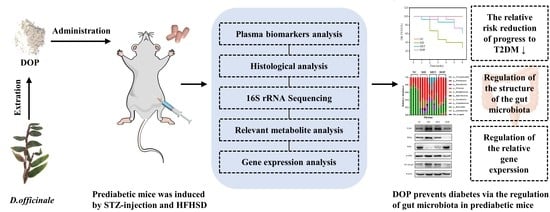
Dendrobium officinale Polysaccharide Prevents Diabetes via the Regulation of Gut Microbiota in Prediabetic Mice
Abstract
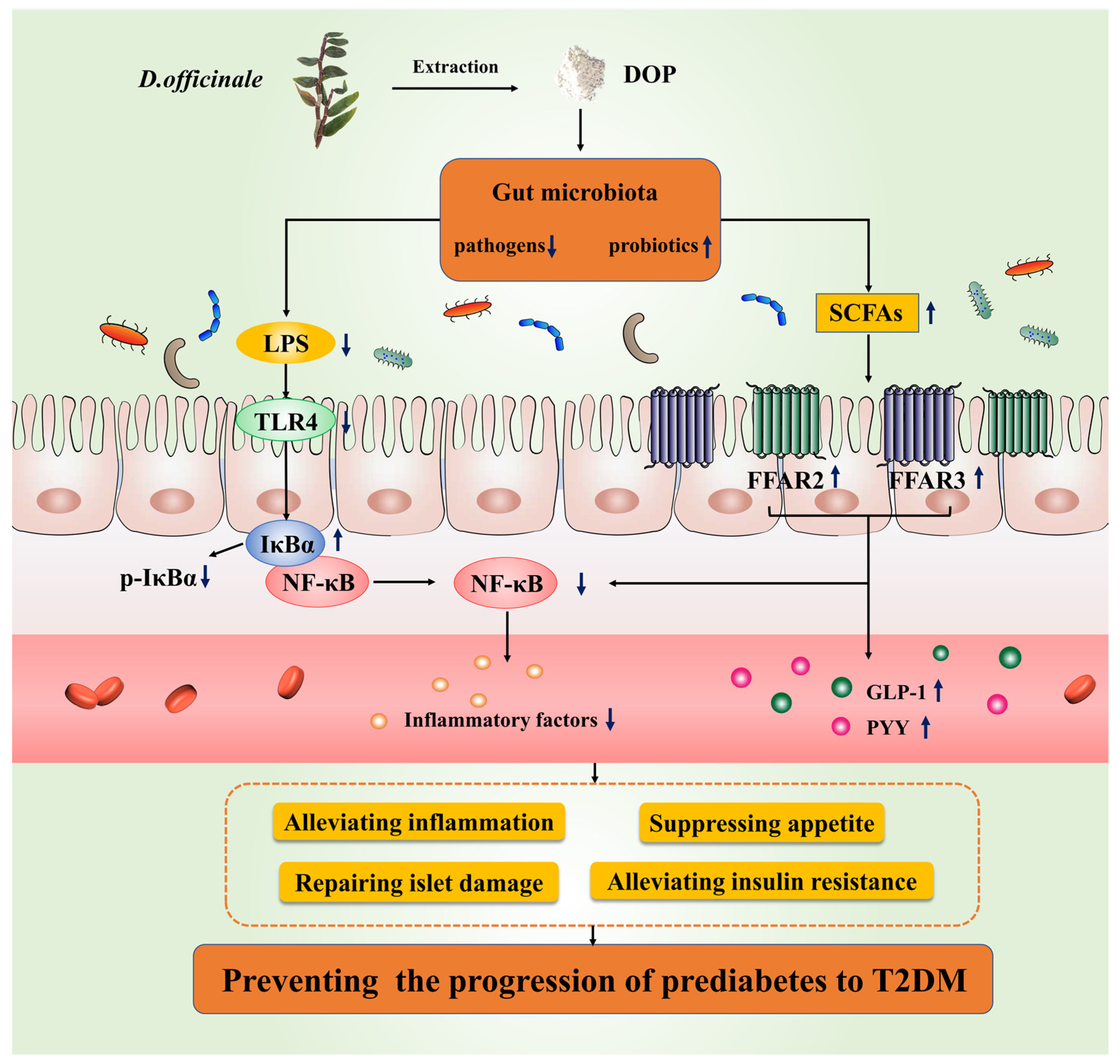
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of DOP
2.3. Purity Determination of DOP
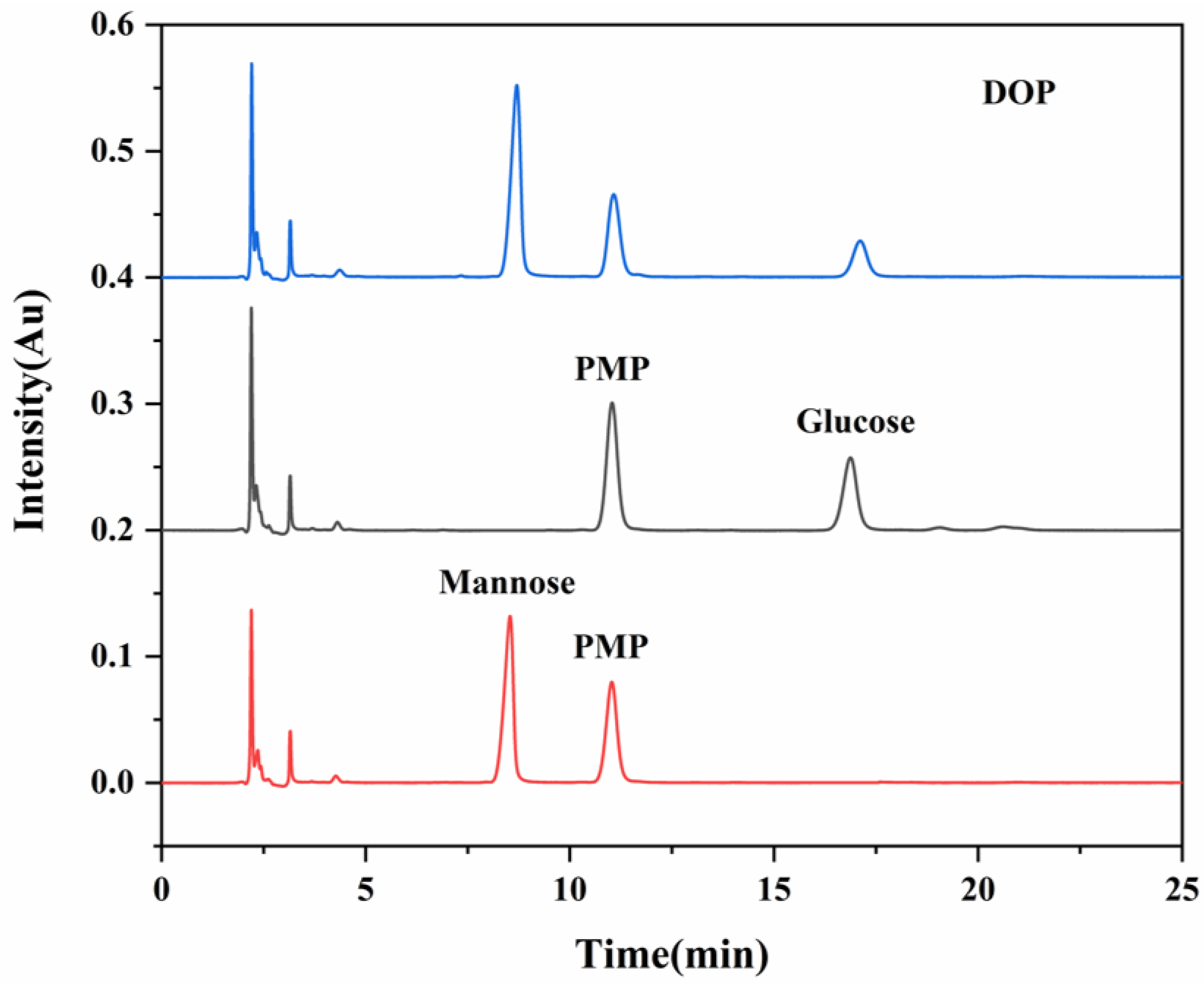
2.4. Analysis of Monosaccharide Composition
2.5. Animals and Experimental Protocols
2.6. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
2.7. Determination of Plasma Biomarkers and HOMA-IR
2.8. Histopathological Observation
2.9. Quantitative Real-Time (RT)-PCR
2.10. Western Blot Analysis
2.11. In Vitro Culture of the Gut Microbiota
2.12. Fecal Microbiota 16S rRNA High-Throughput Sequencing
2.13. Fecal SCFA Measurement
2.14. Statistical Analysis
3. Results
3.1. DOP Characterization
3.2. Effects of DOP on RRRD Value, Glucose Metabolism, and Lipid Profile of Prediabetic Mice
3.3. Effects of DOP on the Damage of Tissues In Vivo
3.4. Effects of DOP on the Gut Microbiota In Vivo and In Vitro
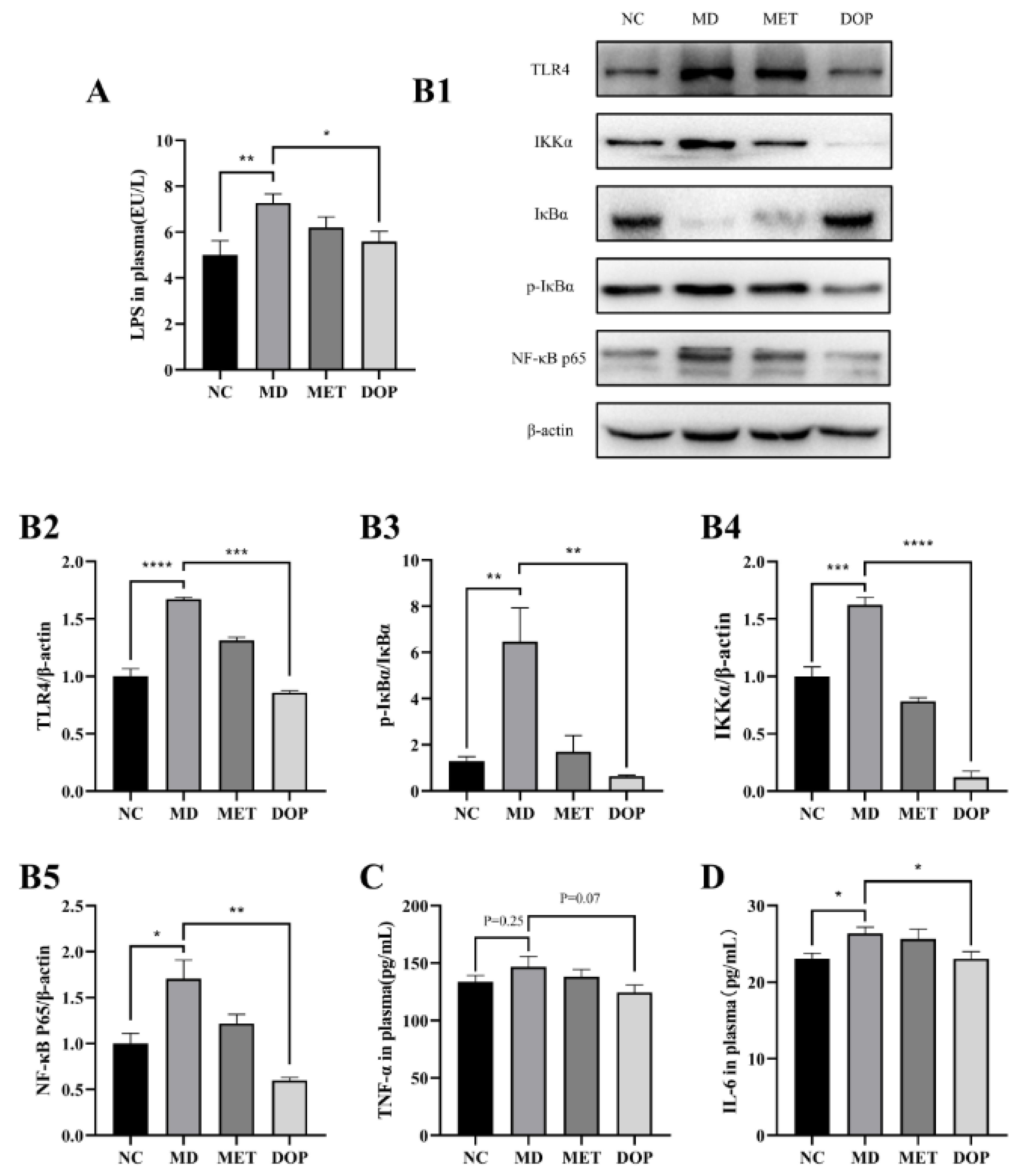
3.5. DOP Improved Insulin Resistance through Regulation of TLR4 Pathway In Vivo
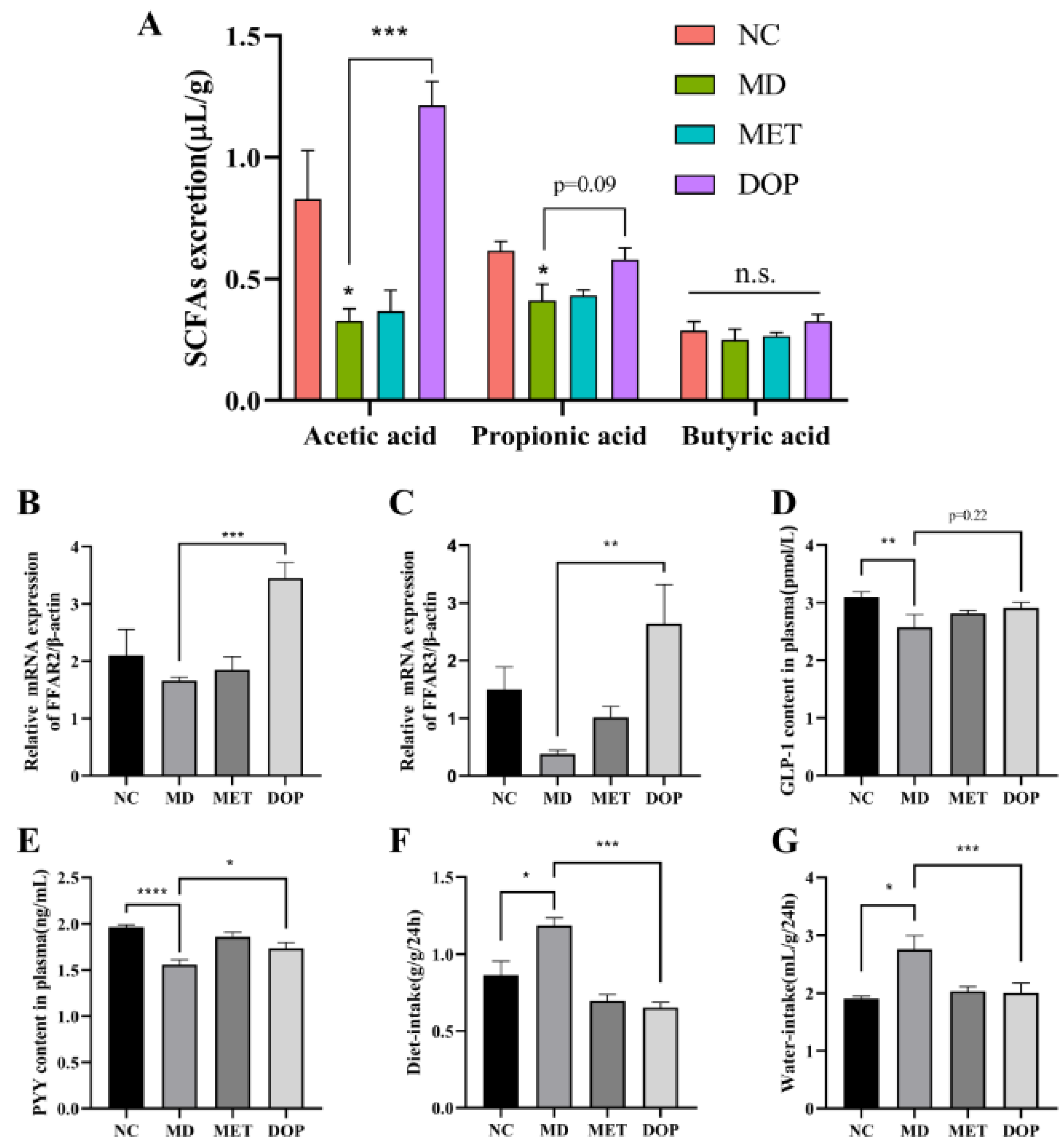
3.6. DOP Alleviated the Pancreatic Islet Damage, Reduced Water and Diet Intake, and Improved Insulin Resistance through the Regulation of the SCFA-FFAR2/FFAR3 Pathway In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Defronzo, R.A.; Abdul-Ghani, M. Assessment and treatment of cardiovascular risk in prediabetes: Impaired glucose tolerance and impaired fasting glucose. Am. J. Cardiol. 2011, 108, 3B–24B. [Google Scholar] [CrossRef]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Mccreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Tolman, K.G. Thiazolidinedione hepatotoxicity: A class effect? Int. J. Clin. Pract. Suppl. 2000, 113, 29–34. [Google Scholar]
