Abstract
Cider is a fermented drink obtained from apple juice. As a function of the used apple cultivar, cider can be classified in four different categories (dry, semi-dry, semi-sweet, sweet), distinguished by the attribute of “dryness,” which reflects the sweetness and softness perceived. The dryness level is defined by scales (IRF, NYCA scales) based on the residual sugar, titratable acidity and tannin contents. Despite some adjustments, these scales show limitations in the prediction of actual perceived dryness, as they cannot consider the complicated interrelation between combined chemical compounds and sensory perception. After defining the perceived sensory dryness and its sensory description by using the quantitative descriptive analysis (QDA) method, a multivariate approach (PLS) was applied to define a predictive model for the dryness and to identify the chemical compounds with which it was correlated. Three models were developed, based on three different sets of chemical parameters, to provide a method that is easily applicable in the ordinary production process of cider. The comparison between the predicted rating and the relative scales scores showed that the models were able to predict the dryness rating in a more effective way. The multivariate approach was found to be the most suitable to study the relation between chemical and sensory data.
1. Introduction
Cider, or “hard” cider, is a fermented drink obtained from apple juice. Any apple cultivar can be used, but those that can contribute to the sensory characteristics of ciders are of particular interest [1,2]. Apple cultivars are classified according to their malic acid and tannin concentrations as “sweet” (low in acid, low in tannin), “sharp” (high in acid, low in tannin), “bittersweet” (low in acid, high in tannin), and “bitter-sharp” (high in acid, high in tannin). The high-tannin “bittersweet” and “bitter-sharp” apples are commonly referred to as cider apples and they contribute to the structure, preservation, finish and perceived complexity of the cider. In the United States, the availability of cider apples is limited, so many cider producers use a combination of low-tannin fruit and high-tannin cider-apple-juice concentrate or other tannin-rich adjuncts to meet the growing demand for cider in the marketplace [3].
According to the most recent survey, conducted in May 2023, there were 1.071 cider producers based in the United States. Most of these cider-producing companies were traditional cideries. The largest collection of U.S. cider-producing companies can be found in New York state, which accounted for 106 of the total (https://www.ciderguide.com accessed on 21 May 2023).
Cider quality and its sensory characteristics are related to several important groups of chemical compounds, including polyphenols, sugars and acids.
Concerning phenolic compounds, researchers have found that apples, when compared to 10 other commonly consumed fruits (avocado, banana, blueberry, white grape, grapefruit, lemon, melon, nectarine, orange and peach), had the highest content of soluble free phenols [4]. Each apple cultivar has its own polyphenolic profile, which is also dependent on harvest year, climatic variables, cultivation and storage conditions [5,6]. The polyphenolic composition of ciders mainly depends on the mixture of apple varieties and the cider-making procedures used for their elaboration. Apple polyphenols can be classified into five main groups: phenolic acids, flavanols, anthocyanins, flavonols, dihydrochalcones. Chlorogenic acid is the most abundant of the phenolic acids reported in apples [7]. The flavanols consist of the monomers (+)-catechin and (−)-epicatechin, and procyanidins (also referred to as condensed tannins), which are the oligomers of monomers [8,9,10]. Anthocyanins are another group of polyphenols responsible for skin pigmentation in red cultivars [11]. Flavanols (mainly quercetin derivatives) and dihydrochalcones (mostly phloretin derivatives) are relatively minor contributors to pigmentation and are present in relatively much lower concentrations than flavanols and phenolic acids [12,13,14].
The phenolic content and profile have important effects on the sensory properties of apple ciders, particularly their color, bitterness, and astringency [15]. Given the composition of cider (water, sugar—mainly fructose—organic acid and phenolic compounds), bitterness and astringency have been considered important attributes to define cider quality. These attributes were studied by Lea and Arnold [16] in bittersweet English cider, which demonstrated that no individual procyanidin can be uniquely identified with bitterness or astringency, while bitterness can be associated with oligomeric procyanidins, overall epicatechin tetramer and astringency to higher-molecular-weight procyanidins. Simoneaux et al. [17] studied the taste and tactile characteristics (bitterness, astringency, sweetness and sourness) of cider and highlighted that they were modified according to the concentration of procyanidins. Their study was performed on model solutions with procyanidins extracted from apples at different levels of polymerization and evaluated by a panel of trained judges. The degree of polymerization of the procyanidins influenced only the bitterness and astringency, but this impact was not the same for all concentrations. The same authors, in another study [18], showed that procyanidins exerted a significant negative effect on bitterness and a positive effect on astringency with interactions due to fructose. Moreover, the presence of ethanol significantly increased the perception of bitterness, while the presence of malic acid and fructose decreased it.
Pando Bedriñana et al. [19] studied the perceived characteristics of ice cider by using the citation-frequencies method and an assessment of quality by a group of experts using a five-point discontinuous scale. The results of their study demonstrated that the perceptions of sweetness, acidity and bitterness were closely interrelated and that these, in turn, were related to the contents of sugars, acids, polyphenols and alcoholic degree. According to the study by Riekstina et al. [20], polyphenols showed moderate negative correlation with the perception of fruity aroma. To improve the structure and complexity of cider, Martin et al. [21] enriched a base cider with the same amounts of endogenous and exogenous tannins of three different types (high-tannin cider apple, grape tannin and gallnut). A panel of 193 consumers were recruited to evaluate the quality of the ciders, but no significant differences in overall enjoyment were detected, demonstrating a wide range of astringency acceptability.
To allow consumers to choose according to their preferences, ciders are classified by a marketable scale derived from the traditional sparkling-wine-style sweetness scale (UE reg. n. 607/2009–encl. XIV), based solely on the quantity of residual sugar content and expressed as “dryness.” The limit of this dryness scale was its low correlation with consumer perception, as it does not consider the relation between sensory attributes such as astringency, bitterness and acidity and other chemical compounds, such as tannins and malic acid. Accordingly, two other scales are now available: the International Riesling Scale (IRF) and the New York Cider Association (NYCA) scale. These better relate the perceived dryness to the chemical composition, taking into consideration not only the residual sugar content, but also the titratable acidity, measured as malic acid, and the tannin concentration.
The study of the relationship between chemical composition and sensory perception is complicated by the fact that every sensory attribute is influenced by many variables. To explore this relationship, a multivariate method, such as partial least squares (PLS), can be appropriate. The use of PLS analysis is a “soft modeling” method to extract “factors” or latent variables, which are linear combinations of one set of variables (such instrumental data) able to predict much of the variation in another set of variables (such as sensory-data-attribute ratings) [22]. The PLS model indicates how well the variables in one data set predict the variation among variables in a second set by validation tests, which determine the percentage of variation in one data set that is accounted for by the other and vice versa [23]. This multivariate method is largely applied to foods and beverages due to the ability to examine samples in their entirety, to untangle all the complicated interactions between the constituents and to understand their combined effects on the whole matrix. Note that the emphasis is on predicting the characteristics and not necessarily on explaining the underlying relationships between the variables [24].
