Physicochemical and In Vitro Digestion Properties of Curcumin-Loaded Solid Lipid Nanoparticles with Different Solid Lipids and Emulsifiers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
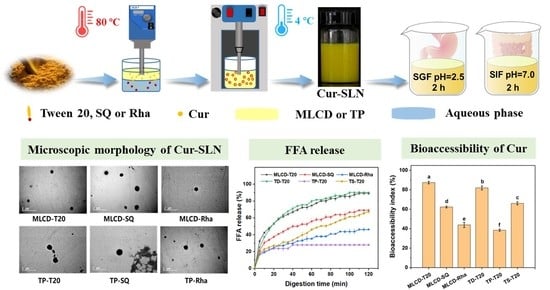
2.2. Cur-SLNs Preparation
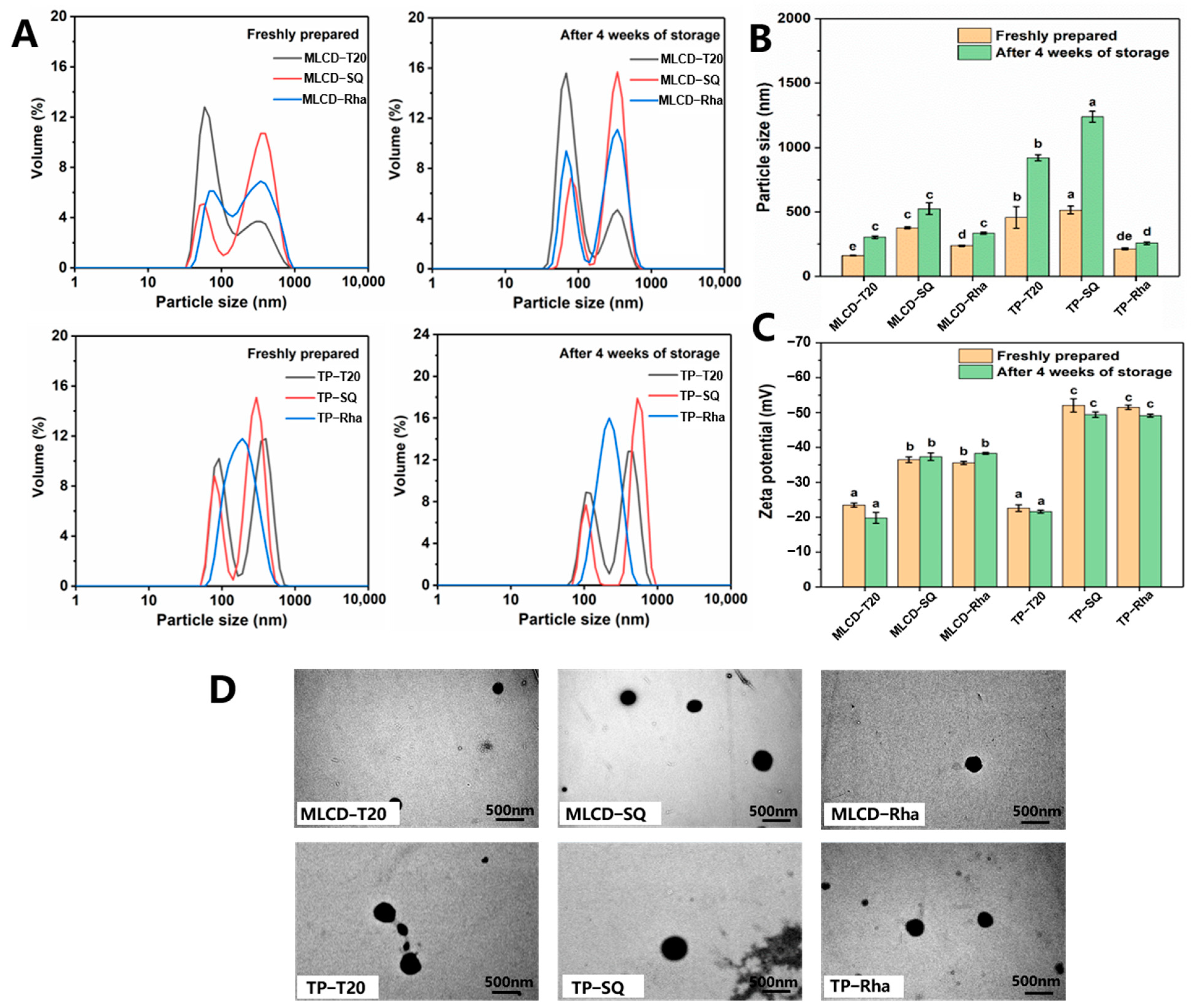
2.3. Particle Size and Zeta Potential of Cur-SLNs
2.4. Transmission Electron Microscopy (TEM) Observation
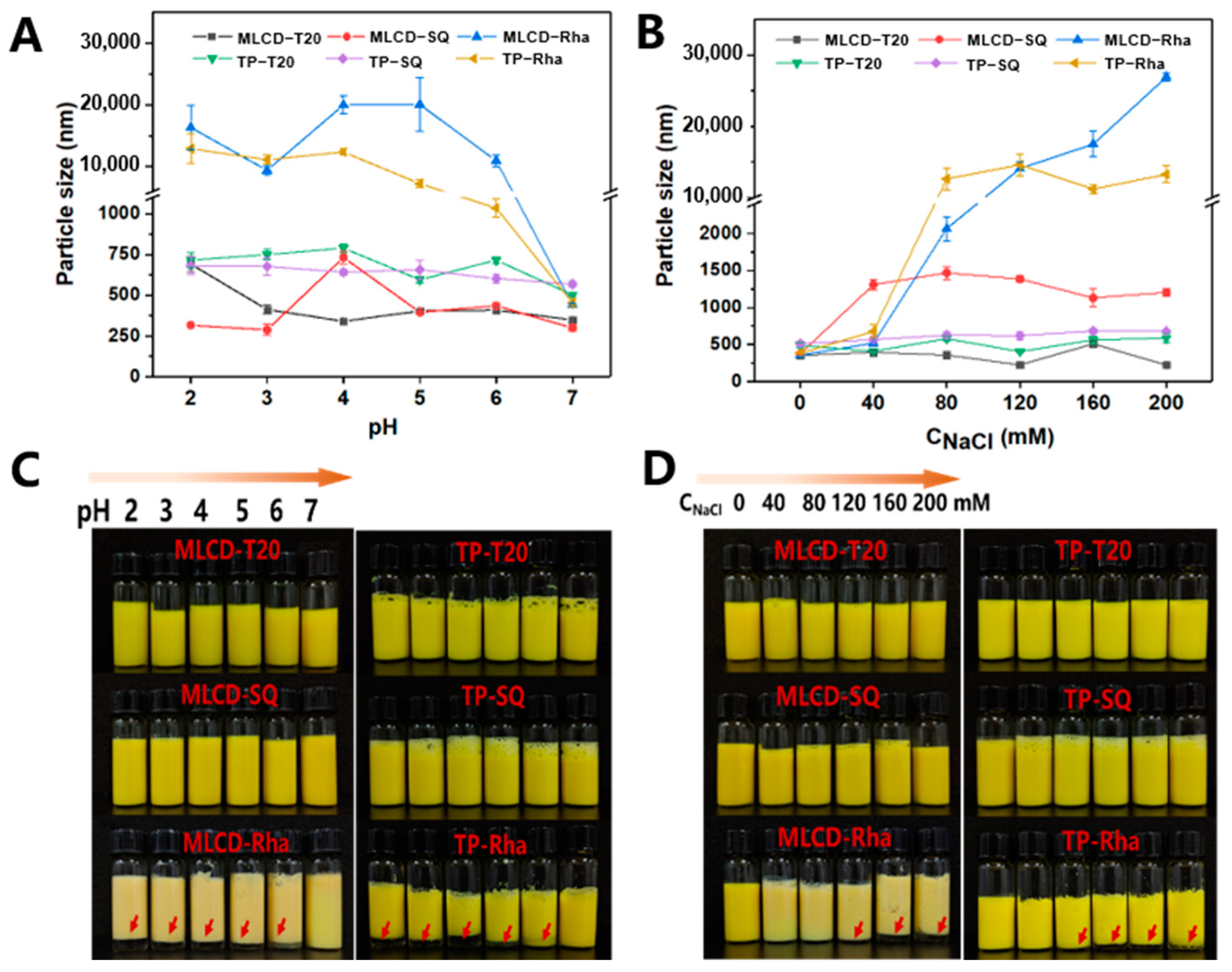
2.5. Stability of Cur-SLNs against pH and Ionic Strength
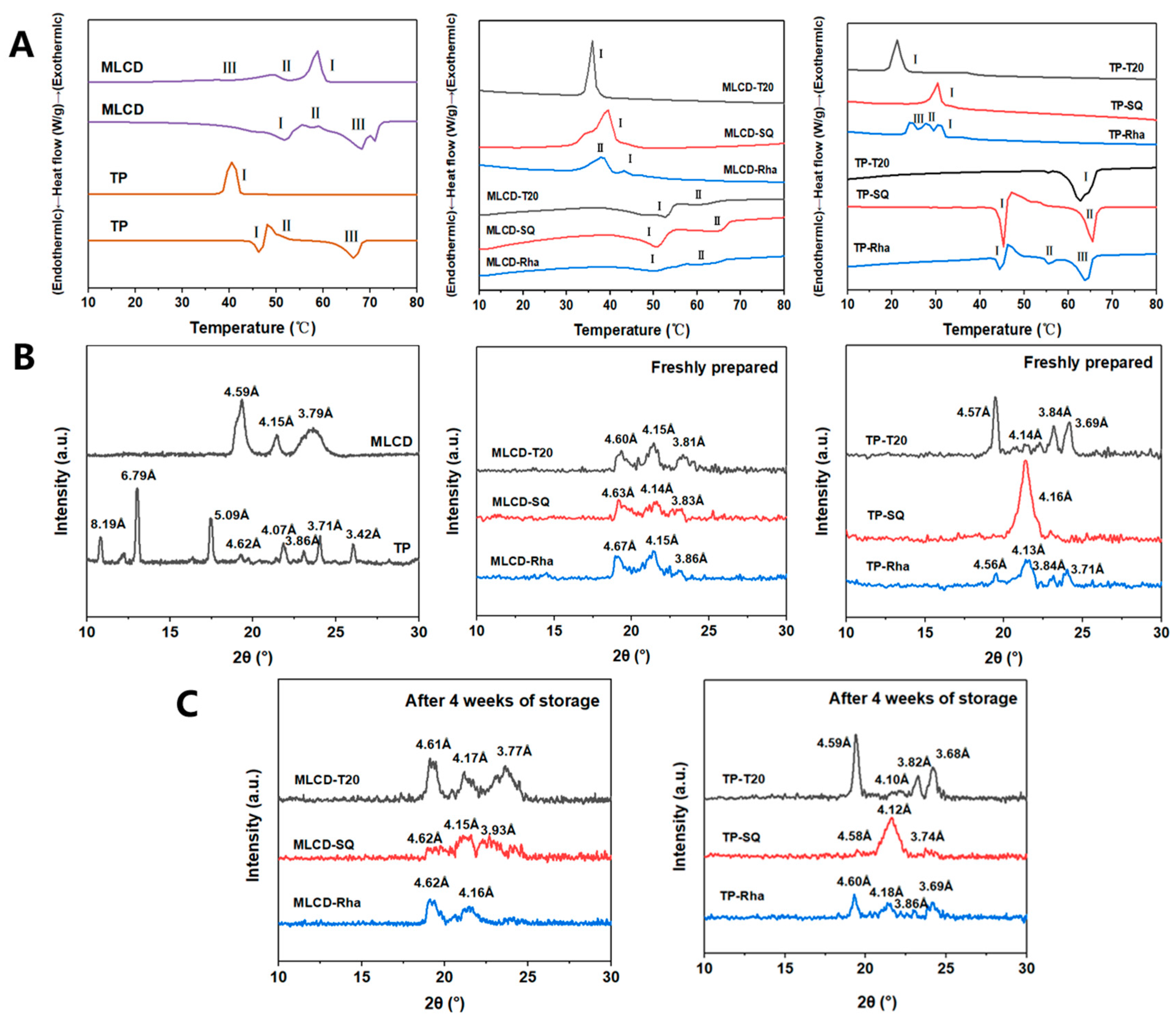
2.6. Thermal Analysis
