Evaluation of Environmentally Relevant Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
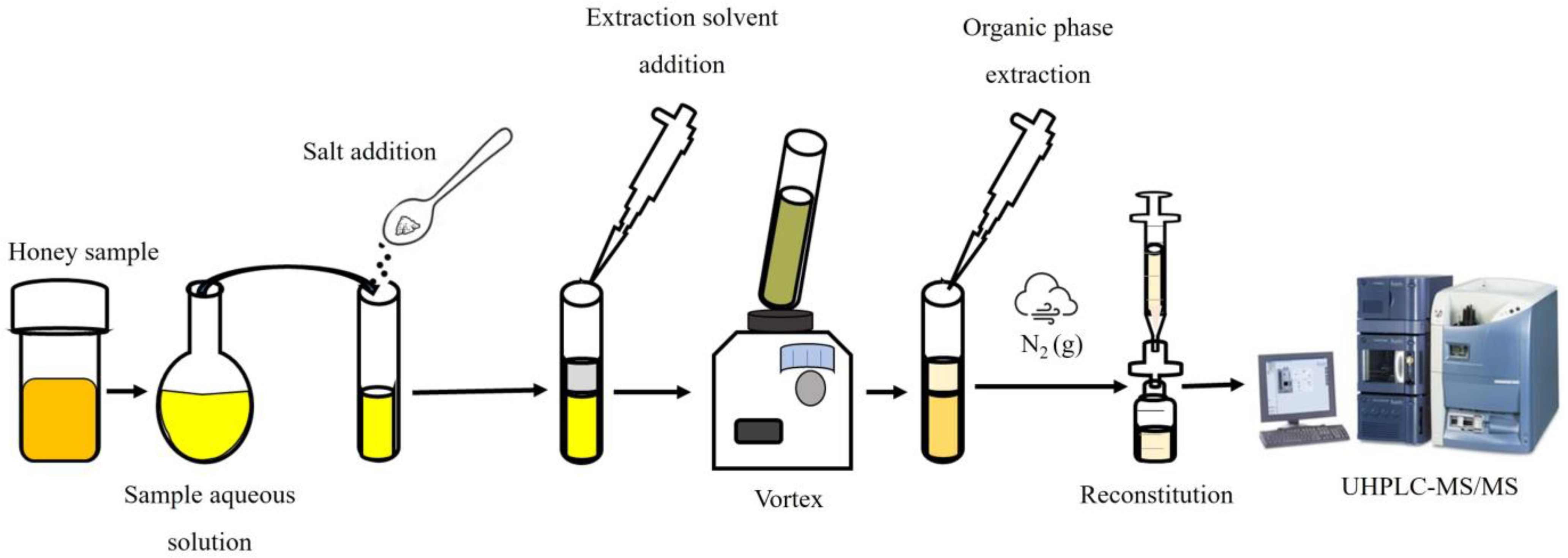
2.3. Sampling and Sample Preparation
2.4. SALLE Procedure
2.5. Optimization and Experimental Design
2.6. Method Validation
2.6.1. Matrix Effect
2.6.2. Analytical Capacity
2.7. Sustainability Assessment
3. Results
3.1. Optimization SALLE