Many authors have applied this method to study the relationships between sensory descriptors and volatile compounds in wines [23,25,26,27]. Regarding cider, PLS was used as a regression method, for example, to study the relation between the composition of the raw material (apples) and cider quality, to ascertain a general prediction rule for the stage of ripening of a set of apples selected for their technological suitability for cider making [28]. PLS model was also applied in a sensory study to predict bitter or non-bitter tastes in cider using six polyphenols as predictor variables [7]. Lobo et al. [29] applied PLS in the development and validation of prediction models to transform frequency signals obtained by Fourier transform infrared (FT-IR) spectroscopy into concentrations of the different components in the cider, which is useful for the routine analysis of samples.
Given the results of the previous studies, this study aimed to characterize hard-apple ciders from a chemical point of view and to study the relationship between the production process and customers’ sensory perceptions. Moreover, a sensory-dryness-prediction model to monitor the production process was proposed. To meet consumer demand and maintain consistent their perceptions of quality, a new marketable scale based on the model was set up, which informed customers about the kind style of cider they intended to purchase. This scale expressed the perceived dryness by considering taste and tactile attributes (astringency, bitterness, acidity and sweetness), which, in turn, were correlated with a set of selected chemical parameters considered as markers. In order to define the most effective chemical variables to predict dryness by an appropriate scale, the present study was articulated in several steps: (i) chemical and sensory characterization of ciders representative of the New York region’s production; (ii) study of the relation between their chemical and sensory profiles; (iii) analysis of the predictive capability of the chemical parameters by using a PLS analysis and the selection of the most effective parameters; (iv) model verification defined by a set of cider samples (calibration set) and by the dryness prediction of another set of cider samples (validation set); (v) comparison of the predicted values with the dryness scores attributed to the same samples, by the current scales, and by the evaluation of a panel of trained judges.
2. Materials and Methods
2.1. Cider Samples
Seventy-six commercial cider samples (0.75-L glass bottle) were collected from twenty-two facilities from the New York State area, representative of the different products and of the relevant production orchards (Table 1).

Table 1.
Cider facilities, codes and number of samples collected from every producer.
2.2. Chemical Analyses
2.2.1. Chemical Standard Parameters
Residual sugar (glucose and fructose) and malic acid were measured using the enzymatic method on an automated chemical analyzer (Konelab 20XT, Thermo Fisher Scientific, Waltham, MA, USA) with test kits for discrete analyzers (Vintessential, Rowe Scientific, Adelaide, SA, Australia). The pH was analyzed using a standard pH probe (Ross Sure-Flow pH electrode, Thermo Orion, Thermo Fisher Scientific, Cleveland, OH, USA), and the titratable acidity was determined through potentiometric titration using 0.1 N NaOH and the Mettler Toledo T90 (1900 Polaris Parkway, Columbus, OH 43240, USA) instrument. Alcohol content was measured by gas chromatography using a Hewlett-Packard 5890 GC (Roseville, CA 95747, USA) combined with a flame-ionization detector (GC-FID) and according to official method of the Association of Official Analytical Chemists, or AOAC (International Method 983.13, Rockville, MD 20850, USA).
2.2.2. Spectrophotometer Indices
Absorbances at 280 nm and 320 nm were measured using a 1-cm-path-length quartz cell and a UV-VIS spectrophotometer (Genesys™ 10S UV-VIS, Thermo Scientific, Cleveland, OH, USA). Samples were centrifuged before the analysis.
2.2.3. Phenolic Analysis
The HPLC analysis was performed using an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA). Each sample was injected (5 μL) and chromatographically separated on a reverse-phase column LiChroCART 250-4 HPLC Cartridge LiChrospher 100 RP-18, endcapped (5 μm, 250 × 4 mm) (Agilent Technologies) and detected using a diode-array detector. Flow was 0.5 mL/min and the time run was 80 min. Solvent and gradients were set up according to Peng et al. [30]. Chromatograms were registered at 280 nm for the determination of tannins and hydroxybenzoic acids, at 316 nm for the determination of hydroxycinnamic acids and at 365 nm for the determination of flavanols. Calibration curves were obtained using the following standard compounds: gallic acid for hydroxybenzoic acids, caftaric acid for hydroxycinnamic acids, rutin for flavanols and (+)-catechin for tannins.
2.3. Sensory Analysis
The sensory tests were carried out in the late morning before lunch time, in a sensory-analysis laboratory equipped with individual cabins (temperature-controlled and combined natural/artificial light), designed in accordance with the ISO standard.
2.3.1. Dryness Evaluation
Seventy-six samples were collected and submitted to a sensory analysis to evaluate the sensory “dryness” perception and the sensory evaluation of sweetness, acidity, astringency and bitterness. The panel consisted of 11 trained judges, who evaluated the cider samples in two separate sessions. The panel was trained and calibrated using the scale applied by the American Cider Association, Beer Judge Certification Program-BJCP, Great Lakes International Cider and Perry Competition (GLINTCAP) and New York Ci-der Association (NYCA), consisting of four categories of sweetness, defined according to the residual sugar content in cider, as follows: dry (<9 g/L), semi-dry (9–18 g/L), semi-sweet (18–45 g/L) and sweet (>45 g/L). In the first session, a category scale of 8 levels was set up. To anchor the dryness level in the perceived intensity of sweetness, three reference standards of sweetness (glucose in standard cider) were submitted to the judges: a total of 9 g/L of glucose, corresponding to a score of 2; 18 g/L, corresponding to a score of 4; and 45 g/L, corresponding to a score of 6. Based on these reference standards, the dryness levels were defined as follows: 0–2, corresponding to dry; 2.1–4, to semi-dry; 4.1–6, to semi-sweet; and 6.1–8. to sweet. According to this scale, the trained judges evaluated the cider samples’ dryness.
