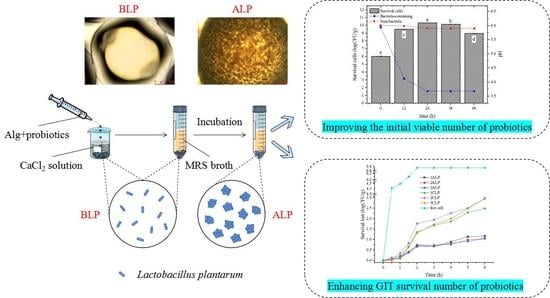
Improved Loading Capacity and Viability of Probiotics Encapsulated in Alginate Hydrogel Beads by In Situ Cultivation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Beads Preparation
2.3. Hydrogel Beads Post-Encapsulation Cultivation
2.4. Particle Size Measurement
2.5. Microscopic and SEM Observation
2.6. Rheology Experiments
2.7. In Vitro Simulation of Gastro-Intestinal Digestion
2.8. Statistical Analysis and Figure Plotting
3. Results and Discussion
3.1. Growth Characteristics of Bacteria Encapsulated in Hydrogel Beads during Incubation
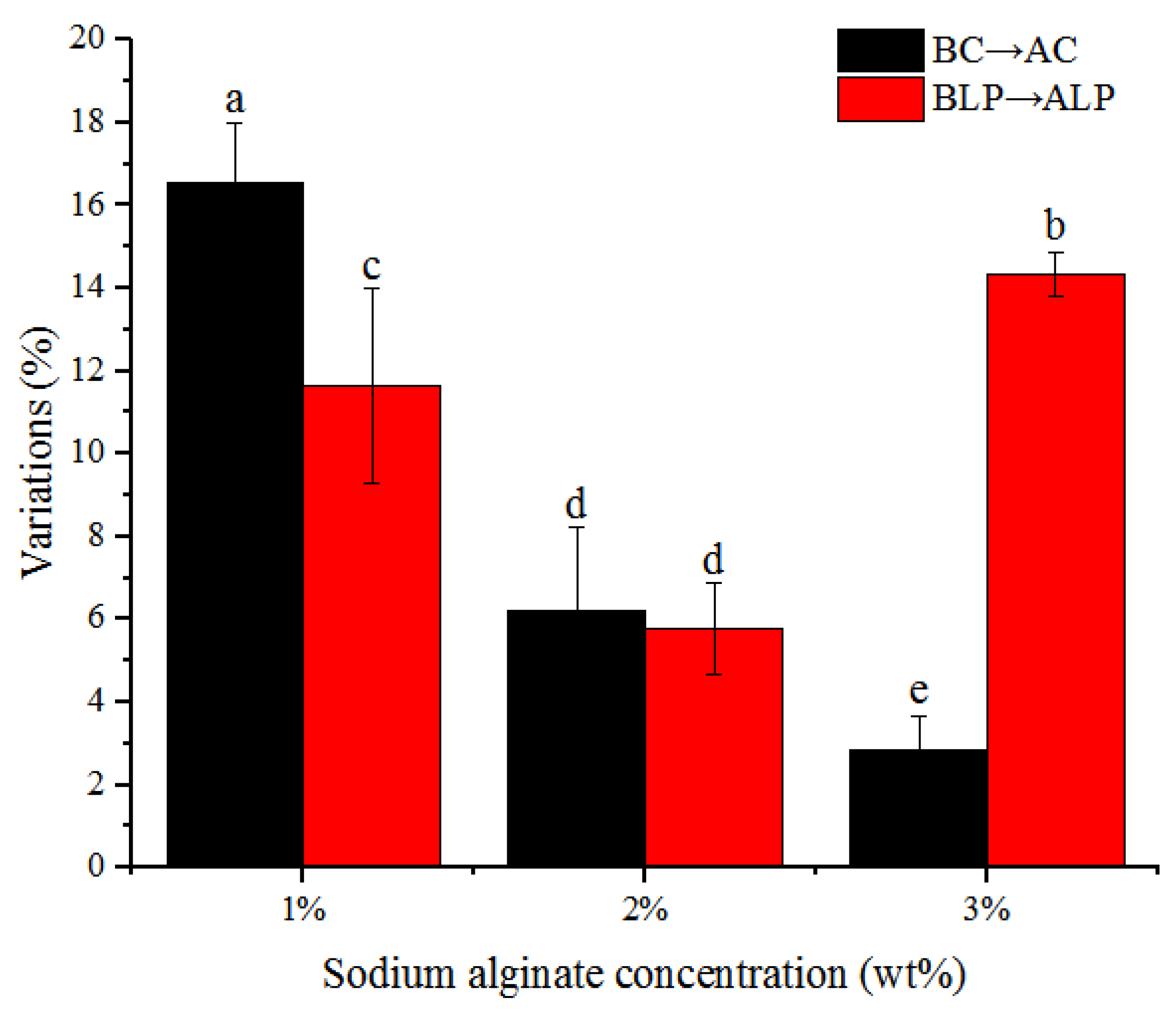
3.2. Particle Size Variations of Hydrogel Beads during Incubation
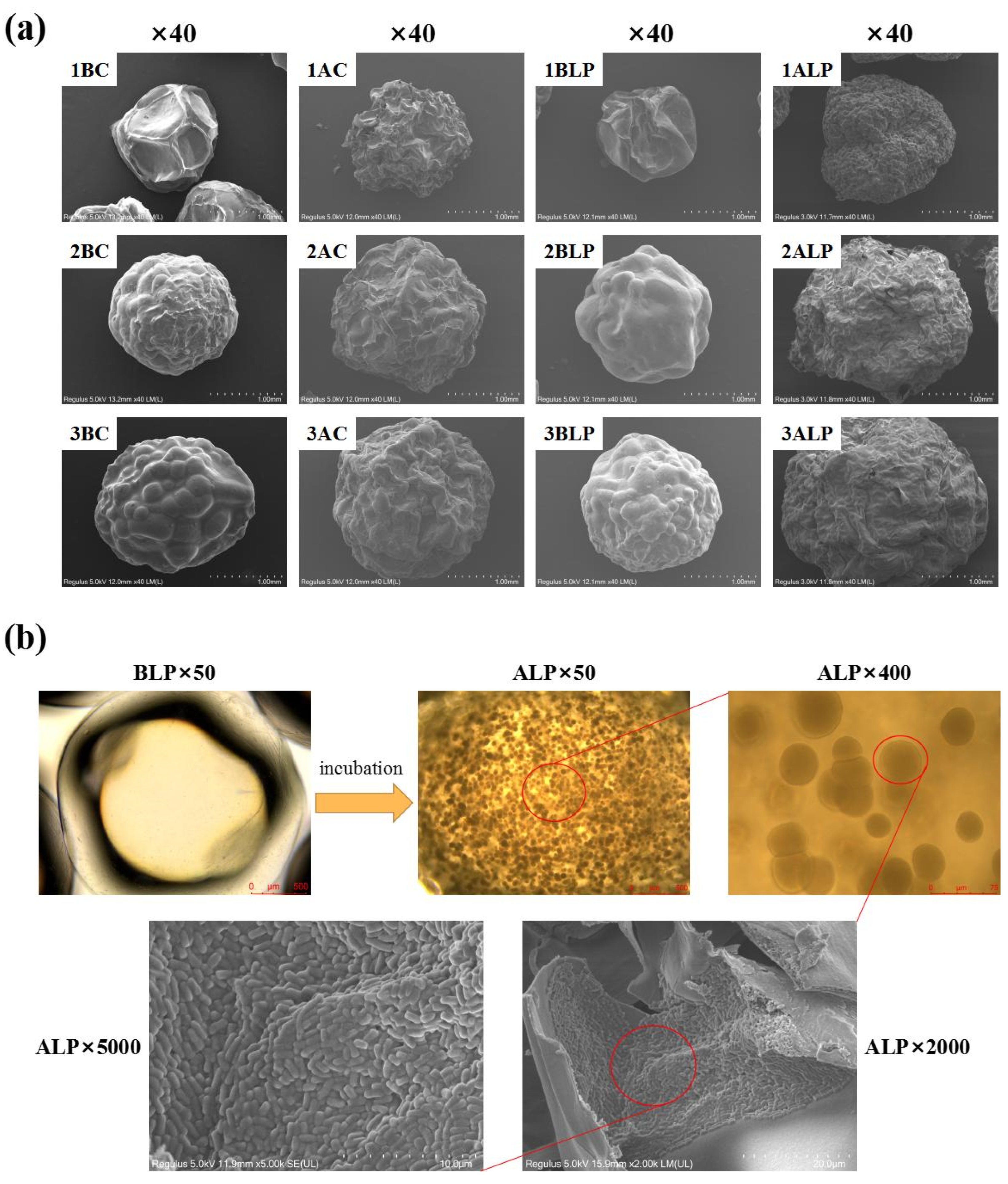
3.3. Microstructure Variations of Hydrogel Beads during Incubation
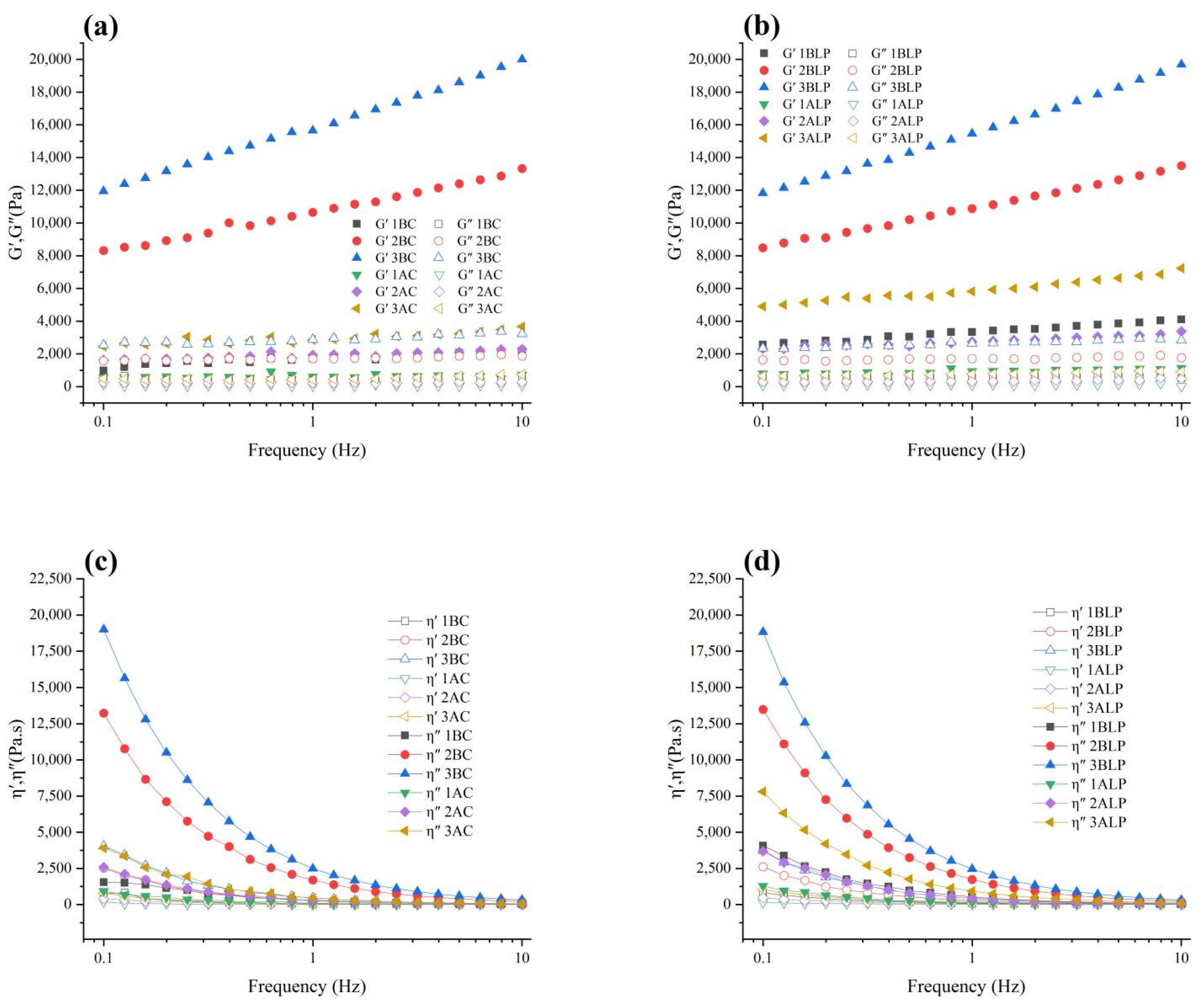
3.4. Rheological Property Analysis
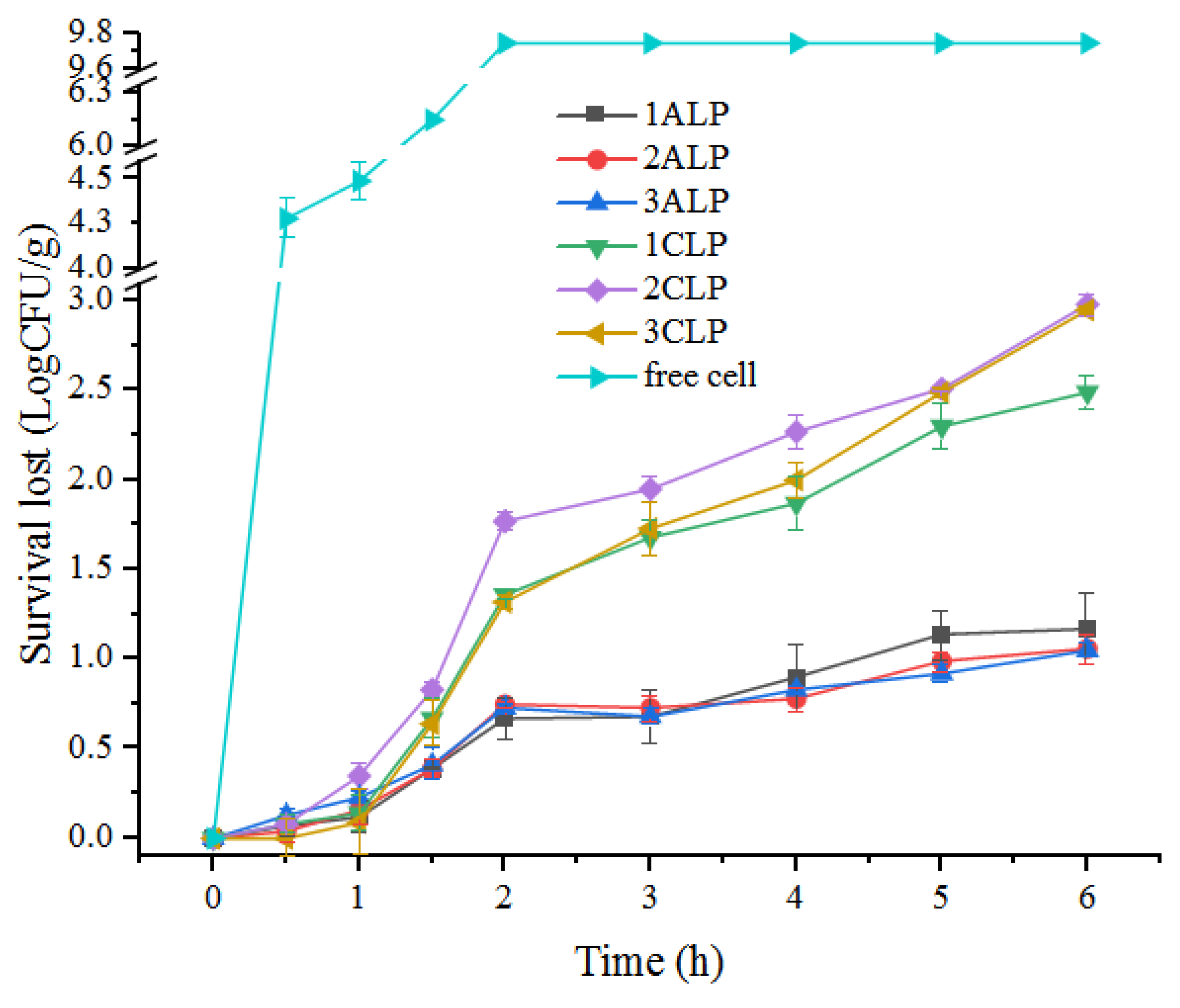
3.5. In Vitro Simulation of Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asgari, S.; Pourjavadi, A.; Licht, T.R.; Boisen, A.; Ajalloueian, F. Polymeric carriers for enhanced delivery of probiotics. Adv. Drug Deliv. Rev. 2020, 