- Sun, W.L.; Sun, J.; Zhang, B.W.; Xing, Y.; Yu, X.X.; Li, X.; Xiu, Z.L.; Dong, Y.S. Baicalein improves insulin resistance via regulating SOCS3 and enhances the effect of acarbose on diabetes prevention. J. Funct. Foods 2017, 37, 339–353. [Google Scholar] [CrossRef]
- Ren, X.X.; Xing, Y.; He, L.Y.; Xiu, Z.L.; Yang, L.; Han, A.Z.; Jia, Q.H.; Dong, Y.S. Effect of 1-Deoxynojirimycin on insulin resistance in prediabetic mice based on next-generation sequencing and intestinal microbiota study. J. Ethnopharmacol. 2022, 289, 115029. [Google Scholar] [CrossRef]
- Sun, W.L.; Zhang, B.W.; Yu, X.X.; Zhuang, C.L.; Li, X.; Sun, J.; Xing, Y.; Xiu, Z.L.; Dong, Y.S. Oroxin A from Oroxylum indicum prevents the progression from prediabetes to diabetes in streptozotocin and high-fat diet induced mice. Phytomedicine 2018, 38, 24–34. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Adejobi, O.I.; Wang, Y.H.; Liu, A.Z. Research advances in multi-omics on the traditional chinese herb Dendrobium officinale. Front. Plant Sci. 2022, 12, 808228. [Google Scholar] [CrossRef]
- Xu, Z.L.; Li, L.; Xu, Y.; Wang, S.S.; Zhang, X.X.; Tang, T.; Yu, J.Z.; Zhao, H.Y.; Wu, S.G.; Zhang, C.R.; et al. Pesticide multi-residues in Dendrobium officinale Kimura et Migo: Method validation, residue levels and dietary exposure risk assessment. Food Chem. 2021, 343, 128490. [Google Scholar] [CrossRef]
