Abstract
Nowadays, the development of innovative food products with positive health effects is on the rise. Consequently, the aim of this study was a formulation of aggregates based on tart cherry juice and dairy protein matrix to investigate whether different amounts (2% and 6%) of protein matrix have an impact on the adsorption of polyphenols as well as on the adsorption of flavor compounds. Formulated aggregates were investigated through high-performance liquid chromatography, spectrophotometric methods, gas chromatography and Fourier transform infrared spectrometry. The obtained results revealed that with an increase in the amount of protein matrix used for the formulation of aggregates, a decrease in the adsorption of polyphenols occurred, and, consequently, the antioxidant activity of the formulated aggregates was lower. The amount of protein matrix additionally affected the adsorption of flavor compounds; thus the formulated aggregates differed in their flavor profiles in comparison with tart cherry juice. Adsorption of both phenolic and flavor compounds caused changes in the protein structure, as proven by recording IR spectra. Formulated dairy-protein-based aggregates could be used as additives which are enriched with tart cherry polyphenols and flavor compounds.
1. Introduction
Lately, there has been a great need for the development of innovative food products possessing many positive health benefits, such as antioxidative, antidiabetic and anti-inflammatory effects [1]. The modern, busy lifestyle has a large impact on eating habits which can lead to a negative impact on health. Quite often, food is related to the fulfillment of desire, and a balance between pleasure and health has to be found [2]. Due to these reasons, functional foods are gaining more attention from consumers; however, their attractiveness cannot be neglected. Cherries are a rich resource of polyphenols and other bioactive food components, including fibers, vitamin C, carotenoids and potassium. There are two main types of cherries, sweet cherries (Prunus avium L.) and tart cherries (Prunus cerasus L.). Most of the sweet cherries are consumed fresh, while 97% of tart cherries are processed into different products for direct consumption or baking or cooking [3]. Natural polyphenols present in fruits and vegetables have poor stability, can be easily degraded and are sensitive to light, heat and oxygen. The application of polyphenols in food products is also limited as a result of their unpleasant taste and formation of off-color [1]. From this perspective, there is a need for the development of alternatives that minimize the nutritional and sensory losses while achieving greater stability of polyphenols [4]. Considering the structural and functional varieties of proteins, different approaches of exploiting proteins for the delivery of bioactives have been recommended [1]. It has been reported that protein-based aggregates provide a protective effect on polyphenols. For instance, the antioxidant activity of proteins increased after modification with certain polyphenols [5]. However, interactions between proteins and polyphenols are various and complicated since they both show high sensitivity to the environment [6]. Larger polyphenols have been reported to form complexes with dairy proteins. This binding can influence the electron donation capacity of polyphenols through a reduction in the number of hydroxyl groups available in the mixture. Caseins are major phosphoproteins of mammalian milk and belong to the group of unstructured proteins with unfolded structure and low hydrophobicity under native conditions [7]. Cyanidin-3-rutinoside, cyanidin-3-glucosyl-rutinoside and cyanidin-3-glucoside are anthocyanins detected in tart cherries, while the most abundant of the phenolic acids is chlorogenic acid [8]. It has been reported that there is a strong relationship between anthocyanin intake and anti-inflammatory activity, protection against neurological diseases and reduction of heart disease, obesity and diabetes. In the food industry, anthocyanins have the potential to be used as natural dyes, as synthetic dyes can cause adverse effects [4]. As consumers’ awareness of the significance and advantages of a healthy diet is on the rise, the use of tart cherry in the development of functional foods is necessary [9]. In the review of Lila et al. [10], the importance of protein/polyphenol particles and their possible utilization in the food industry, as well as the bioaccessibility of bioactive polyphenolic compounds and edible proteins were explored. In the food industry, depending on their properties, proteins have a broad range of applications. They can be used as emulsifiers, stabilizers of foams and colloid systems and film-forming polymers [11,12]. In the past decade, their utilization as microencapsulating carriers of different active compounds has been the focus of many studies, and an especially distinct segment is the encapsulation of phenolics. In this way, stability of phenolics can be achieved and bioactive protein-based aggregates can be formulated [13,14,15,16,17,18]. Usually, during the formulation of protein-based aggregates, when fruit material is used as a resource of phenolic compounds, the adsorption of flavor compounds has been neglected. There have been studies proving that during the formulation of functional additives based on proteins or fibers next to phenolics, flavor compounds were also adsorbed on the applied carrier [19,20,21,22]. Another important factor affecting the overall acceptance of the food products on the market is the characteristic flavor. The overall flavor profile of fruits is affected by the equilibrium between volatile compounds and their concentrations. Tart cherries are known for their specific and pleasant flavor. Different organic compounds such as carbonyls, alcohols, acids, esters and terpenes make up the flavor of tart cherries [23]. During the formulation and preparation of food products including functional additives, changes in flavor composition are often a result of the impact of processing conditions which can cause different chemical changes in flavor compounds and other food constituents, and, additionally, processing conditions can lead to interactions between them. Such protein-based aggregates can be utilized for modulation of the phenolic profile, flavor profile, color and antioxidant potential of different food products to which they are added.
The aim of this research was to formulate aggregates from tart cherry juice and dairy protein matrix to investigate whether different amounts (2% and 6%) of protein matrix have an impact on the adsorption of polyphenols adsorption as well as on the adsorption of flavor compounds. High-performance liquid chromatography (HPLC) was applied for the identification and quantification of polyphenols present in the samples, while the flavor profile was evaluated using gas chromatography with mass spectrometry (GC-MS) analysis. Color parameters were measured, and Fourier transform infrared spectroscopy (FTIR-ATR) screening of aggregates was performed. Additionally, antioxidant activity was determined spectrophotometrically using different assays.
