Properties of Film
3.5. Water Contact Angle of Film
3.6. UV-Vis Light Barrier Property of Film
3.7. Microstructure of Film
3.8. FTIR Analysis of Film
3.9. The Color Changes at Different pH Value of Film
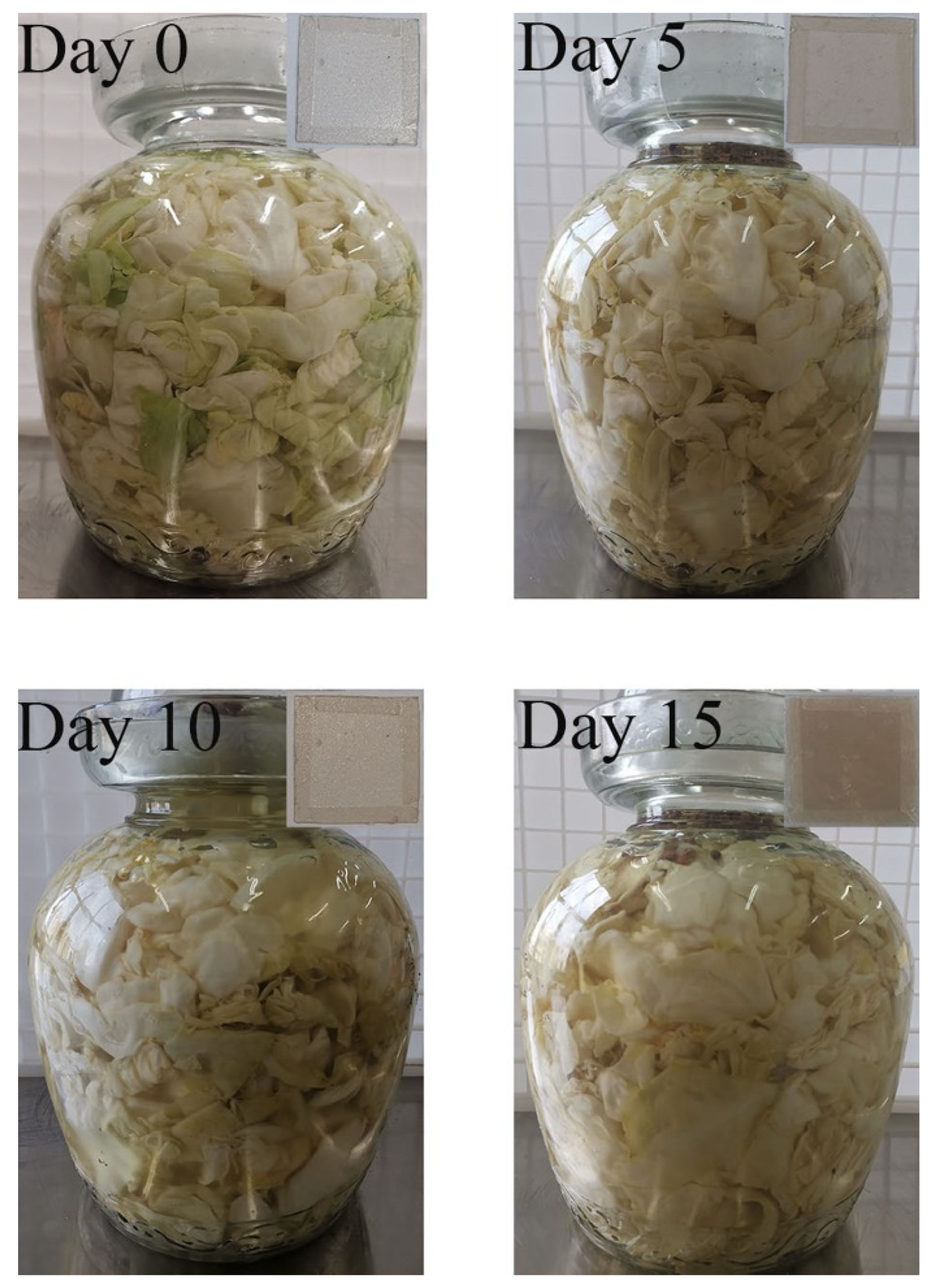
3.10. Changes of the Fermentation Process of Pickle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanjanzadeh, H.; Park, B.D. Covalent immobilization of bromocresol purple on cellulose nanocrystals for use in pH-responsive indicator films. Carbohyd. Polym. 2021, 273, 118550. [Google Scholar] [CrossRef]
- Ezati, P.; Priyadarshi, R.; Bang, Y.J.; Rhim, J.W. CMC and CNF-based intelligent pH-responsive color indicator films integrated with shikonin to monitor fish freshness. Food Control 2021, 126, 108046. [Google Scholar] [CrossRef]
- Yan, J.; Cui, R.; Qin, Y.; Li, L.; Yuan, M. A pH indicator film based on chitosan and butterfly pudding extract for monitoring fish freshness. Int. J. Biol. Macromol. 2021, 177, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, J.; Yan, X.; Cai, Z.; Fu, L.; Gu, Q.; Liu, L.; Jin, H.; Fu, Y. Functional chitosan/zein films with Rosa roxburghii Tratt leaves extracts prepared by natural deep eutectic solvents. Food Packag. Shelf Life 2022, 34, 101001. [Google Scholar] [CrossRef]
- Monte, M.L.; Moreno, M.L.; Senna, J.; Arrieche, L.S.; Pinto, L.A.A. Moisture sorption isotherms of chitosan-glycerol films: Thermodynamic properties and microstructure. Food Biosci. 2018, 22, 170–177. [Google Scholar] [CrossRef]
- Kusmono; Abdurrahim, I. Water sorption, antimicrobial activity, and thermal and mechanical properties of chitosan/clay/glycerol nanocomposite films. Heliyon 2019, 5, e02342. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Razavi, R.; Omer, A.K.; Farhangfar, A.; McClements, D.J. Interactions between nanoparticle-based food additives and other food ingredients: A review of current knowledge. Trends Food Sci. Technol. 2022, 120, 75–87. [Google Scholar] [CrossRef]
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of pomegranate peel extract as a natural additive in foods. Trends Food Sci. Technol. 2021, 115, 380–390. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Jin, L.; Chen, Z.; Jiang, J.; Jackson, A. Development of fruit color in Rubus chingii Hu (Chinese raspberry): A story about novel offshoots of anthocyanin and carotenoid biosynthesis. Plant Sci. 2021, 311, 110996. [Google Scholar] [CrossRef]
- Chandra, S.M.; Price, W.E.; Kelso, C.; Arcot, J.; Probst, Y. Measuring the anthocyanin content of the Australian fruit and vegetables for the development of a food composition database. J. Food Compos. Anal. 2022, 112, 104697. [Google Scholar] [CrossRef]
- Hematian, F.; Baghaei, H.; Nafchi, A.M.; Bolandi, M. Preparation and characterization of an intelligent film based on fish gelatin and Coleus scutellarioides anthocyanin to monitor the freshness of rainbow trout fish fillet. Food Sci. Nutr. 2023, 11, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Tilak, G.; Shruti, D.; DsO, D.H.V.J.; Kumar, V.S. Smart biodegradable films based on chitosan/methylcellulose containing Phyllanthus reticulatus anthocyanin for monitoring the freshness of fish fillet. Int. J. Biol. Macromol. 2021, 187, 451–461. [Google Scholar]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; et al. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control 2022, 131, 108441. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Rose, K.R.; Thomas, K.J.; Sandeep, S.S.J. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2022, 126, 108039. [Google Scholar]