- Li, M.J.; Trapika, I.; Tang, S.; Cho, J.L.; Qi, Y.F.; Li, C.G.; Li, Y.J.; Yao, M.C.; Yang, D.P.; Liu, B.W.; et al. Mechanisms and active compounds polysaccharides and bibenzyls of medicinal Dendrobiums for diabetes management. Front. Nutr. 2022, 8, 811870. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, X.; Wang, K.; Cao, X.Y. Structural characterisation and bioactivity of polysaccharides isolated from fermented Dendrobium officinale. J. Sci. Food Agric. 2022, 102, 280–290. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Chang, H.; Cai, B.; Gao, H.J.; Chen, G.Y.; Hou, W.; Jappar, Z.; Yan, Y.Z. Research progress on extraction, purification, structure and biological activity of Dendrobium officinale polysaccharides. Front. Nutr. 2022, 9, 965073. [Google Scholar] [CrossRef]
- Chen, W.H.; Wu, J.J.; Li, X.F.; Lu, J.M.; Wu, W.; Sun, Y.Q.; Zhu, B.; Qin, L.P. Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol. 2021, 184, 1000–1013. [Google Scholar] [CrossRef]
- Gómez, B.; Míguez, B.; Yáñez, R.; Alonso, J.L. Manufacture and Properties of Glucomannans and Glucomannooligosaccharides Derived from Konjac and Other Sources. J. Agric. Food Chem. 2017, 65, 2019–2031. [Google Scholar] [CrossRef]
- Wang, K.P.; Wang, H.X.; Liu, Y.G.; Shui, W.Z.; Wang, J.F.; Cao, P.; Wang, H.J.; You, R.X.; Zhang, Y. Dendrobiurn officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J. Funct. Foods 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Yang, J.R.; Chen, H.H.; Nie, Q.X.; Huang, X.J.; Nie, S.P. Dendrobium officinale polysaccharide ameliorates the liver metabolism disorders of type II diabetic rats. Int. J. Biol. Macromol. 2020, 164, 1939–1948. [Google Scholar] [CrossRef]