2. Materials and Methods
2.1. Chemicals
Dairy proteins were obtained from Ingredia Functional (Arras, France). Methanol used for analysis was HPLC grade and it was obtained from J.T. Baker (Deventer, The Netherlands). Orthophosphoric acid was also HPLC grade (>85%) and it was purchased from Fisher Scientific (Loughborough, UK). 4-dimethyl-amino-cinnamaldehyd, trolox, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, 2,2-diphenyl-1-picrylhydrazil and standards for HPLC analysis (rutin, quercetin, chlorogenic acid, p-coumaric acid, (−)-epicatechin) were products of Sigma-Aldrich (St. Louis, MO, USA). The standard of cyanidin-3-rutinoside was from Phytolab GmbH (Vestenbergsgreuth, Germany) and the standard of neochlorogenic acid was the product of Extrasynthese (Genay, France). Folin-Ciocalteu reagent and potassium persulfate were products of Kemika (Zagreb, Croatia). Neocuproine, cupric chloride and 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) were products of Acros Organic (Geel, Belgium). Sodium carbonate was bought from T.T.T. (Sveta Nedelja, Croatia) and hydrochloric acid (37%) from Carlo Erba Reagents (Sabadell, Spain).
2.2. Preparation of Tart Cherry/Dairy Protein Aggregates
Two different percentages (2% and 6%) of dairy protein matrix (86% of protein on dry matter with 80% of casein micelles) were used for the preparation of aggregates with tart cherry juice. Mixing of protein matrix and juice was performed on a magnetic stirrer (Stuart US152, Buch and Holm, Hervel, Denmark) for 15 min at ambient temperature. Using centrifugation for 15 min at 4000 rpm, the precipitate was separated from the supernatant. After that, the frozen precipitate was freeze-dried using a freeze dryer, Alpha 1–4 (Christ, Osterode am Harz, Germany). The freeze-drying conditions were previously specified [20].
2.3. Extraction of Polyphenols from Tart Cherry/Dairy Protein Aggregates
Approximately 0.4 g of sample was extracted with 15 mL of extraction solvent (HCl:methanol 1:99). The extraction was conducted for 24 h at ambient temperature. Obtained extracts were evaluated spectrophotometrically and used for HPLC analysis.
2.4. Determination of Total Polyphenol Content, Proanthocyanidin Content and Anthocyanin Content in Formulated Aggregates
Total polyphenol content was assessed through the utilization of a spectrophotometric method [24]. Extract (0.2 mL) was mixed with demineralized water (1.8 mL), Folin–Ciocalteu reagent (10 mL) and 7.5% sodium carbonate solution (8 mL). The reaction mixture was kept in the dark for 120 min and the absorbance was read at a wavelength of 765 nm using a UV/Vis spectrophotometer (Cary 60 UV-Vis, Agilent Technologies, Santa Clara, CA, USA). Gallic acid was used for the expression of results; its calibration curve was constructed, and the obtained results were expressed as g of gallic acid per kg of tart cherry/dairy protein aggregates (g GAE/g). Each sample was measured in triplicate.
The DMAC (4-dimethyl-amino-cinnamaldehyd) method by Prior et al. [25] was used for the determination of proanthocyanidin content. Prepared samples were measured in triplicate and the absorbance was determined at 640 nm. A procyanidin B2 was used for the expression of results; its calibration curve was constructed, and the obtained results were expressed as mg of procyanidin B2 per g of tart cherry/dairy protein aggregates (mg PB2E/g).
Anthocyanin concentration was evaluated with the pH differential method that was previously published by Giusti et al. [26]. Two different buffers were made—0.025 M KCl at pH 1 and 0.4 M sodium acetate at pH 4.5—and 2.8 mL of each buffer was mixed with 0.2 mL of extract. Prepared mixtures were put in a dark place for 15 min. The absorbance was measured at two different wavelengths (515 and 700 nm). Then, the final absorbance of the sample was determined by the following equation:
To determine the anthocyanin concentration, the following equation was used:
where MW is 449.2 g/mol (the molecular weight of cyanidin-3-glucoside), DF is the dilution factor, ε is 26,900 L/mol cm (the molar absorptivity) and l is the 1 cm cuvette length. Finally, the monomeric anthocyanins concentration was expressed as mg of cyanidin-3-glucoside per gram of tart cherry/dairy protein aggregates (mg cyanidin-3-glucoside/g).
2.5. Antioxidant Activity (DPPH, FRAP, CUPRAC, ABTS Assays) of Formulated Aggregates
Antioxidant activity of tart cherry/dairy protein aggregates was achieved spectrophotometrically using DPPH, FRAP, CUPRAC and ABTS assays. All measurements were performed using a UV/Vis spectrophotometer. For all assays, calibration curves were made using Trolox. The results were expressed as µmol of Trolox per 100 g of aggregates (µmol TE/100 g). All aggregates were analyzed in triplicate. The DPPH method was previously described in the study by Brand-Williams et al. [27]. Briefly, DPPH solution at a concentration of 0.5 mM was made, and 3 mL of that solution was added to the extract. After incubation that lasted for 15 min in a dark place, the absorbance was read at 517 nm. To determine ferric reducing ability, FRAP reagent was added to 0.2 mL of the extract, and, after it was incubated for 30 min, the absorbance was read at 593 nm. This method was described in detail elsewhere [28]. The protocol for the CUPRAC assay was previously published by Apak et al. [29]. Briefly, 1 mL of CuCl2 (10 mM), 1 mL of neocuproine (7.5 mM), 1 mL of ammonium acetate buffer (1 M, pH 7.0), extract and distilled water at a total volume of 1.1 mL were mixed and put in a dark place for 30 min, and then the absorbance was read at 450 nm. A slightly modified method from Arnao et al. [30] was used to carry out the ABTS assay. Extracts were mixed with ABTS reagent. After 95 min of incubation, the absorbance was read at 734 nm.