3.1.1. Univariate Optimization
3.1.2. Full Factorial Design
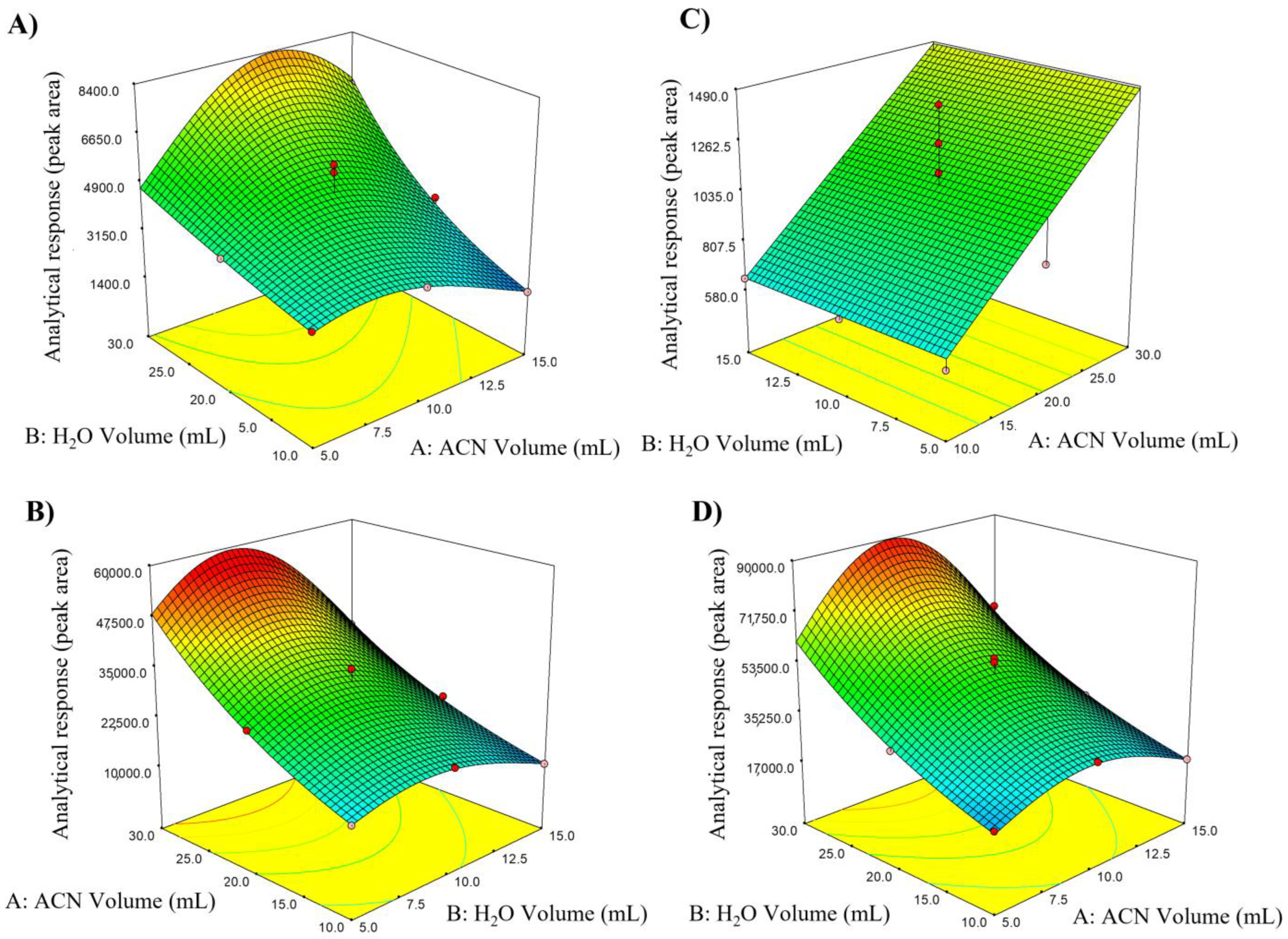
3.1.3. Response Surface Methodology (RSM) and Composite Central Design (DCC)
3.2. Methodological Validation
3.3. Greenness Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agency, U.S.E.P. Issues Terms & Acronyms. Available online: https://www.epa.gov/ (accessed on 3 March 2023).