- Fang, J.; Lin, Y.; Xie, H.; Farag, M.A.; Feng, S.; Li, J.; Shao, P. Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated SCFAs to alleviate type 2 diabetes in adult mice. Food Chem. X 2022, 13, 100207. [Google Scholar] [CrossRef]
- Liu, Y.G.; Yang, L.L.; Zhang, Y.; Liu, X.C.; Wu, Z.J.; Gilbert, R.G.; Deng, B.; Wang, K.P. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020, 248, 112308. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.; Damgaard, M.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M.; et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Kramer, M.; Gummesson, A.; Perkins, R.; Bergstrom, G.; Backhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390. [Google Scholar] [CrossRef]
- Xie, S.Z.; Ge, J.C.; Li, F.; Yang, J.; Pan, L.H.; Zha, X.Q.; Li, Q.M.; Duan, J.; Luo, J.P. Digestive behavior of Dendrobium huoshanense polysaccharides in the gastrointestinal tracts of mice. Int. J. Biol. Macromol. 2018, 107, 825–832. [Google Scholar] [CrossRef]
- Kuang, M.T.; Li, J.Y.; Yang, X.B.; Yang, L.; Xu, J.Y.; Yan, S.; Lv, Y.F.; Ren, F.C.; Hu, J.M.; Zhou, J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohyd. Polym. 2020, 241, 116326. [Google Scholar] [CrossRef]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem.-Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes 2018, 68, 291–304. [Google Scholar] [CrossRef] [Green Version]
- He, L.Y.; Xing, Y.; Ren, X.X.; Zheng, M.J.; Yu, S.Q.; Wang, Y.B.; Xiu, Z.L.; Dong, Y.S. Mulberry leaf extract improves metabolic syndrome by alleviating lipid accumulation in vitro and in vivo. Molecules 2022, 27, 5111. [Google Scholar] [CrossRef]