2.6. Evaluation of Polyphenols by High-Performance Liquid Chromatography (HPLC) in Formulated Aggregates
Concentrations of individual polyphenols in tart cherry/dairy protein aggregates were determined using an Agilent HPLC system 1260 Infinity II (Agilent Technology, Santa Clara, CA, USA). The system consisted of a quaternary pump, a vial sampler and a column (Poroshell 120 EC C-18; 4.6 × 100 mm, 2.7 µm). Mobile phase A was 0.1% orthophosphoric acid, and mobile phas B was 100% methanol. The gradient used was previously published in the study by Buljeta et al. [31]. Before injection into the system, extracts were filtered through PTFE filters (pore size 0.2 μm). The flow rate was set at 1 mL/min and the injected volume was 5 µL. Each extract was injected two times. UV/Vis spectra were recorded in the range from 190 to 600 nm. Peaks were identified by comparing the UV-Vis spectra of peaks in extracts and retention times. The calibration curves were constructed for all standards of polyphenols (cyanidin-3-rutinoside, neochlorogenic acid, chlorogenic acid, p-coumaric acid, rutin, quercetin and (−)-epicatechin) and calibration curve linearity was confirmed by r2 > 0.99. Cyanidin-3-glucosyl-rutinoside was identified according to the literature [32] and quantified using the cyanidin-3-rutinoside curve. The concentration of p-coumaric acid derivate was calculated using the calibration curve constructed for p-coumaric acid. Concentrations of identified polyphenols were expressed as mg of polyphenol per kg of tart cherry/protein aggregates (mg/kg).
2.7. Color Parameters of Formulated Aggregates
A CR-400 chromometer (Konica Minolta, Inc., Osaka, Japan) was used to record the following parameters of color: L* represents lightness (0 is black and 100 is white); a* represents redness (+) or greenness (−); b* indicates yellowness (+) or blueness (−); C* denotes saturation of color and °h represents the hue angle [33]. Formulated aggregates were measured three times. The color difference between the protein matrix and formulated aggregates (ΔE*) was calculated by the equation:
2.8. Evaluation of Flavour Compounds
Flavor compounds were evaluated in tart cherry juice, dairy protein matrix and formulated aggregates. Flavor compounds from samples were extracted using solid-phase microextraction (SPME). A quantity of 0.3 g of each aggregate, together with 4.7 g of water and 1 g of NaCl, were put into the vial. SPME fiber coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) sorbent (50/30 µm, StableFlex™, Supelco, Bellefonte, PA, USA) was applied for the extraction of flavor compounds. The method was described in detail in the study of Vukoja et al. [22]. Confirmation of the compounds was achieved by comparison of their mass spectra with the National Institute of Standards and Technology mass spectral database (NIST, East Amwell Township, NJ, USA) and through retention time and retention index. Two replicates were conducted for each aggregate. Myrtenol was used as an internal standard for quantification, and the results were expressed as µg/kg.
2.9. Fourier Transform Infrared with Attenuated Total Reflection (FTIR-ATR) Spectroscopy Analysis
IR spectra were collected by screening samples on an FTIR-ATR instrument (Cary 630, Agilent, Santa Clara, CA, USA), and evaluation was performed using the software MicroLab Expert (Agilent, Santa Clara, CA, USA). IR spectra of formulated aggregates and protein matrix were screened from 4000 cm−1 to 600 cm−1 with 32 scans at a resolution of 8 cm−1.
2.10. Statistical Analysis
Statistical analysis of all obtained data was carried out with the software STATISTICA 13.1 (StatSoft Inc., Tulsa, OK, USA), through the analysis of variance (ANOVA) and Fisher’s least significant difference test, where the significance was defined at p < 0.05. Additionally, a principal component analysis was carried out.
3. Results
3.1. Phenolic Compounds and Antioxidant Activity of Formulated Aggregates
The total phenolic, proanthocyanidin and monomeric anthocyanin contents, along with the antioxidant activities of the formulated aggregates, are presented in Table 1. Comparing parameters that were evaluated on formulated aggregates, it can be concluded that the amount of protein matrix used for complexation highly affected the adsorption of polyphenols and consequently affected antioxidant activity. Aggregates formulated with 2% of the protein matrix had higher values of all evaluated parameters than aggregates with 6% of the protein matrix. Even though the increase in protein matrix during complexation was threefold, the decrease of adsorption didn’t follow that trend. Total phenolic contents of aggregates were 874.93 mg/100 g and 369.78 mg/100 g for Cas2%_TC and Cas6%_TC, respectively. Aggregates with 2% of protein matrix had a concentration of proanthocyanidins 619.45 mg/100 g and aggregates with 6% of protein matrixhad a concentration of 273.18 mg/100 g. In both cases, the concentration was 2.3 times higher in Cas2%_TC than in Cas6%_TC. A lower decrease with an increase of protein amount was observed for monomeric anthocyanins, from 137.52 mg/100 g to 78.75 mg/100 g, indicating that Cas2%_TC had 1.7 times higher concentration than Cas6%_TC. Additionally, values of antioxidant activities that were determined by the DPPH, ABTS, CUPRAC and FRAP methods also did not follow a trend of threefold increase. For DPPH antioxidant activity, Cas2%_TC had values 1.2 times higher than those of Cas6%_TC, while for all other methods, the antioxidant activity of Cas2%_TC was around 2 times higher than that of Cas6%_TC. These results indicate that methods are based on different mechanisms of action but also that individual phenolic compounds contribute differently to antioxidant activity.

Table 1.
Total phenolic content (TPC), proanthocyanidin content (PAC), monomeric anthocyanins (MA) and antioxidant activity of formulated aggregates.