- Yong, H.; Liu, J.; Kan, J.; Liu, J. Active/intelligent packaging films developed by immobilizing anthocyanins from purple sweetpotato and purple cabbage in locust bean gum, chitosan and κ-carrageenan-based matrices. Int. J. Biol. Macromol. 2022, 211, 238–248. [Google Scholar] [CrossRef]
- Gao, L.; Liu, P.; Liu, L.; Li, S.; Zhao, Y.; Xie, J.; Xu, H. κ-carrageenan-based pH-sensing films incorporated with anthocyanins or/and betacyanins extracted from purple sweet potatoes and peels of dragon fruits. Process Biochem. 2022, 121, 463–480. [Google Scholar] [CrossRef]
- Prata, E.R.B.A.; Oliveira, L.S. Fresh coffee husks as potential sources of anthocyanins. LWT 2007, 40, 1555–1560. [Google Scholar] [CrossRef]
- Rida, S.H.L.; Februadi, B.; Adiansyah, S. Effect of decaffeination and re-fermentation on level of caffeine, chlorogenic acid and total acid in green bean robusta coffee. Earth Environ. Sci. 2021, 807, 022069. [Google Scholar]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Karthik, K.T.V.; Maldonado, A.; Olvera, L.; Hernández, G.A.; Pérez, V.J.; Pozos, G.H. Copper-Doped ZnO Thin Films Deposited by Spray Pyrolysis: Effect of Water Content in Starting Solution on Methylene Blue Degradation by Photocatalysis. J. Electron. Mater. 2021, 50, 5542–5552. [Google Scholar] [CrossRef]
- Jannah, A.B.A.; Anuar, M.A.K. Swelling Behaviour and Water Vapour Transmission Rates of Gellan Gum/Collagen Film Containing Gatifloxacin as Dressing Materials. Mater. Sci. Forum. 2021, 1041, 75–79. [Google Scholar]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Pakdel, P.M. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Li, W.; Tan, L.; Fan, Q.; Wei, W.; Zhou, Z. Effect of storage time and temperature on dissolved state of cellulose in TBAH-based solvents and mechanical property of regenerated films. Rev. Adv. Mater. Sci. 2021, 60, 466–478. [Google Scholar] [CrossRef]
- Mohamad, M.H.; Halim, A.A.; Zulkarnain, Z.; Ngee, L.H. PES-Ag Mixed Matrix Film Photocatalyst for Degradation of Methyl Orange Dye. Polymers 2021, 13, 1746. [Google Scholar]
- Roy, S.; Rhim, J.W. Effect of CuS reinforcement on the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of alginate-based composite films. Int. J. Biol. Macromol. 2021, 164, 37–44. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.; Qin, Y.; Yan, J.; Yang, Q. A novel intelligent film with high stability based on chitosan/sodium alginate and coffee peel anthocyanin for monitoring minced beef freshness. Int. J. Food Sci. Technol. 2022, 57, 4673–4686. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Li, L.; Wang, Y.; Qin, Y.; Chen, H. A pH indicator film based on sodium alginate/gelatin and plum peel extract for monitoring the freshness of chicken. Food Biosci. 2023, 53, 102584. [Google Scholar] [CrossRef]