2.3.2. Descriptive Analysis
In the second session, the evaluation of the samples was performed by using quantitative descriptive analysis (QDA). The panel leader trained the panelists in four 60-min training sessions. In the first session, the panelists were presented with a range of cider samples and invited to describe them through the evaluation of three taste attributes (sweetness, acidity, bitterness), and one mouthfeel descriptor (astringency). In the subsequent sessions, the judges were provided with a subset of samples and reference standards until they reached a consensus as to the attributes and the score-sheet sequencing. The references were prepared using a standard cider with addition of the compounds reported in Table 2 and corresponded to 6 (medium intensity) on an intensity-category scale, ranging from 1, on the left (not detected), to 10, on the right (very intense). The level of training of the panelists was checked by an individual evaluation of a subset of the samples and the statistical analysis of the data. The evaluation of the cider samples by the QDA was performed by serving them at a temperature of 12 °C in plastic test tubes. Each sample contained a constant volume of 30 mL of cider. Judges evaluated three taste attributes (sweetness, acidity, bitterness), and one mouthfeel descriptor (astringency). Samples were blind-tasted and coded with randomized three-digit numbers. Before each evaluation, panelists tasted the taste and mouthfeel standards, which were prepared following the same procedure as the panel training.

Table 2.
Taste and mouthfeel standard recipes: compounds added to the standard cider and their relative concentrations, corresponding to 6 on the intensity scale.
Panelists evaluated all samples in a total of 11 sessions. Each session featured 6 or 7 samples in duplicate, served across judges in a balanced incomplete block design. Given that some cider samples showed very different contents of residual sugar and alcohol and to avoid the influence of outliers, the sessions were organized in such a way that homogeneous group of samples for these compounds were presented. Judges were required to spit all the cider samples, wait 30 s between samples and cleanse their palates with water.
2.4. Statistics
Principal component analysis (PCA) and partial least-squares regression (PLS1) were performed using Unscrambler (ver. 10.3, CAMO Process AS, Bedford, MA, USA). Principal component analysis was performed before PLS models to examine any relevant and interpretable structures in the data and to detect outliers [31]. Principal component analysis was performed on the trained judges’ average scores attributed by QDA to the taste and tactile descriptors (sweetness, acidity, bitterness, astringency) after determination of their significance by ANOVA (Statgraphic Centurion Ver.XV, StatPoint Technologies, Warrenton, VA, USA). For the PLS1 analysis, the x-variables were represented by the selected chemical variables affecting the taste and tactile perception and, therefore, the dryness of the cider (residual sugars, alcohol, titratable acidity, malic acid, pH, tannins, polyphenol content, hydroxybenzoic and hydroxycinnamic acids, 280- and 320-nm absorbances); the y-variable was represented by the dryness scores. Cross-validation with randomization were used for prediction and the Nipals algorithm was applied.
3. Results and Discussion
3.1. Chemical and Sensory Characterization
The cider samples were submitted to chemical analysis for standard parameters such as alcohol content, pH, titratable acidity, malic acid content and residual sugar. Moreover, parameters such as the polyphenol content, hydroxybenzoic and hydroxycinnamic acids and absorbance at 280 nm and 320 nm were determined to define the polyphenol profiles of the samples. The results of the descriptive statistics are reported in Table 3. Samples were divided into two groups according to their chemical composition to form two homogeneous cider samples groups, in order to build and validate a prediction model. Table 3 shows that the two sample sets selected for the calibration and validation models presented similar chemical compositions. However, some exceptions were presented and highlighted by the maximum and minimum values for all the chemical parameters considered. In particular, the residual sugar content was found to be one of the parameters with the highest variability, as several samples showed very high values (i.e., 7689 mg/100 mL for calibration samples, and 17,059 mg/100 mL for validation samples) and, for this reason, they strongly affected the average value. The same trend, albeit less evident, was observed for other chemical parameters, such as the alcohol content, the malic acid and the related titratable acidity, as well as the polyphenol content. These chemical data indicated that the selected cider samples were substantially homogeneous, with some exceptions, which were suitably managed for both the evaluation and the model building.

Table 3.
Concentration ranges and absorbances (max, min, median, lower quartile Q1 (25°), upper quartile Q3 (75°) and average) of calibration and validation cider samples.
This was confirmed by the PCA shown in Figure 1 (the KS2 and KS8 samples presented outliers and were removed from the data), which displays the distribution of the cider samples according to their chemical parameters (alcohol content, pH, titratable acidity, malic acid, hydroxybenzoic acids, hydroxycinnamic acids, polyphenol content, total tannins and residual sugar), their sensory attributes (sweetness, acidity, bitterness and astringency) and their sensory dryness.
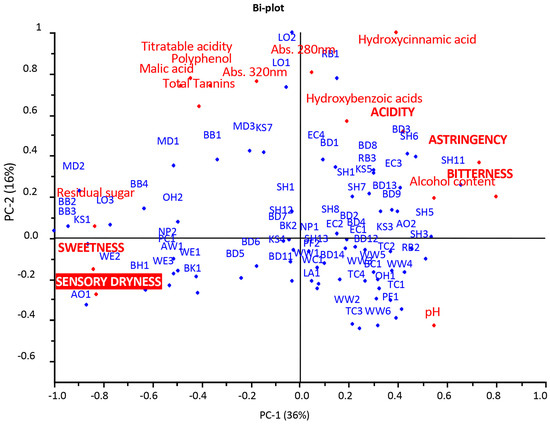
Figure 1.
Principal component analysis (PCA): scores-and-loadings bi-plot of chemical parameters, sensory attributes and dryness.
The PCA results explained 52% of the variance and separated the cider samples along the first dimension (PC1 explained 36% of the variance) according to the chemical parameters of pH, hydroxycinnamic and hydroxybenzoic acids and alcohol on the right side of the graphic and residual sugar, malic acid, titratable acidity, polyphenols and total tannins on the left side. Furthermore, the samples were separated along the second dimension (PC2 explained 16% of the variance) according to the titratable acidity, malic acid, polyphenol content, hydroxycinnamic and hydroxybenzoic acids, total tannins, polyphenols and the spectrophotometric measurements abs 320 nm and 280 nm, which are correlated with polyphenol compounds, and pH on the opposite side. The sensory attributes of bitterness, astringency and acidity were related to the hydroxycinnamic acids and, albeit to a lesser extent, to the hydroxybenzoic acids, pH and alcohol content. Instead, the sweetness and sensory dryness were related to the residual sugar and, to a lesser extent, to the malic acid, titratable acidity, tannins and polyphenols. The distribution of the samples showed that most of them were concentrated between the fourth and the first quadrants and were related to the chemical parameters of pH, alcohol and hydroxycinnamic and hydroxybenzoic acids. The other samples were more spread out, with a different level of relationship with the other chemical parameters: RB1, LO2, LO1, MD3, KS7, BB1 and MD1 were strongly correlated with abs 280 nm, abs 320 nm, polyphenols, total tannins, titratable acidity and malic acid, while BB3, BB2, MD2, KS1, AO1, LO3, BB4, WE2, BH1, WE3, PC1, NP2, OH2 and AW1 were heavily related to chemical parameters such as residual sugar, titratable acidity and malic acid and the sensory attributes of sweetness and sensory dryness.