Specific phenolic compounds were also evaluated in formulated aggregates and are presented in Table 2 (additionally Figures S1–S4). Two anthocyanins were identified, cyanidin-3-glucosyl-rutinoside and cyanidin-3-rutinoside. As it was determined for monomeric anthocyanins, both individual anthocyanins were determined in higher concentration in aggregates with the lower amount (2%) of the protein matrix, 504.66 mg/kg vs. 783.09 mg/kg for aggregates with 6% of protein matrix. These aggregates had 1.1 times higher concentrations of these anthocyanins than aggregates with 6% of protein matrix (458.72 mg/kg and 675.5 mg/kg, respectively). Two flavonoids were identified, rutin and quercetin, and their adsorption was also affected by the amount of protein matrix. The same pattern as for anthocyanins was determined. Cas2%_TC had 1.2 times higher concentration of both flavonoids than Cas6%_TC. Rutin was evaluated in concentrations of 259.79 mg/kg and 209.02 mg/kg in Cas2%_TC and Cas6%_TC, respectively, while quercetin for the same samples was evaluated in lower concentrations, 89.57 mg/kg and 79.33 mg/kg, respectively. Chlorogenic acid, neochlorogenic acid and p-coumaric acid and its derivate were identified from phenolic acids. All of these acids were determined in higher concentrations in Cas2%_TC: 470.23 mg/kg, 352.90 mg/kg, 559.40 mg/kg and 927.04 mg/kg, respectively. In this aggregate, these concentrations were 2.3 higher for chlorogenic and neochlorogenic acids and 1.3 times higher for p-coumaric acid and its derivate than in Cas6%_TC (186.44 mg/kg, 159.88 mg/kg, 454.07 mg/kg and 626.51 mg/kg, respectively). Additionally, epicatechin was identified in aggregates in a concentration of 1156.63 mg/kg in Cas2%_TC, which was 4.4 times higher than in Cas6%_TC (249.26 mg/kg).

Table 2.
Individual phenolic compounds that were identified and quantified in formulated aggregates (mg/kg).
Formulated aggregates differ in their color parameters, which are presented in Table 3. L* value (lightness) of the protein matrix decreased with the adsorption of tart cherry juice components onto it. Cas6%_TC aggregate had a significantly higher L* value than was expected since that sample contained a higher amount of protein matrix. The a* value (redness) increased upon adsorption of juice components on the protein matrix, and this increase was higher for Cas2%_TC aggregate, which is in accordance with the concentration of anthocyanins that adsorbed onto the protein matrix. Adsorption of juice components caused a decrease in b* values (yellowness) of aggregates, and this decrease was more pronounced for Cas6%_TC. The °h value decreased with the adsorption of juice components; however, there was no difference between aggregates. The C* value (saturation of color) increased, and a higher increase was evaluated for Cas6%_TC.

Table 3.
Parameters of color of the dairy protein matrix and formulated aggregates.
3.2. Flavor Compounds and Flavor Profile of Formulated Aggregates
The identified flavor compounds of all samples and their properties are presented in Table 4. Flavor compounds of tart cherry juice, dairy protein powder and formulated aggregates were determined by GC-MS (Table 5) and compounds were grouped into aldehydes and ketones, alcohols, terpenes and esters. Tart cherry juice is rich in flavor compounds; however, during its complexation with dairy protein powder and the formulation of aggregates, partial adsorption of fruit flavor compounds onto the protein matrix occurred. Dairy protein powder also contained some flavor compounds, so the final flavor of the formulated aggregates was the combination of the flavors of both applied ingredients. However, some flavor compounds of the initial ingredients were completely lost during the complexation and formulation of the aggregates. Additionally, it was observed that the amount of protein matrix used for complexation affected the adsorption of flavor compounds. In tart cherry juice, 13 flavor compounds from the aldehyde and ketone groups were detected; in protein powder, nine were detected; and in formulated aggregates, only six were detected. Tart cherry juice contained hexanal, 2-heptanone, heptanal, benzaldehyde, 1-octen-3-one, 6-methyl-5-hepten-2-one, nonanal, decanal, 4-propylbenzaldehyde, dodecanal, geranylacetone, myristaldehyde and hexylcinnamal. The protein matrix contained some of the same flavor compounds as tart cherry juice (hexanal, benzaldehyde, nonanal, decanal, geranylacetone, myristaldehyde and hexylcinnamal) but also two compounds that were not identified in juice (octanal and lillial). In formulated aggregates, only six compounds from this chemical group were identified. Hexanal, benzaldehyde, nonanal, decanal and geranylacetone originated from both initial ingredients and octanal and lillial from the protein matrix. Hexanal was identified in both initial ingredients and in formulated aggregates, and it was determined that its concentration in formulated aggregates was higher than in the initial ingredients. Probably, alongside the adsorption of hexanal onto the protein matrix, the transformation of other compounds into it also occurred. A similar effect was observed for octanal that originated from the protein matrix. Additionally, this compound was found in a higher concentration when a higher amount (6%) of protein matrix was used for complexation. The remaining aldehydes and ketones in the formulated aggregates were determined in lower concentrations than in the initial ingredients. Benzaldehyde, which is one of the key flavor compounds of the overall flavor of tart cherries, was identified in all samples. Comparing its adsorption on the protein matrix, it is evident that higher adsorption was achieved when a lower amount (2%) of protein matrix was used for complexation. Of the alcohols, five were identified in tart cherry juice (2-ethyl-1-hexanol, benzyl alcohol, phenethyl alcohol, decanol and perillyl alcohol) and three in the protein matrix (octen-3-ol, 2-ethyl-1-hexanol and 1-octanol). In the formulated aggregates, five compounds were identified, namely 2-ethyl-1-hexanol, benzyl alcohol, 1-octanol, decanol and perillyl alcohol, meaning that phenethyl alcohol from juice was not adsorbed on the protein matrix and that octen-3-ol was lost from the protein matrix during complexation. Next to benzaldehyde, benzyl alcohol and phenethyl alcohol are also very important compounds that contribute to the overall flavor of the tart cherry. While benzyl alcohol was adsorbed on the protein matrix (with higher adsorption observed when 2% of the protein matrix was used for complexation), phenethyl alcohol was not adsorbed at all. Terpenes are generally very important fruit flavor contributors. Twelve flavor compounds from this group were identified in tart cherry juice (D-limonene, α-terpinolene, linalool, α-campholenal, nerol, geraniol, vitispirane, eugenol, α-ionol, β-damascenone, α-ionone and β-ionone), while in protein matrix, only 3 of them (geraniol, trans-chariophyllene and β-ionone) were identified. Seven terpenes were identified in formulated aggregates, namely D-limonene, linalool, geraniol, eugenol, α-ionol, α-ionone and β-ionone, which mostly originated from juice, with the exception of geraniol. Comparing the impact of protein matrix amount during complexation, it can be concluded that all terpenes adsorbed in higher amounts when a lower amount (2%) of the matrix was used for complexation, with the exception of D-limonene. Several esters were also identified in the samples: 2-ethylhexyl acetate, ethyl benzoate and ethyl decanoate in tart cherry juice and ethyl decanoate in the protein matrix. Interestingly, none of those esters were identified in the formulated aggregates. On the contrary, probably due to the transformation of other compounds, methyl acetate was determined in aggregates with a higher concentration when 2% of protein matrix was used for complexation.