- Xing, Y.; Ren, X.X.; Li, X.; Sui, L.P.; Shi, X.; Sun, Y.; Quan, C.S.; Xiu, Z.L.; Dong, Y.S. Baicalein enhances the effect of acarbose on the improvement of nonalcoholic fatty liver disease associated with prediabetes via the inhibition of de novo lipogenesis. J. Agric. Food Chem. 2021, 69, 9822–9836. [Google Scholar] [CrossRef]
- Dong, Y.S.; Sui, L.P.; Yang, F.; Ren, X.X.; Xing, Y.; Xiu, Z.L. Reducing the intestinal side effects of acarbose by baicalein through the regulation of gut microbiota: An in vitro study. Food Chem. 2022, 394, 133561. [Google Scholar] [CrossRef]
- Yue, H.; Zeng, H.; Ding, K. A review of isolation methods, structure features and bioactivities of polysaccharides from Dendrobium species. Chin. J. Nat. Med. 2020, 18, 1–27. [Google Scholar] [CrossRef]
- Lilly, M.; Godwin, M. Treating prediabetes with metformin Systematic review and meta-analysis. Can. Fam. Physician 2009, 55, 363–369. [Google Scholar]
- Ye, J.P. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Afoods-12-02310sia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.T.; Li, Y.Q.; Li, P.F.; Wang, M.; Wang, J.K.; Tang, Z.Y.; Wang, T.; Luo, L.L.; Wang, C.G.; Zhao, B.S. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Saad, M.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, P.W.; Shen, L.Y.; Niu, L.L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.X.; Li, X.W.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Prentice, K.J.; Wheeler, M.B. FFAR out new targets for diabetes. Cell Metab. 2015, 21, 353–354. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Martin, R.J.; Raggio, A.M.; Shen, L.; Mccutcheon, K.; Keenan, M.J. The importance of GLP-1 and PYY in resistant starch’s effect on body fat in mice. Mol. Nutr. Food Res. 2015, 59, 1000–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Q.X.; Chen, H.H.; Hu, J.L.; Fan, S.T.; Nie, S.P. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. 2019, 59, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Neyrinck, A.M.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Kaji, L.; Karaki, S.; Kuwahara, A. Short-Chain Fatty Acid Receptor and Its Contribution to Glucagon-Like Peptide-1 Release. Digestion 2014, 89, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.; Nawrocki, A.R.; Lu, M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.R.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [Green Version]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.M.; Ding, Y.H.; Xu, H.Y.; Shen, C.; Chen, X.; Li, C.P. Effects of sodium butyrate supplementation on inflammation, gut microbiota, and short-chain fatty acids in Helicobacter pylori-infected mice. Helicobacter 2021, 26, e12785. [Google Scholar] [CrossRef] [PubMed]
- Leibovitzh, H.; Lee, S.H.; Xue, M.Y.; Garay, J.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered gut microbiome composition and function are associated with gut barrier dysfunction in healthy relatives of patients with crohn’s disease. Gastroenterology 2022, 163, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Herp, S.; Raj, A.; Silva, M.S.; Woelfel, S.; Stecher, B. The human symbiont Mucispirillum schaedleri: Causality in health and disease. Med. Microbiol. Immun. 2021, 210, 173–179. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef] [Green Version]