The sensory dryness was found to be strictly correlated to the sweetness perception, which, in turn, was directly correlated with the residual sugar content and negatively correlated with the pH, alcohol content, hydroxycinnamic and hydroxybenzoic acids and abs 280 nm. The relationship between the sensory attributes and chemical parameters demonstrated that sweetness hindered acidity and astringency, with some exceptions [32] and only when the residual sugar content was low (lower than ~15 g/L) but, overall, when hydroxycinnamic and hydroxybenzoic acids were high (generally more than about 50 mg/L for hydroxycinnamic acid and about 20 mg/L for hydroxybenzoic acid), the acidity and astringency became perceptible, which was also due to the synergism between these two oral perceptions [33]. The hydroxybenzoic and hydroxycinnamic acids, combined with high alcohol content, showed a greater ability to interfere with the sweetness perception than acid compounds, such as malic acid, and the titratable acidity. This confirmed that sweet taste suppressed astringency perception and that it was the only taste to be slightly or not affected by astringency [34]. On the other hand, these findings confirmed those of other studies, in which the ethanol significantly increased the perception of bitterness, while the acidic compounds decreased it [35]. The similarity in the trends of bitterness and astringency attributes could have been due, beyond the low sweetness level, to the fact that these two sensations were often induced by the same compounds, such as polyphenols [36,37]. Moreover, the correlation of the pH with the astringency and bitterness sensations seemed to confirm the findings of other studies on wine, in which the increase in the pH led to an increase in the perception of these attributes [38].
3.2. Definition of the Dryness Rating by Marketable Scales and Sensory Evaluation
In Table 4, the scores and the relative rating of the dryness attributed to the cider samples are reported as resulting from the application of four different scales: the International Riesling Scale (IRF) with and without pH correction, the New York Cider Association (NYCA) scale and scores attributed by the Quantitative Descriptive Analysis (QDA). Although the IRF rating demonstrated that the ratio between the residual sugar and the malic acid had to be corrected as a function of the pH value, the IRF scores without pH correction were also considered as they represented the base value for the calculation of the NYCA-scale rating (see Table 5 and Table 6).

Table 4.
Scores and ratings of the cider samples: IRF scale with and without pH adjustment; NYCA scale; sensory dryness by the panel of trained judges.

Table 5.
IRF scale: technical guidelines for the calculation of the cider samples’ dryness.

Table 6.
IRF tannin adjustment for the calculation of the NYCA rating (RS: residual sugar; MA: malic acid).
The values attributed to the ciders by the four scales were quite similar for most of the samples, although some relevant exceptions were detected (Table 4).
The IRF scale without pH adjustment expressed the dryness as the ratio between the residual sugar and the titratable acidity (expressed as the malic acid content), assuming that the perceived intensities of sweet and sour tastes were different when both tastes were in the same mixture. Mixture suppression is a phenomenon in which the perceived intensity of two tastes in a mixture is lower than if they were unmixed, at the same concentration level [39]. Generally, sourness is suppressed by sweetness with a stable pattern, depending on the levels of both components. Previous studies showed that the overall perceived intensity was the result of “perceptual additivity,” i.e., the additivity of the taste intensity, that is, the perceived taste within the mixture, rather than the “stimulus intensity” [40,41,42,43]. Moreover, the influence of the intensity concentration on the taste interaction was modeled according to a psychophysical function, in which the ratio between different intensity perceptions is different at low, medium, and high levels of perception [44]. This function can also be applied to sweetness, the most heavily related taste to perceived dryness (see PCA Figure 1), and it is generally considered an especially effective suppressor of sourness, which is also affected by the alcohol, which generally enhances the perception of sweetness [32]. Therefore, the ratio between the concentrations of the two stimuli (residual sugar and malic acid), given that their intensity perception in a complex mixture such as cider was unknown, represented a poor predictor of the overall perceived intensity of the related taste. The IRF scale presented another critical point in relation to the calculation modality of the rating: when the malic acid concentration was low or very low (a fraction of mg per liter), the ratio and, hence, the scores, became very high (WW5, SH3, WW1, PF1, WW4, SH5 and TC3), although the residual sugar of the sample was not relevant in terms of its perception (Table 4). For example, the sample WW5 had a ratio of 5, corresponding to the IRF rating of “sweet,” resulting from the ratios of 5 mg/100 mL of residual sugar and 0.001 g/L of malic acid. The score for the same sample obtained by the sensory evaluation of dryness, given the concentration of the residual sugar (5 mg/100 mL), was probably more realistic and noticeably lower (2.9), corresponding to a “semi-dry” rating. A similar situation occurred with the sample SH3, with a ratio of 9, corresponding to the rating of “sweet,” resulting from the ratios of 90 mg/100 mL of residual sugar and 0.001 g/L of malic acid, presenting a score obtained by the sensory evaluation of dryness of 2, which is equivalent to a “dry” rating. The most evident case was that of the sample SH5, with a residual sugar level of 178 mg/100 mL and a similarly low content of malic acid (0.001 g/L), which presented a very high IRF value of 178, corresponding to a “sweet” rating, while the sensory dryness score attributed by the panel of trained judges was 2.7, corresponding to “semi-dry”.
The IRF scale with pH correction demonstrated that the ratio between the residual sugar and the malic acid had to be adjusted as a function of the pH of the sample (Table 5), assuming that the higher the pH, the higher the dryness perception and that other acid compounds than malic acid significantly contributed to the perceived acidity. The studies on this topic provided conflicting results. Stone et al. [45] found that in an aqueous solution, reducing the pH from 5.8 to 4.0 had little effect on the sweetness of glucose and fructose, but a reduction from 4.0 to 2.7 caused a 50% reduction in the relative sweetness.
The study by Stevens [46] found that weak concentrations of citric acid (e.g., below 10−3 M) had little effect on sucrose thresholds, while higher concentrations (2 × 10−3 M and above) slightly elevated sucrose thresholds; the pH values were not reported. Schimann et al. [47] evaluated the effect of pH on sweetness, at five different levels (pH 3.0, 4.0, 5.0, 6.0 and 7.0) in aqueous solutions of HCl or NaOH. No significant changes in perceived sweetness were found over a pH range from 3 to 7, but the acidity and astringency perceptions increased with decreasing pH values. The latter finding could in some way support the IRF scale with the pH correction, given that the perceived dryness was a sensation which could contribute to both the perception of sweetness and to the acidity. Compared to the others, the scores obtained by this scale were found to be the higher for almost all the samples, indicating that the pH adjustment had a strong effect and noticeably increased the rating, however it seems to have led to oversized values, since almost none the resulting scores were confirmed by those of the panel of trained judges.