Table 4.
Volatile compounds identified in tart cherry juice (TC), dairy protein powder (Cas) and formulated aggregates (Cas_TC).

Table 5.
Concentration of volatile compounds (µg/kg) in tart cherry juice (TC), dairy protein powder (Cas) and formulated aggregates (Cas2%_TC and Cas6%_TC).
Since each flavor compound is characterized by its odor descriptor and flavor compounds were detected in different concentrations in samples, this resulted in different flavor profiles of initial ingredients and formulated aggregates (Figure 1). According to odor descriptors of flavor compounds, fruity, citrus, floral, green, fatty, earthy, woody and spice flavor notes were identified in samples. In the overall flavor profile of tart cherry juice, the fruity flavor note prevailed (49%), followed by citrus and floral flavor notes (around 23%) with a slight scent of green, spicy and fatty flavor notes (<3%). The protein matrix was characterized by fruity, floral, citrus and green flavor notes, which contributed almost equally to its final flavor (27%, 23%, 21% and 20%, respectively), with a slight scent of woody and earthy flavor notes (8% and 1%, respectively). The formulated aggregates were a combination of the flavor notes of initial ingredients, and the amount of protein matrix affected the final overall profile of aggregates. Generally, the fruity flavor note prevailed in aggregates and it was more pronounced in aggregates prepared with 2% of protein matrix (61%) in contrast to 55% in aggregates prepared with 6%. The fruity flavor note was followed by the citrus one, but the opposite effect was observed with regard to the amount of protein matrix. The citrus flavor note was more pronounced in aggregates prepared with 6% of protein matrix (26%) in contrast to 17% in aggregates prepared with 2%. The floral flavor note followed the trend that was obtained for the fruity one (12% for aggregates with 2% of protein matrix and 8% for aggregates with 6%). Green, fatty and spicy flavor notes were also noted but there was no difference between aggregates with different amounts of protein matrix used for complexation (8%, 2% and 2%, respectively).
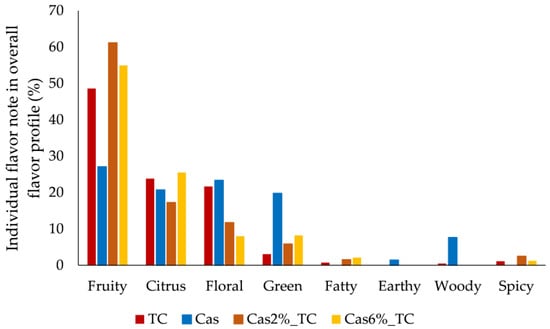
Figure 1.
Contribution of individual flavor notes in the overall flavor profile of tart cherry juice (TC), protein matrix (Cas) and formulated aggregates (Cas2%_TC and Cas6%_TC).
Additionally, flavor notes were analyzed through principal component analysis (Figure 2). To the total variance, PC1 accounted for 73.31% and PC2 for 16.31%. The protein matrix was on the positive side of PC1 while all the other samples were on the negative side. Tart cherry juice was on the negative side of PC2 and all the other samples were on the positive side, so it can be also concluded that the initial ingredients were quite different in their flavor profiles, while the formulated aggregates were a combination of flavor notes of both initial ingredients.
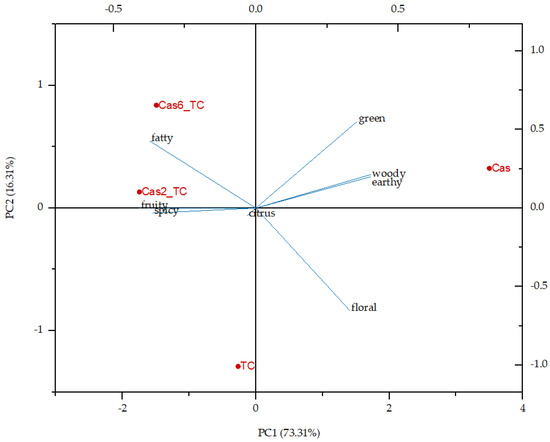
Figure 2.
Principal component analysis of individual flavor notes in the overall flavor profile of tart cherry juice (TC), protein matrix (Cas) and formulated aggregates (Cas2_TC and Cas6_TC—aggregates with 2% and 6% of protein matrix).