- Scheithauer, T.; Dallinga-Thie, G.M.; de Vos, W.M.; Nieuwdorp, M.; van Raalte, D.H. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol. Metab. 2016, 5, 759–770. [Google Scholar] [CrossRef]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef]
- Huang, W.; Man, Y.; Gao, C.L.; Zhou, L.P.; Gu, J.L.; Xu, H.W.; Wan, Q.; Long, Y.; Chai, L.; Xu, Y.H.; et al. Short-Chain Fatty Acids Ameliorate Diabetic Nephropathy via GPR43-Mediated Inhibition of Oxidative Stress and NF-kappa B Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 4074832. [Google Scholar] [CrossRef]
- Wang, K.P.; Yang, X.W.; Wu, Z.J.; Wang, H.J.; Lie, Q.; Mei, H.; You, R.X.; Zhang, Y. Dendrobium officinale polysaccharide protected CCl4-induced liver fibrosis through intestinal homeostasis and the LPS-TLR4-NF-kappa B signaling pathway. Front. Pharmacol. 2020, 11, 240. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Zhan, L.H.; Lu, T.T.; Zhou, C.; Chen, X.; Dong, Y.J.; Lv, G.Y.; Chen, S.H. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-kappa B signaling pathway. Cytokine 2020, 130, 155058. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Zhang, K.; Nie, S. Glucomannans Alleviated the Progression of Diabetic Kidney Disease by Improving Kidney Metabolic Disturbance. Mol. Nutr. Food Res. 2019, 63, e1801008. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Yin, J.; Nie, S. Multiomics Approach to Explore the Amelioration Mechanisms of Glucomannans on the Metabolic Disorder of Type 2 Diabetic Rats. J. Agric. Food Chem. 2021, 69, 2632–2645. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jayachandran, M.; Xu, B. Antidiabetic effect of konjac glucomannan via insulin signaling pathway regulation in high-fat diet and streptozotocin-induced diabetic rats. Food Res. Int. 2021, 149, 110664. [Google Scholar] [CrossRef]