The NYCA scale (Table 4) was used to calculate the perceived dryness according to the ratio of the residual sugar content to the malic acid (corresponding to IRF score), adjusted as a function of the tannin concentration. This scale assumed that tannins were the main compounds responsible for astringency and, hence, that they are able to decrease the sweetness perception. Polyphenols (tannins and hydroxybenzoic and hydroxycinnamic acids) play an important role in cider quality, since they are responsible for its color and the balance of bitterness and astringency, which defines the “overall mouthfeel” of ciders. Several studies confirmed that astringency depends mainly on tannin content [19,20,21,35] and that its perception can be enhanced or depressed as a function of different factors. However, there were conflicting results regarding the effects of sweetness on astringency. Lyman et al. [48] found that the addition of 0.5 M of sucrose to 1000 mg/L tannic acid solution reduced the perception of dryness, while Brannan et al. [34]’s results showed that in a model solution of alum and tannin at low concentrations, astringency was not affected by sweetness, while at high concentrations, sweet taste suppressed astringency perception. The authors concluded that sweetness was the only taste to be slightly or not affected by astringency.
Studies on pH state that in general, the higher the pH, the lower the perceived astringency [49,50,51]. Furthermore, studies on the effect of total acidity on the perceived astringency have hypothesized that it was not significant [51,52] and that the astringency elicited by acids was a function of pH and not of acid concentration [53,54].
The NYCA scale provided the values that must be subtracted from the ratio between the residual sugar and the malic acid, in relation to the concentration of tannins (Table 6). This scale, did not change the calculated IRF-dryness score when the tannin concentration was lower than 500 mg/L and decreased it for higher values as a function of their concentration, up to a maximum of ¾ of a unit, for values equal to or more than 1000 mg/L tannins. Although it seemed to have a good relationship with the perceived dryness evaluated by the panel of trained judges, when there was a very high tannin content, the scale appeared to lose its ability to detect their effect on the perception. This was the case with the sample MD1, which had an IRF value of 4.009, corresponding to a “sweet” rating but, given the high content of tannins (5000 mg/L), a final value on the NYCA scale was 3.32, which corresponded to a “semi-sweet” rating. In fact, the perceived dryness assigned by the scale determined by the panel of trained judges was clearly lower (3.6), corresponding to the “semi-dry” rating. This can be explained by the fact that the very high content of tannins had a strong effect on the perceived dryness, while the NYCA tannin correction affected the score values both at 1000 and 5000 mg/L of tannins (Table 6) in the same way.
The sensory evaluation of the cider samples by the QDA method confirmed that the dryness rating was directly correlated with the perceived sweetness (Table 4). Moreover, given the mixture’s effect [39] on the perceived sweetness represented by the results of the interactions among the different tastes and tactile stimuli contained in the mixture, QDA is the most effective method for providing the final perception of the overall intensity according to the tastes perceived after any and all peripheral and central interactions occur [40,42,43].
According to these results, it is possible to highlight that the marketable scales discussed here, despite the improvements introduced (see the NYCA scale), take into consideration the different chemical compounds that directly influence the perception of dryness without consider their interactions. To better correlate the scores with the actual perception of dryness, including the interaction effects of the different tastes and tactile sensations, a multivariate approach can be exploited.
3.3. Dryness Prediction by Chemical Parameters: Set-Up of the Model and Validation
To define a model for the prediction of the dryness, it was necessary to focus on the relation between the perceived dryness and the chemical parameters related to its perception. Given the studies on this topic, several chemical parameters were selected as a function of their direct or indirect influence on taste and tactile perception: residual sugars and alcohol for sweetness; titratable acidity, malic acids and pH for acidity; and tannins, polyphenol content, hydroxybenzoic and hydroxycinnamic acids and 280-nm and 320-nm absorbances for astringency and bitterness.
Partial least squares (PLS1) regression was applied to compare the dryness scores with the chemical compounds of a set of cider samples (calibration set), with the goal of setting up a model to predict the dryness of a new set of ciders (validation set). When the first model was set up, the Hotelling T2 Test showed that there was an outlier (KS8) and a new model was set up without that sample.
The resulting model is reported in Figure 2a, while Figure 2b reports the Bw regression coefficients. The values of explained variance (24%, 20% for Factor-1 and 71%, 10% for Factor-2) were at their maximum after Factor-3, so these factors were considered for the prediction. The predicted-versus-reference plot (Figure 2a) showed that it was a good-quality model (R2 = 0.909; RMSEC = 0.472; SEC = 0.478). The predictions made during the calibration were checked against the predicted-versus-measured plot to examine the ability of the single chemical variables to model the dryness, but their importance was better summarized as weighted regression coefficients (Bw) (Figure 2b). The Bw coefficients made it possible to explain their weight and the quality of the relationship with the dryness parameter. Moreover, in order to improve the model’s efficiency, it was more convenient to select the most predictive chemical variables, i.e., those whose regression coefficients had estimated uncertainty limits within the 95% confidence interval (Figure 2b, bars marketed with grid). The variables with uncertainty limits crossing the zero line were not significant even if they presented large regression coefficients because the estimated uncertainty was consistent with the relationship between such variables and Y (dryness), since only some of the samples spanned the range. The regression coefficients showed that the residual sugar, malic acid, abs 320 nm and hydroxybenzoic acid had a direct relationship with the predicted dryness, while the total tannins, polyphenols, hydroxycinnamic acid, abs 280 nm, pH and alcohol content were negatively correlated. The uncertainty-test limits and the Bw coefficients indicated that the residual sugar, hydroxycinnamic acid and abs 280 nm were the chemical variables that were able to significantly predict the dryness, whose predicted values are shown in Table 7. The defined model was tested by the prediction of the dryness scores of a second set of ciders, the validation set, after their transformation into the corresponding rating values. The predicted ratings were compared to the sensory dryness ratings obtained through the QDA analysis and the NYCA scale. The IRF scale was not considered.
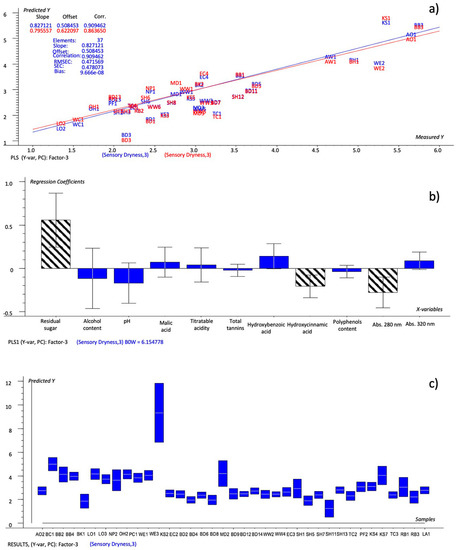
Figure 2.