161–162, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fan, L.; Li, S.; Sun, X.; Di, Q.; Zhang, H.; Li, B.; Liu, X. Validation of Layer-by-Layer Coating as a Procedure to Enhance Lactobacillus plantarum Survival during In Vitro Digestion, Storage, and Fermentation. J. Agric. Food Chem. 2023, 71, 1701–1712. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Ribas, M.M.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Microcapsules loaded with the probiotic Lactobacillus paracasei BGP-1 produced by co-extrusion technology using alginate/shellac as wall material: Characterization and evaluation of drying processes. Food Res. Int. 2016, 89, 582–590. [Google Scholar] [CrossRef]
- Khan, N.H.; Korber, D.R.; Low, N.H.; Nickerson, M.T. Development of extrusion-based legume protein isolate–alginate capsules for the protection and delivery of the acid sensitive probiotic, Bifidobacterium adolescentis. Food Res. Int. 2013, 54, 730–737. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; García-Galindo, H.; Alvarez-Ramírez, J.; Vernon-Carter, E. Textural properties of alginate–pectin beads and survivability of entrapped Lb. casei in simulated gastrointestinal conditions and in yoghurt. Food Res. Int. 2010, 43, 111–117. [Google Scholar] [CrossRef]
- Park, J.K.; Jin, Y.B.; Chang, H.N. Reusable biosorbents in capsules from zoogloea ramigera cells for cadmium removal. Biotechnol. Bioeng. 1999, 63, 116–121. [Google Scholar] [CrossRef]
- Heumann, A.; Assifaoui, A.; Da Silva Barreira, D.; Thomas, C.; Briandet, R.; Laurent, J.; Beney, L.; Lapaquette, P.; Guzzo, J.; Rieu, A. Intestinal release of biofilm-like microcolonies encased in calcium-pectinate beads increases probiotic properties of Lacticaseibacillus paracasei. NPJ Biofilms Microbiomes 2020, 6, 44. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.; Kim, H.; Kim, H.J.; Yang, Y.-H.; Kim, Y.H.; Jung, S.-K.; Kan, E.; Lee, S.H. Alginate/bacterial cellulose nanocomposite beads prepared using Gluconacetobacter xylinus and their application in lipase immobilization. Carbohydr. Polym. 2017, 157, 137–145. [Google Scholar] [CrossRef]
- Dai, Z.; Yang, X.; Wu, F.; Wang, L.; Xiang, K.; Li, P.; Lv, Q.; Tang, J.; Dohlman, A.; Dai, L.; et al. Living fabrication of functional semi-interpenetrating polymeric materials. Nat. Commun. 2021, 12, 3422. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Sun, L.; Rehman, R.U.; Wang, Y. In vitro and in vivo study of sodium polyacrylate grafted alginate as microcapsule matrix for live probiotic delivery. J. Funct. Foods 2016, 24, 429–437. [Google Scholar] [CrossRef]