- Tester, R.; Al-Ghazzewi, F. Glucomannans and nutrition. Food Hydrocoll. 2017, 68, 246–254. [Google Scholar] [CrossRef]



| Genes | Primer (5′-3′) | |
|---|---|---|
| FFAR2 | forward | GGACCAGAGGAGAACCAGGTAGAAG |
| reverse | GCCGTGAGGATCAAGGAACTGTG | |
| FFAR3 | forward | CCACACTGCTCATCTTCTTCGTCTG |
| reverse | ACGGACTCTCACGGCTGACATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xing, Y.; Wang, Y.; Ren, X.; Zhang, D.; Dai, J.; Xiu, Z.; Yu, S.; Dong, Y. Dendrobium officinale Polysaccharide Prevents Diabetes via the Regulation of Gut Microbiota in Prediabetic Mice. Foods 2023, 12, 2310. https://doi.org/10.3390/foods12122310
Liu H, Xing Y, Wang Y, Ren X, Zhang D, Dai J, Xiu Z, Yu S, Dong Y. Dendrobium officinale Polysaccharide Prevents Diabetes via the Regulation of Gut Microbiota in Prediabetic Mice. Foods. 2023; 12(12):2310. https://doi.org/10.3390/foods12122310
Chicago/Turabian StyleLiu, Haodong, Yan Xing, Yinbo Wang, Xinxiu Ren, Danyang Zhang, Jianying Dai, Zhilong Xiu, Shiqiang Yu, and Yuesheng Dong. 2023. "Dendrobium officinale Polysaccharide Prevents Diabetes via the Regulation of Gut Microbiota in Prediabetic Mice" Foods 12, no. 12: 2310. https://doi.org/10.3390/foods12122310
APA StyleLiu, H., Xing, Y., Wang, Y., Ren, X., Zhang, D., Dai, J., Xiu, Z., Yu, S., & Dong, Y. (2023). Dendrobium officinale Polysaccharide Prevents Diabetes via the Regulation of Gut Microbiota in Prediabetic Mice. Foods, 12(12), 2310. https://doi.org/10.3390/foods12122310