PLS 1 plots: (a) Prediction model (sensory dryness predicted by all chemical variables); (b) weighted regression coefficients—Bw (important variables reported as black-striped bars) with estimated 95% confidence intervals; (c) predicted of dryness values for cider samples by the prediction model with uncertainty limits.

Table 7.
Predicted dryness rating by the PLS1 models (model #1: all chemical variables; model #2: important chemical variables; model #3: selected chemical variables) and comparison with NYCA and QDA ratings.
The values predicted by the model set up based on all the chemical variables (Table 7—model #1: residual sugar, titratable acidity, malic acid, tannins, polyphenols, hydroxycinnamic and hydroxybenzoic acids, abs 280, abs 320, alcohol content and pH), were found to be more similar to the evaluated sensory dryness than the NYCA scale. In fact, the NYCA scale predicted different values with respect to the evaluated dryness for twenty samples, three of which (SH5, SH11 and TC3) had two different ratings, while the predicted model’s values showed different ratings for 18 samples, none of which had more than one level. A large deviation from the predicted value was also detected for the sample KS2, but this can be explained by the very high content of residual sugar (177 g/L), which made it an outlier and, hence, difficult to predict with precision within of this category of cider.
The model set up by the important variables (Factor-1: 50%, 39%; Factor-2: 75%, 6%; R2 = 0.901; RMSEC = 0.490; SEC = 0.497), which were the variables whose regression coefficients had estimated uncertainty limits within the 95% confidence interval (Figure 3: residual sugar, pH and abs 280 nm), showed an even better performance, as 14 rating levels were found to be different from the sensory dryness scores, versus 20 on the NYCA scale and 18 from the model defined by all the chemical variables (Table 7; model #2). Although the samples SH11, RB1 and RB3 presented a larger range of variation, the dryness values predicted by the defined model suggest that it was possible to obtain a good level of prediction by using three defined chemical variables for the tested cider, i.e., the residual sugar, hydroxycinnamic acid and abs 280 nm.
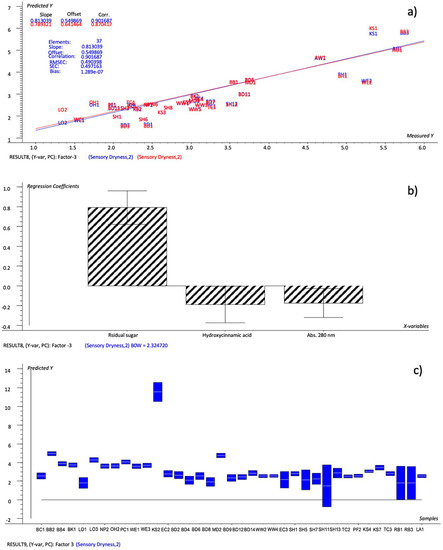
Figure 3.
PLS 1 plots: (a) Prediction model (sensory dryness predicted by important chemical variables); (b) weighted regression coefficients—Bw (important variables reported as black-striped bars) with estimated 95% confidence intervals; (c) dryness values predicted for cider samples by the prediction model with uncertainty limits.
Selected-Variables Model
Given the purposes of this study, which were to set up a model to predict dryness based on appropriate chemical parameters and to explore the possibility of refine the model itself by making it easily applicable in the cider-production process, the choice of chemical variables was considered in light of their readiness for use in an ordinary laboratory analysis of a production company.
Residual sugar is a common, easy and inexpensive parameter, determined by a specific enzymatic method and spectrophotometric analysis. The polyphenols presented some difficulties, as they were measured by a HPLC method, a very expensive instrument that can only be handled by specialized technicians; moreover, the polyphenols included a pool of chemical compounds (which also included hydroxybenzoic and hydroxycinnamic acids), which had a different kind and level of influence on the perception of sweetness. The measurement of abs 280 nm is in fact, an easy and widely used method determinate the polyphenols content, together with abs 320 nm, correlated to hydroxybenzoic and hydroxycinnamic acids [55]. For these reasons, this study was performed to test the predictive power of easily measurable parameters correlated with polyphenol contents, such as 280-nm and 320-nm absorbance, and the chemical compounds and parameters influencing sweetness perception beyond residual sugar, such as pH and titratable acidity, which are usually determined in the quality-control process and correlated with the perception of sweetness.
The new model is reported in Figure 4. The explained variance (35%, 73% for Factor-1 and 28%, 10% for Factor-2) was at its maximum after Factor-3, so these factors were considered for the prediction. The predicted-versus-reference plot (Figure 4a), with R2 equal to 0.857, RMSEC to 0.441 and SEC to 0.447, showed a good level of prediction. The uncertainty test and Bw coefficients made it possible to identify the chemical variables that significantly predicted the dryness, i.e., the residual sugar was positively correlated and the pH and titratable acidity were negatively correlated with the dryness. These results confirmed the relation, evidenced by the PCA (Figure 1), between acidity (together with astringency), and sweetness in the perception of the dryness.
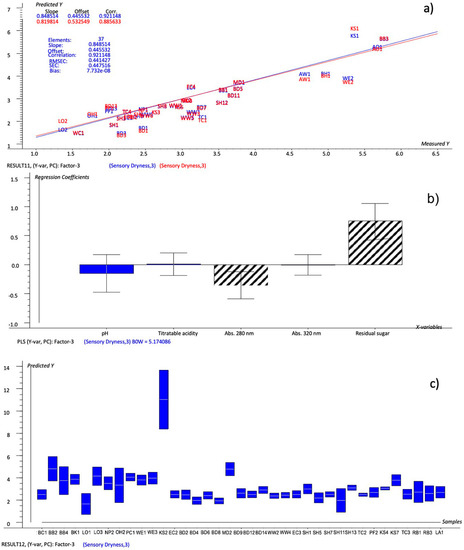
Figure 4.
PLS 1 plots: (a) Prediction model (sensory dryness predicted by selected chemical variables); (b) weighted regression coefficients—Bw (important variables reported as black stripes bars) with estimated 95% confidence intervals; (c) dryness values predicted for cider samples by the prediction model with uncertainty limits.