3.3. IR Spectra of Formulated Aggregates
Characteristic amide bands were defined in regions at wavenumbers of 1700–1600 cm−1, 1600–1500 cm−1, 1380–1200 cm−1 and 3300–3500 cm−1, and these were associated with amide I (C-O stretching), amide II (N-H bending and C-H stretching), amide III (N-H in plane bending coupled with C-N stretching) and amide A structures of proteins, respectively [34,35,36,37]. The IR spectra of protein matrix and formulated aggregate are presented in Figure 3. Protein powder and formulated aggregates had these characteristic amide regions; however, the intensity of bands on the formulated aggregates was lower. Changes in the amide I region, which reflect the secondary structure of the protein, were detected. The band at the protein matrix on 1640 cm−1 due to the adsorption of compounds from tart cherry juice shifted to 1632 cm−1, and, additionally, a slight shoulder formed at 1647 cm−1. Different secondary structures can be detected on proteins such as α-helix (1658–1650 cm−1), β-sheet (1640–1610 cm−1), β-turn (1700–1660 cm−1) and random coil structure (1650–1640 cm−1) [36]. From our IR spectra, it was apparent that the change in β-sheet occurred with a slight change in the random coil structure. Changes in amide II structure were not detected but there were changes in the amide III region. The band at 1237 cm−1 shifted to 1230 cm−1 and broadened with a slight shoulder at 1200 cm−1. These changes indicated changes in β-sheet [34]. Bands in the region 1060–890 cm−1 are mostly assigned to C–C stretching with 1080 cm−1, 1038 cm−1 and 933 cm−1, and related to α-helices and C–O stretching vibration [38]. We also observed a change in this region that could be associated with α-helices: the band at 1073 cm−1 shifted to 1051 cm−1 upon adsorption of tart cherry compounds. Additionally, upon adsorption of tart cherry compounds on the protein matrix, the band at 991 cm−1 completely disappeared and two bands, at 916 cm−1 and 879 cm−1, combined into one at 900 cm−1. Also, on the protein matrix, the band at 1730 cm−1, which was assigned to C = O, disappeared.
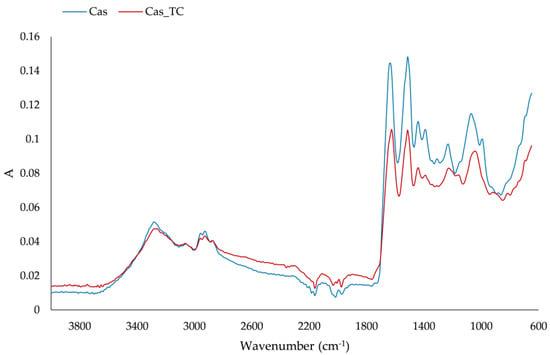
Figure 3.
IR spectra of protein matrix (Cas) and formulated aggregate (Cas_TC).
4. Discussion
Results of other studies that were conducted with the aim of creating protein/polyphenol enriched matrices proved that that type of protein matrix as well as the polyphenol source has a great impact on the adsorption of these valuable bioactives on the selected carrier. Additionally, the concentrations of the initial ingredients and the conditions during the formulation of such matrices was of great importance. Through the study on the formulation of delivery systems of raspberry phenolics based on brown rice protein matrix, it was concluded that, with a increase in the amount of protein matrix during the formulation of protein aggregates, a decrease in adsorption capacity occurred [16,17,18,20]. Our results were in accordance with those results. However, in this case, a higher decrease in adsorption capacity with an increase in protein matrix was observed for total phenolic compounds and anthocyanins. The importance of the protein source and the amount of proteins in this source have been proven in the study of the binding of cranberry polyphenols on defatted soy flour (50% of proteins), soy protein isolate (70% of proteins), hemp protein isolate (70% of proteins), medium-roast peanut flour (50% of proteins) and pea protein isolate (85% of proteins) [16]. The highest adsorption of total phenolic of cranberries was determined for hemp protein isolate, and the lowest for soy protein isolate, while for anthocyanins the highest adsorption was observed on defatted soy flour and the lowest, again for soy protein isolate [16]. In the study of the adsorption of blueberry polyphenols on defatted soybean flour in combination with white whole wheat, brown rice or corn flours, it was concluded that proteins and other insoluble components of used plant flours were included in the overall adsorption of blueberry polyphenols. More precisely, it was concluded that the protein content of the utilized flours was not linearly related with the binding efficiency for blueberry anthocyanins [18], which is in accordance with the results of this study. Therefore, the authors conclude that, in their case, carbohydrates (the major components of flours) were also involved in the overall adsorption of phenolics. Additionally, when they individually tested the mentioned carriers, they determined that defatted soy flour, with the highest content of proteins, had the highest adsorption capacity of blueberry anthocyanins, followed by wheat, rice and corn flours [18]. For our protein aggregates, it can be concluded that, through the complexation of protein matrix and tart cherry juice, when a higher amount of protein matrix was used, additional interactions (hydrogen bonding and/or hydrophobic interactions) between protein chains occurred. In this way, the number of binding sites on proteins for polyphenols decreased. On the other hand, tart cherry juice contained flavor compounds that also bonded on protein molecules, additionally diminishing the number of free binding sites on proteins. Generally, the reactivity of phenolics depends on the number of hydroxyl groups, but it also depends on their position, so this is also valid for interactions between phenolics and proteins and, additionally, the protein fraction—i.e., the free binding sites on them is very important [39,40,41]. Interactions of both a covalent and non-covalent nature are important between phenolics and proteins [42,43]. Considering non-covalent binding between phenolics and protein hydrophobic association, hydrogen bonds, electrostatic attraction and van der Waals forces can be involved. However, hydrophobic interaction and the formation of hydrogen bonds were considered the most relevant non-covalent interactions for the complexation of phenolics with proteins [44].