- Zhu, B.; Lu, W.; Qin, Y.; Cheng, G.; Yuan, M.; Li, L. An intelligent pH indicator film based on cassava starch/polyvinyl alcohol incorporating anthocyanin extracts for monitoring pork freshness. J Food Process. Preserv. 2021, 45, e15822. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.Y.; Yoon, S.R.; Lee, H.W.; Ha, J.H. Regression analysis for predicting the fermentation state of packaged Kimchi using a colorimetric indicator. J. Food Eng. 2019, 240, 65–72. [Google Scholar] [CrossRef]
- Huang, J.Y.; Liu, J.L.; Chen, M.Y.; Yao, Q.; Hu, Y.Q. Immobilization of roselle anthocyanins into polyvinyl alcohol/hydroxypropyl methylcellulose film matrix: Study on the interaction behavior and mechanism for better shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 184, 666–677. [Google Scholar] [CrossRef]
- Qin, Y.; Yun, D.W.; Xu, F.F.; Chen, D.; Kan, J.; Liu, J. Smart packaging films based on starch/polyvinyl alcohol and Lycium ruthenicum anthocyanins-loaded nano-complexes: Functionality, stability and application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar] [CrossRef]
- Chen, M.; Yan, T.; Huang, J.; Zhou, Y.; Hu, Y. Fabrication of halochromic smart films by immobilizing red cabbage anthocyanins into chitosan/oxidized-chitin nanocrystals composites for real-time hairtail and shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 179, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Huang, J.Y.; Ying, Y.B.; Hu, Y.P.; Hu, Y.Q. pH-sensitive and antibacterial films developed by incorporating anthocyanins extracted from purple potato or roselle into chitosan/polyvinyl alcohol/nano-ZnO matrix: Comparative study. Int. J. Biol. Macromol. 2021, 178, 104–112. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium amoenum anthocyanins into bacterial cellulose film: A novel colorimetric pH indicator for freshness/spoilage monitoring of shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicator film based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Peralta, J.; Bitencourt-Cervi, C.M.; Maciel, V.B.V.; Yoshida, C.M.P.; Carvalho, R.A. Aqueous hibiscus extract as a potential natural pH indicator incorporated in natural polymeric films. Food Packag. Shelf Life 2019, 19, 47–55. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, F.; Yuan, L.; Hu, H.; Yao, X.; Liu, J. Comparison of the physical and functional properties of starch/polyvinyl alcohol films containing anthocyanins and/or betacyanins. Int. J. Biol. Macromol. 2020, 163, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.N.; Lim, S.A.; Navaranjan, N. Development of sago (Metroxylon sagu)-based colorimetric indicator incorporated with butterfly pea (Clitoria ternatea) anthocyanin for intelligent food packaging. J. Food Saf. 2020, 40, e12807. [Google Scholar] [CrossRef]
- Coelho, G.O.; Batista, M.J.A.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Development and characterization of biopolymeric films of galactomannans recovered from spent coffee grounds. J. Food Eng. 2021, 289, 110083. [Google Scholar] [CrossRef]