- Carroquino, M.; Pena-Fernandez, A.; Duarte-Davidson, R.; Ordonez, J.; Martín-Olmedo, P. Risk Characterization; Sociedad Española de Sanidad Ambiental: Madrid, Spain; Escuela Andaluza de Salud Pública: Granada, Spain, 2016. [Google Scholar]
- Harvey, R.G. Environmental Chemistry of PAHs. In PAHs and Related Compounds: Chemistry; Neilson, A.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–54. [Google Scholar] [CrossRef]
- Shen, H. Polycyclic Aromatic Hydrocarbons: Their Global Atmospheric Emissions, Transport, and Lung Cancer Risk; Springer: Berlin/Heidelberg, Germany, 2016; p. 117. [Google Scholar] [CrossRef]
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, S.; Gong, G.J. Trends of research on polycyclic aromatic hydrocarbons in food: A 20-year perspective from 1997 to 2017. Trends Food Sci. Technol. 2019, 83, 86–98. [Google Scholar] [CrossRef]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Zimmermann, R.; Van Langenhove, H. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos. Environ. 2010, 44, 1831–1846. [Google Scholar] [CrossRef]
- Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E.; Jaffrezo, J. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys: Part 1: Concentrations, sources and gas/particle partitioning. Atmos. Environ. 2008, 42, 43–54. [Google Scholar] [CrossRef]
- Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E.; Jaffrezo, J. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys Part 2: Particle size distribution. Atmos. Environ. 2008, 42, 55–64. [Google Scholar] [CrossRef]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, I.B.; Öberg, L.; Haglund, P.; Tysklind, M.J. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. AMBIO A J. Hum. Environ. 2007, 36, 475–485. [Google Scholar] [CrossRef]
- Vione, D.; Barra, S.; De Gennaro, G.; De Rienzo, M.; Gilardoni, S.; Perrone, M.G.; Pozzoli, L. Polycyclic aromatic hydrocarbons in the atmosphere: Monitoring, sources, sinks and fate. II: Sinks and fate. Ann. Chim. 2004, 94, 257–268. [Google Scholar] [CrossRef]
- Pozzoli, L.; Gilardoni, S.; Perrone, M.G.; De Gennaro, G.; De Rienzo, M.; Vione, D. Polycyclic aromatic hydrocarbons in the atmosphere: Monitoring, sources, sinks and fate. I: Monitoring and sources. Ann. Chim. 2004, 94, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Wincent, E.; Jonsson, M.E.; Bottai, M.; Lundstedt, S.; Dreij, K.J. Aryl hydrocarbon receptor activation and developmental toxicity in zebrafish in response to soil extracts containing unsubstituted and oxygenated PAHs. Environ. Sci. Technol. 2015, 49, 3869–3877. Available online: https://pubs.acs.org/doi/10.1021/es505588s (accessed on 3 March 2023). [CrossRef] [PubMed]
- Dasgupta, S.; Cao, A.; Mauer, B.; Yan, B.; Uno, S.; McElroy, A. Genotoxicity of oxy-PAHs to Japanese medaka (Oryzias latipes) embryos assessed using the comet assay. Environ. Sci. Pollut. Res. 2014, 21, 13867–13876. [Google Scholar] [CrossRef]
- Velázquez Gómez, M. Organic Pollutants in Indoor Dust: Method Development, Site-Specific Monitoring and Human Exposure. Ph.D. Thesis, Universitat de Barcelona, Catalonia, Spain, 2019. Available online: http://hdl.handle.net/2445/145118 (accessed on 3 March 2023).
- Zheng, X.; Huo, X.; Zhang, Y.; Wang, Q.; Zhang, Y.; Xu, X.J. Cardiovascular endothelial inflammation by chronic coexposure to lead (Pb) and polycyclic aromatic hydrocarbons from preschool children in an e-waste recycling area. Environ. Pollut. 2019, 246, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Varshney, J.G.; Agarwal, T.J. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Environ. Pollut. 2016, 199, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Massous, A.; Ouchbani, T.; Lo Turco, V.; Litrenta, F.; Nava, V.; Albergamo, A.; Potortì, A.G.; Di Bella, G.J. Monitoring Moroccan Honeys: Physicochemical Properties and Contamination Pattern. Foods 2023, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Gutierrez-Gines, M.J.; Maxfield, A.; Gaw, S.; Dickinson, N.; Horswell, J.; Robinson, B. Chemical elements and the quality of mānuka (Leptospermum scoparium) honey. Foods 2021, 10, 1670. [Google Scholar] [CrossRef]
- ANMAT, Código Alimentario Argentino (CAA). Chapter X: Sugary Foods. Available online: https://www.argentina.gob.ar/anmat/codigoalimentario (accessed on 3 March 2023).
- Cabrera, M.; Andrada, A.; Gallez, L. Floración de especies con potencial apícola en el Bosque Nativo Formoseño, distrito Chaqueño Oriental (Argentina). Boletín De La Soc. Argent. De Botánica 2013, 48, 477–491. [Google Scholar] [CrossRef]
- National Institute of Agricultural Technology (INTA). The Beekeeping Market. Available online: https://inta.gob.ar/apicultura (accessed on 3 March 2023).