In most cases, possible additional adsorption of flavor compounds next to phenolics is neglected. However, it has been proven that during the complexation of brown rice protein with raspberry juice, both phenolics and flavor compounds from juice adsorbed onto the protein matrix [20]. The same trend was observed when cellulose was used for the encapsulation of raspberry [22] or apple fibers for blackberry juice phenolics [19,21]. Our results are in accordance with those studies. It is evident that the amount of dairy protein matrix used affected the adsorption of flavor compounds from tart cherry juice, as it has been proven for protein and fiber carriers applied for the formulation of functional additives in those studies. Also, the behavior of flavor compounds, i.e., their adsorption onto the carrier, depended on the properties of the flavor compounds and their affinity towards the carrier. Similar interactions between proteins and flavor compounds occur, as already explained for the interaction between proteins and phenolics. In this case, also, there is no universal mechanism that has been put forward as a favored one. Reversible hydrogen bonding, ionic bonding, van der Waal’s forces, and irreversible chemical binding via covalent linkages with amino and sulfhydryl groups can be possible interactions between proteins and flavor compounds [45]. To explain the complete binding mechanism between those compounds, a combination of different molecular forces/interactions as a function of the chemical structure of flavor compounds as well as matrix composition is necessary to explain, and should be taken into consideration [46,47]. Changes in the interactions of proteins with other compounds highly depend on the microenvironment of amino acid residues, hydrophobicity and the secondary structures of proteins [48]. These interactions cause differences in the concentrations of flavor compounds in samples and the ratios among them. Since each flavor compound is characterized by flavor notes, the previously mentioned factor has a high impact on overall flavor profile, which was evident from our research as well as previous ones.
As proof of the adsorption of phenolic and flavor compounds of tart cherry juice on dairy protein matrix, IR spectra of samples were recorded. Changes on the protein matrix due to different interactions between those initial ingredients were observed, as was the case in other studies dealing with the formulation of diverse protein-based microparticles. Those changes varied from minor to larger, depending on the type and properties of the adsorbed compounds and the protein matrix [20,49,50,51].
5. Conclusions
The aim of this research was to formulate functional protein aggregates based on dairy proteins and tart cherry juice. In most studies, additional adsorption of flavor compounds next to polyphenols from fruit sources is neglected; thus the investigation of both types of compounds on formulated aggregates was investigated. Our results showed that, next to polyphenols from tart cherry juice, flavor compounds were also adsorbed on the protein matrix, altering the protein matrix flavor profile, not only the polyphenol profile and color. Formulated dairy-protein-based aggregates can be applied for the enrichment of different products (fruit, dairy, and bakery products) with proteins, as well as polyphenols and flavor compounds. This type of additive could be beneficial in the formulation of novel innovative food products and the improvement of existing ones.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12112104/s1, Figure S1: HPLC chromatogram at 520 nm of sample Cas2%_TC (1—cyanidin-3-glucosyl-rutinoside; 2—cyanidin-3-rutinoside); Figure S2 HPLC chromatogram at 360 nm of sample Cas2%_TC (3—rutin; 4—quercetin); Figure S3 HPLC chromatogram at 320 nm of sample Cas2%_TC (5—chlorogenic acid; 6—p-coumaric acid derivate; 7—neochlorogenic acid; 8—p-coumaric acid); Figure S4 HPLC chromatogram at 210 nm of sample Cas2%_TC (9—(−)-epicatechin).
Author Contributions
Conceptualization, M.K. and J.Š.; methodology, A.P., M.K. and J.Š.; formal analysis, I.B., I.I., V.K. and I.Ć.; investigation, I.B., I.Ć., I.I. and V.K.; data curation, I.B., I.Ć. and I.I.; writing—original draft preparation, M.K. and I.Ć.; writing—review and editing, A.P. and J.Š.; supervision, M.K. and A.P.; project administration, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was part of the project PZS-2019-02-1595, which has been fully supported by the “Research Cooperability” Program of the Croatian Science Foundation, funded by the European Union from the European Social Fund under the Operational Program for Efficient Human Resources 2014–2020. I.Ć. acknowledges support from the Croatian Science Foundation program for Training New Doctoral Students (DOK-2020-01-4205).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oancea, A.; Aprodu, I.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. A bottom-up approach for encapsulation of sour cherries anthocyanins by using β-lactoglobulin as matrices. J. Food Eng. 2017, 210, 83–90. [Google Scholar] [CrossRef]
- Rivero, R.; Archaina, D.; Sosa, N.; Leiva, G.; Coronela, B.B.; Schebor, C. Development of healthy gummy jellies containing honey and propolis. J. Sci. Food Agric. 2020, 100, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.; Adkins, Y.; Laugero, K. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.G.; Cazarin, C.B.B.; Marostica Junior, M.R. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Tian, D.; Hu, C.Y.; Guo, Y.R.; Meng, Y.H. Improving antioxidant ability of functional emulsifiers by conjugating polyphenols to sodium caseinate. LWT 2022, 154, 112668. [Google Scholar] [CrossRef]
- Yin, X.; Cheng, H.; Wusigale, H.; Huang, W.; Liang, L. Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability. Antioxidants 2022, 11, 647. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.A. Interaction of milk α- and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, 67–72. [Google Scholar] [CrossRef]
- Siddiq, M.; Iezzoni, A.; Khan, A.; Breen, P.; Sebolt, A.M.; Dolan, K.D.; Ravi, R. Characterization of New Tart Cherry (Prunuscerasus L.): Selections Based on Fruit Quality, Total Anthocyanins, and Antioxidant Capacity. Int. J. Food Prop. 2011, 14, 471–480. [Google Scholar] [CrossRef]