- Cui, C.; Jiang, H.; Guan, M.; Ji, N.; Xiong, L.; Sun, Q. Characterization and in vitro digestibility of potato starch encapsulated in calcium alginate beads. Food Hydrocoll. 2022, 126, 107458. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.; Wang, Y.; Xie, L.; Liang, S.; Li, D.; Wang, Y.; Wang, J.; Zhan, X. Carboxymethyl konjac glucomannan-chitosan complex nanogels stabilized double emulsions incorporated into alginate hydrogel beads for the encapsulation, protection and delivery of probiotics. Carbohydr. Polym. 2022, 289, 119438. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Ladányi, M.; Omran, M.; Papp, V.; Ronkainen, V.-P.; Pónya, Z.; Papp, I.; Némedi, E.; Kiss, A. Co-encapsulation of probiotic Lactobacillus acidophilus and Reishi medicinal mushroom (Ganoderma lingzhi) extract in moist calcium alginate beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Guan, M.; Dai, L.; Ji, N.; Qin, Y.; Xu, X.; Xiong, L.; Shi, R.; Sun, Q. Fabrication of starch-based emulsion gel beads by an inverse gelation technique for loading proanthocyanidin and curcumin. Food Hydrocoll. 2023, 137, 108336. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Woods, R.J. Solvent interactions determine carbohydrate conformation. Proc. Natl. Acad. Sci. USA 2001, 98, 10541–10545. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W. Conformational properties of acidic oligo- and disaccharides and their ability to bind calcium: A molecular modeling study. Carbohydr. Res. 2012, 357, 111–117. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhat, M.A.; Shalla, A.H. Multicomponent interpenetrating metal based Alginate-Carrageenan biopolymer hydrogel beads substantiated by graphene oxide for efficient removal of methylene blue from waste water. Chem. Eng. Res. Des. 2022, 182, 604–615. [Google Scholar] [CrossRef]
- Priftis, D.; Laugel, N.; Tirrell, M. Thermodynamic Characterization of Polypeptide Complex Coacervation. Langmuir 2012, 28, 15947–15957. [Google Scholar] [CrossRef]
- Deng, P.; Yao, L.; Chen, J.; Tang, Z.; Zhou, J. Chitosan-based hydrogels with injectable, self-healing and antibacterial properties for wound healing. Carbohydr. Polym. 2021, 276, 118718. [Google Scholar] [CrossRef]
- Tang, B.; Yang, X.; Zhang, A.; Wang, Q.; Fan, L.; Fang, G. Polypseudorotaxane hydrogel based on Tween 80 and α-cyclodextrin for sustained delivery of low molecular weight heparin. Carbohydr. Polym. 2022, 297, 120002. [Google Scholar] [CrossRef]
- Bastos, D.S.; Barreto, B.N.; Souza, H.K.; Bastos, M.; Rocha-Leão, M.H.M.; Andrade, C.T.; Gonçalves, M.P. Characterization of a chitosan sample extracted from Brazilian shrimps and its application to obtain insoluble complexes with a commercial whey protein isolate. Food Hydrocoll. 2010, 24, 709–718. [Google Scholar] [CrossRef]
- Mousavi, S.M.R.; Rafe, A.; Yeganehzad, S. Structure-rheology relationships of composite gels: Alginate and Basil seed gum/guar gum. Carbohydr. Polym. 2019, 232, 115809. [Google Scholar] [CrossRef] [PubMed]
- Marapureddy, S.G.; Hivare, P.; Sharma, A.; Chakraborty, J.; Ghosh, S.; Gupta, S.; Thareja, P. Rheology and direct write printing of chitosan—Graphene oxide nanocomposite hydrogels for differentiation of neuroblastoma cells. Carbohydr. Polym. 2021, 269, 118254. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
 ) and pH of external MRS broth during incubation with (
) and pH of external MRS broth during incubation with ( ) or without (
) or without ( ) inoculation of probiotic bacteria. Columns with different letters (a–e) are significantly different (p < 0.05).