- Pakeeza, M.K.; Zaib, J.; Ghufrana, S.; Arshad, H.; Tahir, A. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2021, 40, e12725. [Google Scholar]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohyd Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2021, 87, 858–868. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2020, 94, 80–92. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crop Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R.M. Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J. Mater. Res. Technol. 2020, 11, 144–157. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- An, F.; Sun, H.; Wu, J.; Zhao, C.; Li, T.; Huang, H.; Fang, Q.; Mu, E.; Wu, R. Investigating the core microbiota and its influencing factors in traditional Chinese pickles. Food Res. Int. 2021, 147, 110543. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Maruthupandy, M.; Lee, K.; Kim, D.; Seo, J. Preparation and characterization of a poly(ether-block-amide) film–based CO2 indicator for monitoring kimchi quality. React. Funct. Polym. 2018, 131, 75–83. [Google Scholar] [CrossRef]
- Kim, J.; Bang, J.; Beuchat, L.R.; Kim, H.; Ryu, J.H. Controlled fermentation of kimchi using naturally occurring antimicrobial agents. Food Microbiol. 2012, 32, 20–31. [Google Scholar] [CrossRef]
- Xiang, W.L.; Zhang, N.D.; Lu, Y.; Zhao, Q.H.; Xu, Q.; Rao, Y.; Liu, L.; Zhang, Q. Effect of Weissella cibaria co-inoculation on the quality of Sichuan Pickle fermented by Lactobacillus plantarum. LWT 2020, 121, 108975. [Google Scholar] [CrossRef]







| Film Sample | CS–GL | CS–GL–RCP10 | CS–GL–RCP15 | CS–GL–RCP20 |
|---|---|---|---|---|
| L | 65.45 ± 0.01 d | 62.91 ± 0.02 c | 61.88 ± 0.01 b | 59.66 ± 0.10 a |
| a | 3.38 ± 0.01 a | 4.21 ± 0.06 b | 6.74 ± 0.03 c | 8.93 ± 0.08 d |
| b | 4.42 ± 0.01 a | 7.86 ± 0.01 b | 9.91 ± 0.02 c | 10.56 ± 0.05 d |
| ΔE | 26.20 ± 0.01 a | 29.12 ± 0.01 b | 31.00 ± 0.01 c | 33.81 ± 0.08 d |
| Thicknesses (μm) | 38.0 ± 2.00 a | 41.6 ± 1.52 b | 47.2 ± 1.79 c | 51.2 ± 1.92 d |
| Water content (%) | 19.92 ± 0.55 d | 15.50 ± 0.23 c | 14.29 ± 0.07 b | 11.09 ± 0.85 a |
| Swelling ratio (%) | 202 ± 1.28 d | 195 ± 0.84 c | 191 ± 0.53 b | 185 ± 0.78 a |
| WVP (10−10·g·m−1·s−1·Pa −1) | 1.87 ± 0.07 a | 1.92 ± 0.03 a | 2.11 ± 0.02 b | 2.29 ± 0.08 c |
| Tensile strength (MPa) | 29.68 ± 0.29 d | 21.56 ± 0.28 c | 16.69 ± 0.16 b | 11.36 ± 0.10 a |
| Elongation at break (%) | 9.28 ± 0.10 a | 13.72 ± 0.23 b | 18.68 ± 0.18 c | 23.76 ± 0.21 d |
| Water contact angle (°) | 62.1 ± 1.84 d | 56.9 ± 0.51 c | 48.3 ± 1.02 b | 41.9 ± 1.37 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Yu, H.; Yang, Z.; Li, L.; Qin, Y.; Chen, H. Development of Smart Films of a Chitosan Base and Robusta Coffee Peel Extract for Monitoring the Fermentation Process of Pickles. Foods 2023, 12, 2337. https://doi.org/10.3390/foods12122337
Yan J, Yu H, Yang Z, Li L, Qin Y, Chen H. Development of Smart Films of a Chitosan Base and Robusta Coffee Peel Extract for Monitoring the Fermentation Process of Pickles. Foods. 2023; 12(12):2337. https://doi.org/10.3390/foods12122337
Chicago/Turabian StyleYan, Jiatong, Hongda Yu, Zhouhao Yang, Lin Li, Yuyue Qin, and Haiyan Chen. 2023. "Development of Smart Films of a Chitosan Base and Robusta Coffee Peel Extract for Monitoring the Fermentation Process of Pickles" Foods 12, no. 12: 2337. https://doi.org/10.3390/foods12122337
APA StyleYan, J., Yu, H., Yang, Z., Li, L., Qin, Y., & Chen, H. (2023). Development of Smart Films of a Chitosan Base and Robusta Coffee Peel Extract for Monitoring the Fermentation Process of Pickles. Foods, 12(12), 2337. https://doi.org/10.3390/foods12122337