- Lila, M.A.; Hoskin, R.T.; Grace, M.H.; Xiong, J.; Strauch, R.; Ferruzzi, M.; Iorizzo, M.; Kay, C. Boosting the bioaccessibility of dietary bioactives by delivery as protein−polyphenol aggregate particles. J. Agric. Food Chem. 2022, 70, 13017–13026. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825. [Google Scholar] [CrossRef] [PubMed]
- Bohin, M.C.; Vincken, J.-P.; van der Hijden, H.T.W.M.; Gruppen, H. Efficacy of food proteins as carriers for flavonoids. J. Agric. Food Chem. 2012, 60, 4136–4143. [Google Scholar] [CrossRef]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Lasterc, S.; Foegeding, E.A. Polyphenol-enriched berry extracts naturally modulate reactive proteins in model foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef]
- Han, J.; Chang, Y.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Lacroix, M. Interactions of phenolic compounds with milk proteins. Eur. Food Res. Technol. 2019, 245, 1881–1888. [Google Scholar] [CrossRef]
- Grace, M.H.; Guzman, I.; Roopchand, D.E.; Moskal, K.; Cheng, D.M.; Pogrebnyak, N.; Raskin, I.; Howell, A.; Lila, M.A. Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J. Agric. Food Chem. 2013, 61, 6856–6864. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Grace, M.H.; Esposito, D.; Lila, M.A. Wild blueberry polyphenol-protein food ingredients produced by three drying methods: Comparative physico-chemical properties, phytochemical content, and stability during storage. Food Chem. 2017, 235, 76–85. [Google Scholar] [CrossRef]
- Roopchand, D.; Grace, M.H.; Kuhen, P.; Cheng, D.; Plundrich, N.; Pouleva, A.; Lila, M.A. Efficient sorption of polyphenols to soybean four enables natural fortification of foods. Food Chem. 2012, 131, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Nosić, M.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Apple fibers as carriers of blackberry juice polyphenols: Development of natural functional food additives. Molecules 2022, 27, 3029. [Google Scholar] [CrossRef]
- Kelemen, V.; Pichler, A.; Ivić, I.; Buljeta, I.; Šimunović, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. Technol. 2022, 57, 1866–1874. [Google Scholar] [CrossRef]
- Kopjar, M.; Buljeta, I.; Nosić, M.; Ivić, I.; Šimunović, J.; Pichler, A. Encapsulation of blackberry phenolics and volatiles using apple fibers and disaccharides. Polymers 2022, 14, 2179. [Google Scholar] [CrossRef] [PubMed]
- Vukoja, J.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Cellulose as a delivery system of raspberry juice volatiles and their stability. Molecules 2020, 25, 2624. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, E.; Pichler, A.; Kopjar, M. Disaccharides: Influence on Volatiles and Phenolics of Sour Cherry Juice. Molecules 2017, 22, 1939. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotonutric acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry Current Protocols; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1994, 239, 70–76. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Sci. Food Agric. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polyphenols and Antioxidant Activity of Citrus Fiber/Blackberry Juice Complexes. Molecules 2021, 26, 4400. [Google Scholar] [CrossRef]
- Lončarić, A.; Pichler, A.; Trtinjak, I.; Piližota, V.; Kopjar, M. Phenolics and antioxidant activity of freeze-dried sour cherry puree with addition of disaccharides. LWT 2016, 73, 391–396. [Google Scholar] [CrossRef]
- Kopjar, M.; Jakšić, K.; Piližota, V. Influence of sugars and chlorogenic acid addition on anthocyanin content, antioxidant activity and color of blackberry juice during storage. J. Food Process. Preserv. 2012, 36, 545–552. [Google Scholar] [CrossRef]
- Singh, B.R.; DeOliveira, D.B.; Fu, F.-N.; Fuller, M.P. Fourier transform infrared analysis of amide III bands of proteins for the secondary structure estimation. Biomol. Spectrosc. III 1993, 1890, 47–55. [Google Scholar] [CrossRef]
- Jia, Z.; Zheng, M.; Tao, F.; Chen, W.; Huang, G.; Jiang, J. Effect of covalent modification by (−)-epigallocatechin-3-gallate on physicochemical and functional properties of whey protein isolate. LWT 2016, 66, 305–310. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Wang, W.; Dong, K. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Song, F.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Roles of Soft Segment Length in Structure and Property of Soy Protein Isolate/Waterborne Polyurethane Blend Films. Ind. Eng. Chem. Res. 2016, 55, 1229–1235. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein-phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Erdem, K.E. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes and the future trends in dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Interaction of whey proteins with phenolic derivatives under neutral and acidic pH conditions. J. Food Sci. 2017, 82, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Guichard, E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Tromelin, A.; Andriot, I.; Guichard, E. Protein–flavour interactions. In Flavour in Food; Voilley, A., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 172–207. [Google Scholar] [CrossRef]
- Wang, K.; Arntfield, S.D. Probing the molecular forces involved in binding of selected volatile flavour compounds to saltextracted pea proteins. Food Chem. 2016, 211, 235–242. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xiong, S.B.; Xie, B.J.; Qin, L.H. Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Sci. 2008, 80, 632–639. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Li, L.; Baokun, Q.; Mengnan, J.; Yue, X.; Yan, Z.; Xiaonan, S. Covalent conjugates of anthocyanins to soy protein: Unravelling their structure features and in vitro gastrointestinal digestion fate. Food Res. Int. 2019, 120, 603–609. [Google Scholar] [CrossRef]
- Ali, M.; Homann, T.; Khalil, M.; Kruse, H.P.; Rawel, H. Milk whey protein modification by coffee-specific phenolics: Effect on structural and functional properties. J. Agric. Food Chem. 2013, 61, 6911–6920. [Google Scholar] [CrossRef]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).