) inoculation of probiotic bacteria. Columns with different letters (a–e) are significantly different (p < 0.05).
 ) and pH of external MRS broth during incubation with (
) and pH of external MRS broth during incubation with ( ) or without (
) or without ( ) inoculation of probiotic bacteria. Columns with different letters (a–e) are significantly different (p < 0.05).
) inoculation of probiotic bacteria. Columns with different letters (a–e) are significantly different (p < 0.05).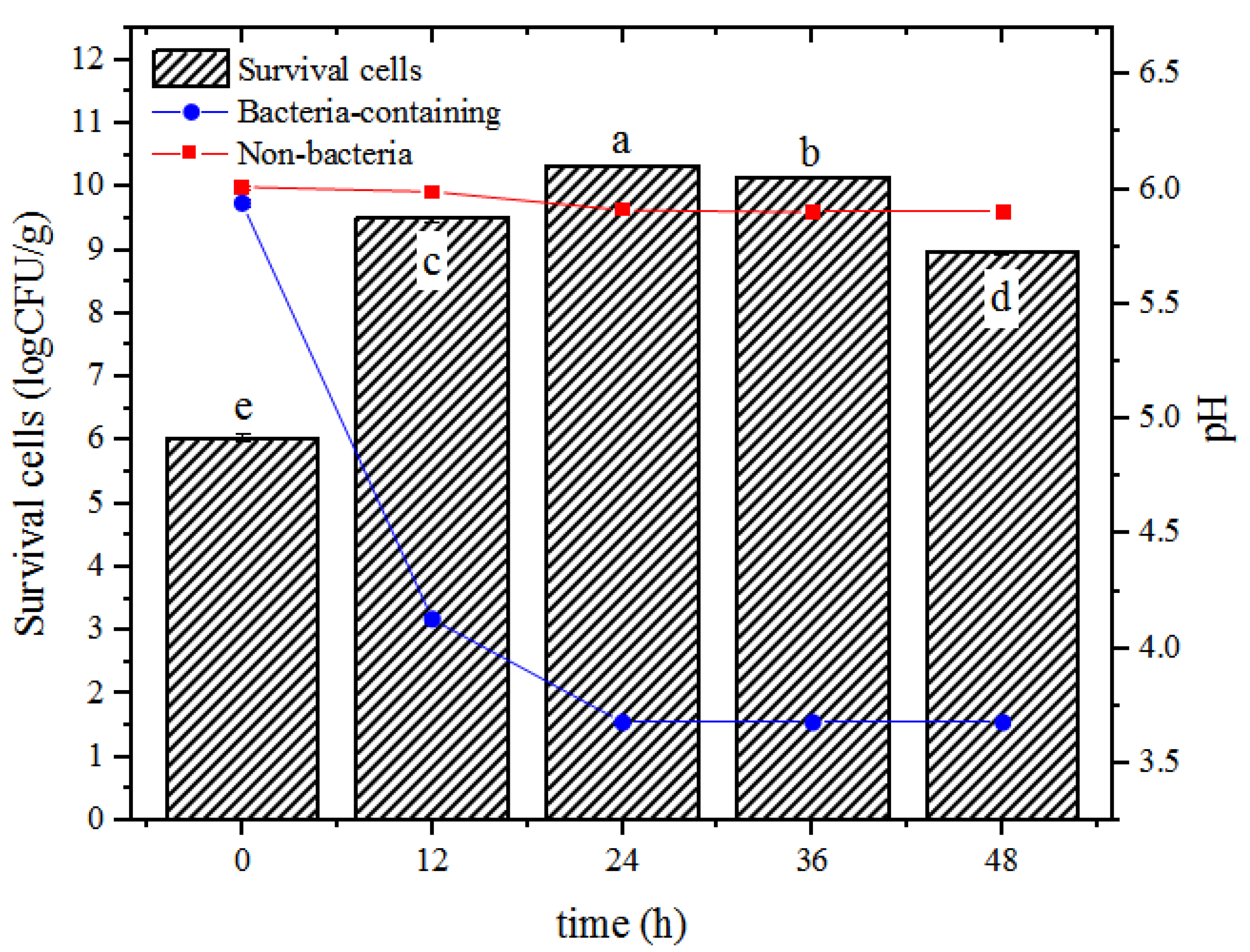

| Group | Sample Name | Sodium Alginate (wt%) | Concentration of Probiotics in the Sample (LogCFU/g) | Incubation Time (h) |
|---|---|---|---|---|
| Before incubation Non-bacteria group | 1BC | 1 | / | / |
| 2BC | 2 | |||
| 3BC | 3 | |||
| Before incubation Contains low concentration of bacteria group | 1BLP | 1 | 6 | / |
| 2BLP | 2 | |||
| 3BLP | 3 | |||
| After incubation Non-bacteria group | 1AC | 1 | / | 24 |
| 2AC | 2 | |||
| 3AC | 3 | |||
| After incubation Bacteria-containing group | 1ALP | 1 | Change with time | 24 |
| 2ALP | 2 | |||
| 3ALP | 3 | |||
| Control group | 1CLP | 1 | 9 | / |
| 2CLP | 2 | |||
| 3CLP | 3 |
| Before Incubation | After Incubation | |||
|---|---|---|---|---|
| Name | Diameter (mm) | Name | Diameter (mm) | |
| Non-bacteria group | 1BC | 2.109 ± 0.035 h | 1AC | 2.458 ± 0.069 f |