The model set up based on the selected chemical variables (model #3—residual sugar, titratable acidity, abs 280, abs 320 and pH) was tested in the same way as the others, by predicting the dryness values of the validation set and comparing the resulting ratings with those calculated by all the variables, the important variables model and the NYCA scale. The comparison made it possible to conclude that the model was more similar to the evaluated sensory dryness than the NYCA scale, and even more effective than the important-variables-based model itself. In fact, the ratings predicted by model #3 showed a different rating from the sensory evaluated dryness for just 11 samples, none of which for more than one level, compared with the 18 samples with different ratings on the NYCA scale and the 14 samples in the important-variables model. Using the latter model, the sample SH11 also demonstrated better predictability, while the large range of values of the sample KS2 was confirmed. The model equation used to calculate the dryness score is as follows (Equation (1)):
4. Conclusions
The traditional scales applied so far (IRF and NYCA scales) to define the dryness of cider cannot comprehend the complexity of the relationship between chemical and sensory profiles when using a univariate approach, in which single compounds are considered separately. On the other hand, sensory evaluation is complex, expensive and time-consuming and cannot be used as an ordinary tool to define marketable product parameters. Given that the relationships between sensory perception and chemical composition are complex, a predictive model for dryness using the multivariate method of PLS was applied, using several chemical variables of cider that are correlated with dryness. The PLS method is an important tool to study the relationship between chemical and sensory data, with important practical applications. The PLS methodology makes it possible to untangle the complicated interactions between chemical constituents to understand their combined effects on perceptions and to explore the relationships between chemical compounds and the sensory perception of dryness.
The determination of cider dryness by using chemical-composition data is challenging because the chemical interactions are complex. Studies that account for this complexity using statistical correlation could be of interest for this purpose.
However, further research is needed, as this topic has not yet been completely explored. It is important to note that to obtain an efficient and effective predictive model and, given the large variability of several chemical parameters, specific studies on different kinds of cider are needed. As with every predictive model, the extension and application of this predictive model over time will be necessary for its optimization.
Author Contributions
Conceptualization, V.C. and M.P.; methodology, V.C. and M.P.; formal analysis, M.W., J.S. and F.O.; data curation, M.W., P.D., V.C. and M.P.; writing—original draft preparation, V.C. and M.P.; writing—review and editing B.Z., V.C. and M.P.; supervision, V.C. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thompson-Witrick, K.A.; Goodrich, K.M.; Neilson, A.P.; Hurley, E.K.; Peck, G.M.; Stewart, A.C. Characterization of the poly- phenol composition of 20 cultivars of cider, processing, and dessert apples (Malus × domestica Borkh.) grown in Virginia. J. Agric. Food Chem. 2014, 62, 10181–10191. [Google Scholar] [CrossRef] [PubMed]
- Valois, S.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization of fermented cider apple cultivars grown in upstate New York. J. Am. Pomol. Soc. 2006, 60, 113–128. [Google Scholar]
- Ewing, B.L.; Peck, G.M.; Ma, S.; Neilson, A.P.; Stewart, A.C. Management of apple maturity and postharvest storage conditions to increase polyphenols in cider. Hort Science 2019, 54, 143–148. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Łata, B.; Tomala, K. Apple peel as a contributor to whole fruit quantity of potentially healthful bioactive compounds. Cultivar and year implication. J. Agric. Food Chem. 2007, 55, 10795–10802. [Google Scholar] [CrossRef] [PubMed]
- Mangas, J.J.; Rodríguez, R.; Suárez, B.; Picinelli, A.; Dapena, E. Study of the phenolic profile of cider apple cultivars at maturity by multivariate techniques. J. Agric. Food Chem. 1999, 47, 4046–4052. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.-F. Variability of the polyphenolic composition of cider apple (Malus domestica) fruits and juices. J. Agric. Food Chem. 2003, 51, 6240–6247. [Google Scholar] [CrossRef]
- Verdu, C.F.; Childebrand, N.; Marnet, N.; Lebail, G.; Dupuis, F.; Laurens, F.; Guilet, D.; Guyot, S. Polyphenol variability in the fruits and juices of a cider apple progeny. J. Sci. Food Agric. 2014, 94, 1305–1314. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; GaweL, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine—Like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.J.; Aubert, S.Y. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Dupont, N.; Guillermin, P. Concentrations and characteristics of procyanidins and other phenolics in apples during fruit growth. Phytochemistry 2007, 68, 1128–1138. [Google Scholar] [CrossRef]
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.F. Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.G.; Arnold, G.M. The phenolics of ciders: Bitterness and astringency. J. Sc. Food Agric. 1978, 29, 478–483. [Google Scholar] [CrossRef]
- Symoneaux, R.; Baron, A.; Marnet, N.; Bauduin, R.; Chollet, S. Impact of apple procyanidins on sensory perception in model cider (part 1): Polymerisation degree and concentration. LWT-Food Sci. Technol. 2014, 57, 22–27. [Google Scholar] [CrossRef]
- Symoneaux, R.; Chollet, S.; Bauduin, R.; Le Quéré, J.M.; Baron, A. Impact of apple procyanidins on sensory perception in model cider (part 2): Degree of polymerization and interactions with the matrix components. LWT-Food Sci. Technol. 2014, 57, 28–34. [Google Scholar] [CrossRef]
- Bedriñana, R.P.; Lobo, A.P.; Madrera, R.R.; Valles, B.S. Characteristics of ice juices and ciders made by cryo-extraction with different cider apple varieties and yeast strains. Food Chem. 2020, 310, 125831. [Google Scholar] [CrossRef]
- Riekstina-Dolge, R.; Kruma, Z.; Dimins, F.; Straumite, E.; Karklina, D. Phenolic composition and sensory properties of ciders produced from Latvian apples. Proc. Latv. Univ. Agr. 2014, 31, 39–45. [Google Scholar] [CrossRef]
- Martin, M.; Padilla-Zakour, O.I.; Gerling, C. Tannin additions to improve the quality of hard cider made from dessert apples. N. Y. State Hort. Soc. 2017, 25, 25–28. [Google Scholar]