- Pardo, L.; Jiménez, L. Observación de rangos de vuelo de Bombus atratus (Hymenoptera: Apidae) en ambientes urbanos. Acta Biol. Colomb. 2006, 11, 131–136. [Google Scholar]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-based technique for fast and reliable extraction of organic contaminants from food, with a special focus on hydrocarbon contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, H.W.; Kim, G.H.; Kim, Y.Y.; Kang, M.J.; Shin, H.S. Risk Assessment and Evaluation of Analytical Method of Polycyclic Aromatic Hydrocarbons (PAHs) for Deep-Fat Fried Pork Products in Korea. Foods 2022, 11, 1618. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Wu, S. Rapid Determination of Oxygenated and Parent Polycyclic Aromatic Hydrocarbons in Milk Using Supercritical Fluid Chromatography-Mass Spectrometry. Foods 2022, 11, 3980. [Google Scholar] [CrossRef]
- Sun, C.; Qu, L.; Wu, L.; Wu, X.; Sun, R.; Li, Y. Advances in analysis of nitrated polycyclic aromatic hydrocarbons in various matrices. TrAC Trends Anal. Chem. 2020, 127, 115878. [Google Scholar] [CrossRef]
- Krzyszczak, A.; Czech, B. Occurrence and toxicity of polycyclic aromatic hydrocarbons derivatives in environmental matrices. Sci. Total Environ. 2021, 788, 147738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, H.; Kim, E.; El-Shourbagy, T. Salting-out assisted liquid/liquid extraction with acetonitrile: A new high throughput sample preparation technique for good laboratory practice bioanalysis using liquid chromatography–mass spectrometry. Biomed. Chromatogr. 2009, 23, 419–425. [Google Scholar] [CrossRef]
- de la Guardia, M.; Ruzicka, J. Towards environmentally conscientious analytical chemistry through miniaturization, containment and reagent replacement. Analyst 1995, 120, 7. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory Practice; Oxford University Press: Oxford, UK, 2000; pp. 1–158. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J.J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Guiñez, M.; Bazan, C.; Martinez, L.D.; Cerutti, S. Determination of nitrated and oxygenated polycyclic aromatic hydrocarbons in water samples by a liquid–liquid phase microextraction procedure based on the solidification of a floating organic drop followed by solvent assisted back-extraction and liquid chromatography–tandem mass spectrometry. Microchem. J. 2018, 139, 164–173. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Lavagnini, I.; Magno, F. A statistical overview on univariate calibration, inverse regression, and detection limits: Application to gas chromatography/mass spectrometry technique. Mass Spectrom. Rev. 2006, 26, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Ciemniak, A.; Witczak, A.; Mocek, K. Assessment of honey contamination with polycyclic aromatic hydrocarbons. J. Environ. Sci. Health Part B 2013, 48, 993–998. [Google Scholar] [CrossRef]
- Corredera, L.; Bayarri, S.; Pérez-Arquillué, C.; Lázaro, R.; Molino, F.; Herrera, A. Multiresidue determination of carcinogenic polycyclic aromatic hydrocarbons in honey by solid-phase extraction and high-performance liquid chromatography. J. Food Prot. 2011, 74, 1692–1699. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Zacharis, C.K.; Fytianos, K. A validated liquid chromatographic method for the determination of polycyclic aromatic hydrocarbons in honey after homogeneous liquid–liquid extraction using hydrophilic acetonitrile and sodium chloride as mass separating agent. J. Chromatogr. A 2015, 1377, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Di Serafino, G.; Giacomelli, A.; Medrzycki, P.; Sabatini, A.G.; Persano Oddo, L.; Marinelli, E.; Amorena, M. Monitoring of polycyclic aromatic hydrocarbons in bees (Apis mellifera) and honey in urban areas and wildlife reserves. J. Agric. Food Chem. 2009, 57, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramos, M.; García-Valcárcel, A.; Tadeo, J.; Fernández-Alba, A.; Hernando, M. Screening of environmental contaminants in honey bee wax comb using gas chromatography–high-resolution time-of-flight mass spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- Ringuet, J.; Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E. Diurnal/nocturnal concentrations and sources of particulate-bound PAHs, OPAHs and NPAHs at traffic and suburban sites in the region of Paris (France). Sci. Total Environ. 2012, 437, 297–305. [Google Scholar] [CrossRef] [PubMed]
| Compounds | r2 | Linear Range (ng g−1) | LOD (ng g−1) | LOQ (ng g−1) | EF (folds) | R (%) | Precision (RSD%) n = 3 |
|---|---|---|---|---|---|---|---|
| NPAH | |||||||
| 1-NPYR | 0.998 | 1.6–150.0 | 0.54 | 1.64 | 55 | 92.0 | 6.40 |
| 2-NFLU | 0.992 | 2.1–500.0 | 0.70 | 2.10 | 57 | 95.7 | 8.90 |
| 3-NFLUANTH | 0.989 | 0.8–150.0 | 0.26 | 0.79 | 59 | 99.4 | 1.90 |
| 9-NANTHR | 0.986 | 12.5–300.0 | 7.42 | 12.49 | 60 | 100.1 | 5.40 |
| OPAH | |||||||
| 5,12-NAPHTONA | 0.974 | 0.1–500.0 | 0.04 | 0.12 | 53 | 90.6 | 6.01 |
| 9,10-ANTHRONA | 0.993 | 20.4–300.0 | 9.77 | 20.36 | 57 | 95.9 | 6.80 |
| 2-FLUCHO | 0.998 | 0.3–750.0 | 0.09 | 0.27 | 55 | 92.6 | 7.90 |
| Reagents Penalty Points (PPR) | Energy (PPE) | Risk (Occupational Hazard) | Waste (PPW) | Total (100-PP) | Green Certificate * | Green Metric Score | |||
|---|---|---|---|---|---|---|---|---|---|
| Regents Type | Regent Amount (mL)—(g) | Hazards | Subtotal | ||||||
| Acetonitrile | 13.5 mL | 1.6 | 6.2 | 2 (>1.5 kWh/sample) | 0 | 5 | 13.2 | B | 86.8 |
| NaCl | 6 g | 1 | 0 | ||||||
| Compounds | Concentrations Determined in Honey Samples (ng g −1) a | ||||||
|---|---|---|---|---|---|---|---|
| ES-A (Handcrafted) | ES-B (Handcrafted) | SL-A (Commercial) | SL-B (Handcrafted) | SL-C (Organic) | SL-D (Handcrafted) | M-A (Commercial) | |
| 1-NPYR | N.D. b. | b | 5 ± 1 | 9 ± 1 | b | 21 ± 1 | 23 ± 2 |
| 2-NFLU | b | b | 82 ± 1 | 152 ± 0.1 | b | 405 ± 1 | 398 ± 10 |
| 3-NFLUANTH | 42 ± 2 | 25 ± 2 | 36 ± 6 | 39 ± 4 | 26 ± 2 | 31 ± 1 | 33 ± 5 |
| 9-NANTHR | b | b | b | b | b | b | b |
| 5,12-NAPHTONA | 300 ± 32 | 305 ± 7 | 194 ± 22 | 162 ± 10 | b | 183 ± 10 | 190 ± 10 |
| 9,10-ANTHRONA | b | b | b | b | b | b | b |
| 2-FLUCHO | 625 ± 51 | 287 ± 4 | 104 ± 9 | 587 ± 78 | 2.0 ± 0.1 | 487 ± 27 | 270 ± 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandelli, A.; Guiñez, M.; Cerutti, S. Evaluation of Environmentally Relevant Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Honey. Foods 2023, 12, 2205. https://doi.org/10.3390/foods12112205
Mandelli A, Guiñez M, Cerutti S. Evaluation of Environmentally Relevant Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Honey. Foods. 2023; 12(11):2205. https://doi.org/10.3390/foods12112205
Chicago/Turabian StyleMandelli, Alejandro, María Guiñez, and Soledad Cerutti. 2023. "Evaluation of Environmentally Relevant Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Honey" Foods 12, no. 11: 2205. https://doi.org/10.3390/foods12112205
APA StyleMandelli, A., Guiñez, M., & Cerutti, S. (2023). Evaluation of Environmentally Relevant Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Honey. Foods, 12(11), 2205. https://doi.org/10.3390/foods12112205