| 2BC | 2.466 ± 0.078 f | 2AC | 2.617 ± 0.034 e | |
| 3BC | 2.787 ± 0.051 c | 3AC | 2.867 ± 0.055 b | |
| Bacteria-containing group | 1BLP | 2.111 ± 0.096 h | 1ALP | 2.358 ± 0.129 g |
| 2BLP | 2.69 ± 0.036 d | 2ALP | 2.846 ± 0.065 bc | |
| 3BLP | 2.644 ± 0.046 de | 3ALP | 3.024 ± 0.047 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhang, L.; Hu, J.; Liu, H. Improved Loading Capacity and Viability of Probiotics Encapsulated in Alginate Hydrogel Beads by In Situ Cultivation Method. Foods 2023, 12, 2256. https://doi.org/10.3390/foods12112256
Huang Y, Zhang L, Hu J, Liu H. Improved Loading Capacity and Viability of Probiotics Encapsulated in Alginate Hydrogel Beads by In Situ Cultivation Method. Foods. 2023; 12(11):2256. https://doi.org/10.3390/foods12112256
Chicago/Turabian StyleHuang, Yachun, Lin Zhang, Jielun Hu, and Huan Liu. 2023. "Improved Loading Capacity and Viability of Probiotics Encapsulated in Alginate Hydrogel Beads by In Situ Cultivation Method" Foods 12, no. 11: 2256. https://doi.org/10.3390/foods12112256
APA StyleHuang, Y., Zhang, L., Hu, J., & Liu, H. (2023). Improved Loading Capacity and Viability of Probiotics Encapsulated in Alginate Hydrogel Beads by In Situ Cultivation Method. Foods, 12(11), 2256. https://doi.org/10.3390/foods12112256