- Noble, A.C.; Ebeler, S.E. Use of multivariate statistics in understanding wine flavor. Food Rev. Int. 2002, 18, 1–20. [Google Scholar] [CrossRef]
- Canuti, V.; Picchi, M.; Zanoni, B.; Fia, G.; Bertuccioli, M. A multivariate methodological approach to relate wine to characteristics of grape composition: The case of typicality. Am. J. Enol. Vitic. 2017, 68, 49–59. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cynkar, W.U.; Shah, N.; Dambergs, R.G.; Smith, P.A. A brief introduction to multivariate methods in grape and wine analysis. Int. J. Wine Res. 2009, 1, 123–130. [Google Scholar] [CrossRef]
- Álvarez, M.G.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC–MS. Food Chem. 2011, 129, 890–898. [Google Scholar] [CrossRef]
- Niimi, J.; Tomic, O.; Næs, T.; Jeffery, D.W.; Bastian, S.E.; Boss, P.K. Application of sequential and orthogonalised-partial least squares (SO-PLS) regression to predict sensory properties of Cabernet Sauvignon wines from grape chemical composition. Food Chem. 2018, 256, 195–202. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Nieuwoudt, H.; Aleixandre, J.L.; Du Toit, W.J. Robust ultraviolet–visible (UV–Vis) partial least-squares (PLS) models for Tannin quantification in red wine. J. Agric. Food Chem. 2015, 63, 1088–1098. [Google Scholar] [CrossRef]
- Mangas, J.J.; Moreno, J.; Picinelli, A.; Blanco, D. Characterization of cider apple fruits according to their degree of ripening. A chemometric approach. J. Agric. Food Chem. 1998, 46, 4174–4178. [Google Scholar] [CrossRef]
- Lobo, A.P.; Valles, B.S.; Tascon, N.F.; Madrera, R.R.; García, O.F. Calibration models for routine analysis of cider by mid-infrared spectroscopy. LWT-Food Sci. Technol. 2006, 39, 1026–1032. [Google Scholar] [CrossRef]
- Peng, Z.; Iland, P.G.; Oberholster, A.; Sefton, M.A.; Waters, E.J. Analysis of pigmented polymers in red wine by reverse phase HPLC. Aust. J. Grape Wine Res. 2002, 8, 70–75. [Google Scholar] [CrossRef]
- Cozzolino, D.; Smyth, H.E.; Lattey, K.A.; Cynkar, W.; Janik, L.; Dambergs, R.G.; Gishen, M. Relationship between sensory analysis and near infrared spectroscopy in Australian Riesling and Chardonnay wines. Anal. Chim. Acta. 2005, 539, 341–348. [Google Scholar] [CrossRef]
- Zamora, M.C.; Goldner, M.C.; Galmarini, M.V. Sourness–sweetness interactions in different media: White wine, ethanol and water. J. Sens. Stud. 2006, 21, 601–611. [Google Scholar] [CrossRef]
- Corrigan Thomas, C.J.; Lawless, H.T. Astringent subquality in acids. Chem. Sens. 1995, 20, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Brannan, G.D.; Setser, C.S.; Kemp, K.E. Interaction of astringency and taste characteristics. J. Sens. St. 2001, 16, 179–197. [Google Scholar] [CrossRef]
- Symoneaux, R.; Le Quéré, J.M.; Baron, A.; Bauduin, R.; Chollet, S. Impact of CO2 and its interaction with the matrix components on sensory perception in model cider. LWT-Food Sci. Technol. 2015, 63, 886–891. [Google Scholar] [CrossRef]
- Lee, C.B.; Lawless, H.T. Time-course of astringent sensations. Chem. Senses 1991, 16, 225–238. [Google Scholar] [CrossRef]
- Pires, M.A.; Pastrana, L.M.; Fuciños, P.; Abreu, C.S.; Oliveira, S.M. Sensorial perception of astringency: Oral mechanisms and current analysis methods. Foods 2020, 9, 1124. [Google Scholar] [CrossRef]
- Lawless, H.T.; Horne, J.; Giasi, P. Astringency of organic acids is related to pH. Chem. Senses 1996, 21, 397–403. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Anatomy and Physiology and Functions of Taste. In Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010; pp. 30–33. [Google Scholar]
- Green, B.G.; Lim, J.; Osterhoff, F.; Blacher, K.; Nachtigal, D. Taste mixture interactions: Suppression, additivity, and the predominance of sweetness. Physiol. Behav. 2010, 101, 731–737. [Google Scholar] [CrossRef]
- Frijters, J.E.; Schifferstein, H.N. Perceptual interactions in mixtures containing bitter tasting substances. Physiol. Behav. 1994, 56, 1243–1249. [Google Scholar] [CrossRef]
- Frank, R.A.; Archambo, G. Intensity and hedonic judgments of taste mixtures: An information integration analysis. Chem. Senses 1986, 11, 427–438. [Google Scholar] [CrossRef]
- Schifferstein, H.N.; Frijters, J.E. Sensory integration in citric acid/sucrose mixtures. Chem. Senses 1990, 15, 87–109. [Google Scholar] [CrossRef]
- Keast, R.S.; Breslin, P.A. An overview of binary taste–taste interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef]
- Stone, H.; Oliver, S.; Kloehn, J. Temperature and pH effects on the relative sweetness of suprathreshold mixtures of dextrose fructose. Percept. Psychophys. 1969, 5, 257–260. [Google Scholar] [CrossRef]
- Stevens, J.C. Detection of tastes in mixture with other tastes: Issues of masking and aging. Chem. Senses 1996, 21, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Sattely-Miller, E.A.; Graham, B.G.; Bennett, J.L.; Booth, B.J.; Desai, N.; Bishay, I. Effect of temperature, pH, and ions on sweet taste. Physiol. Behav. 2000, 68, 469–481. [Google Scholar] [CrossRef]
- Lyman, B.J.; Green, B.G. Oral astringency: Effects of repeated exposure and interactions with sweeteners. Chem. Senses 1990, 15, 151–164. [Google Scholar] [CrossRef]
- Guinard, J.X.; Pangborn, R.M.; Lewis, M.J. The time-course of astringency in wine upon repeated ingestion. Am. J. Enol. Viticul. 1986, 37, 184–189. [Google Scholar] [CrossRef]
- Brossaud, F.; Cheynier, V.; Noble, A.C. Bitterness and astringency of grape and wine polyphenols. Aust. J. Grape Wine Res 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Fontoin, H.; Saucier, C.; Teissedre, P.L.; Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Pref. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Kallithra, S.; Bakker, J.; Clifford, M.N. Red wine and model wine astringency as affected by malic acid and lactic acid. J. Food Sci. 1997, 2, 416–442. [Google Scholar] [CrossRef]
- Sowalsky, R.A.; Noble, A.C. Comparison of the effects of concentration, pH and anion species on astringency and sourness of organic acids. Chem. Senses 1998, 23, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Salces, R.M.; Herrero, C.; Barranco, A.; López-Márquez, D.M.; Berrueta, L.A.; Gallo, B.; Vicente, F. Polyphenolic compositions of Basque natural ciders: A chemometric study. Food Chem. 2006, 97, 438–446. [Google Scholar] [CrossRef]
- Ibern-Gómez, M.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Waterhouse, A.L. Rapid HPLC analysis of phenolic compounds in red wines. Am. J. Enol. Vitic. 2002, 53, 218–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).