2.7. X-ray Diffraction (XRD)
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
2.9. Entrapment Efficiency (EE) of Cur in SLNs
2.10. Chemical Stability of Cur in SLNs
2.11. Simulated Digestion
2.11.1. Gastrointestinal Digestion Model In Vitro
2.11.2. Measurements of the Particle Size and Zeta Potential of Digesta
2.11.3. Morphology of Cur-SLNs after Digestion
2.11.4. Cur Bioavailability
2.12. In Vitro Release Kinetics of Cur
2.13. Statistical Analysis
3. Results and Discussions
3.1. Characterization of SLNs
3.2. pH and Ionic Stability of Cur-SLNs
3.3. Thermodynamic Properties and Crystal Polymorphism of SLNs
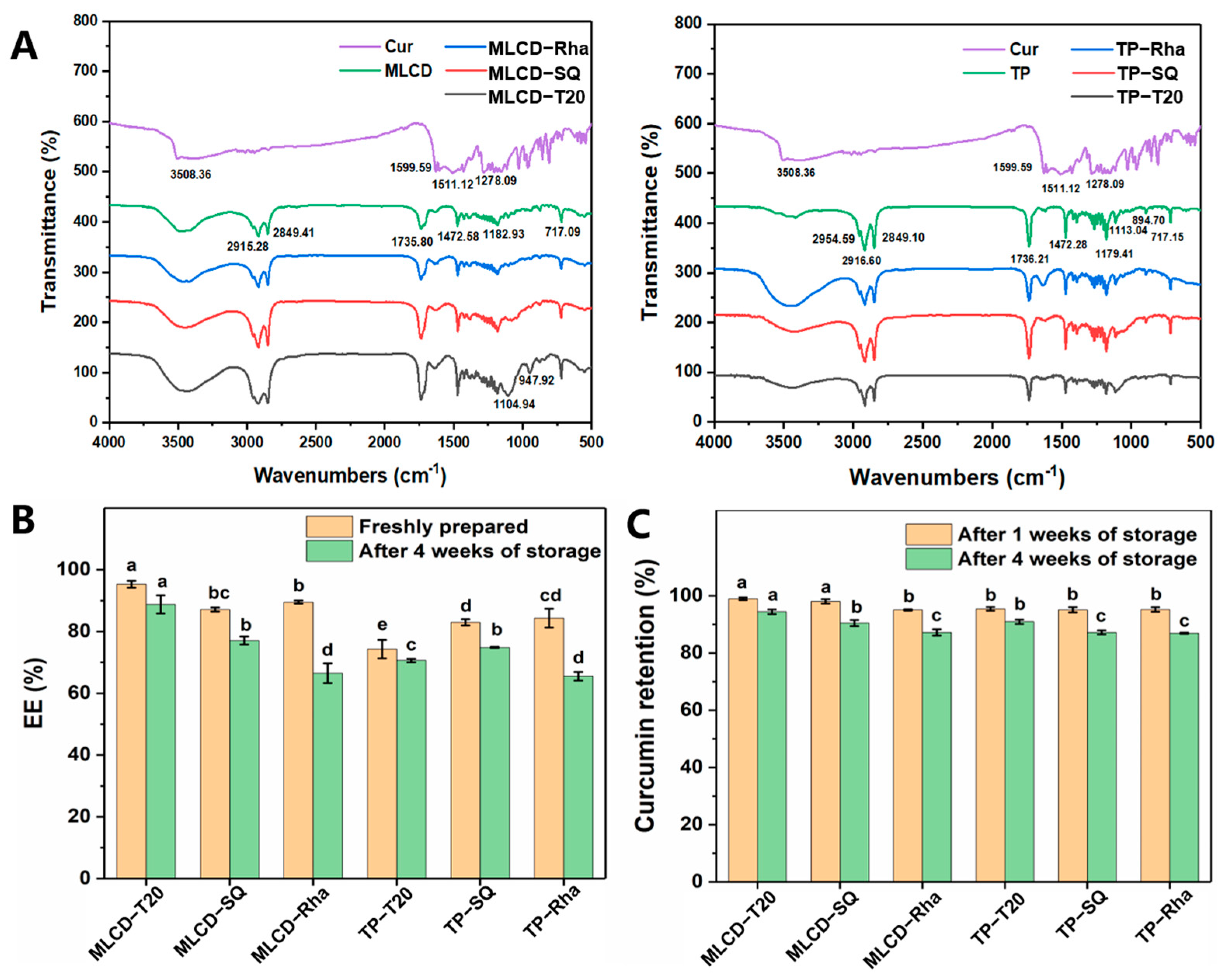
3.4. FT-IR Spectra, Encapsulation and Stability of Cur-SLNs
3.5. Encapsulation Efficiency and Chemical Stability of Cur-SLNs
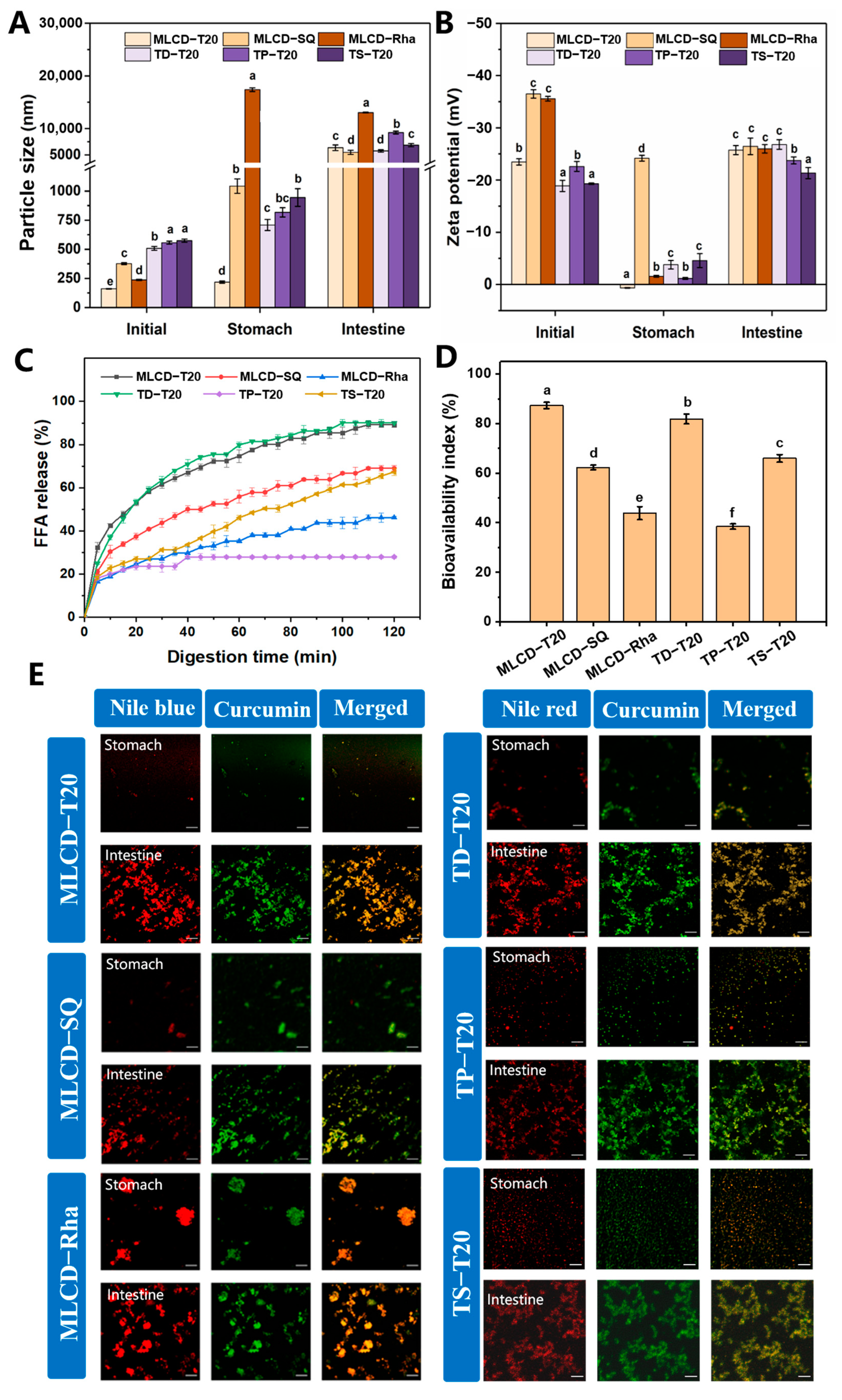
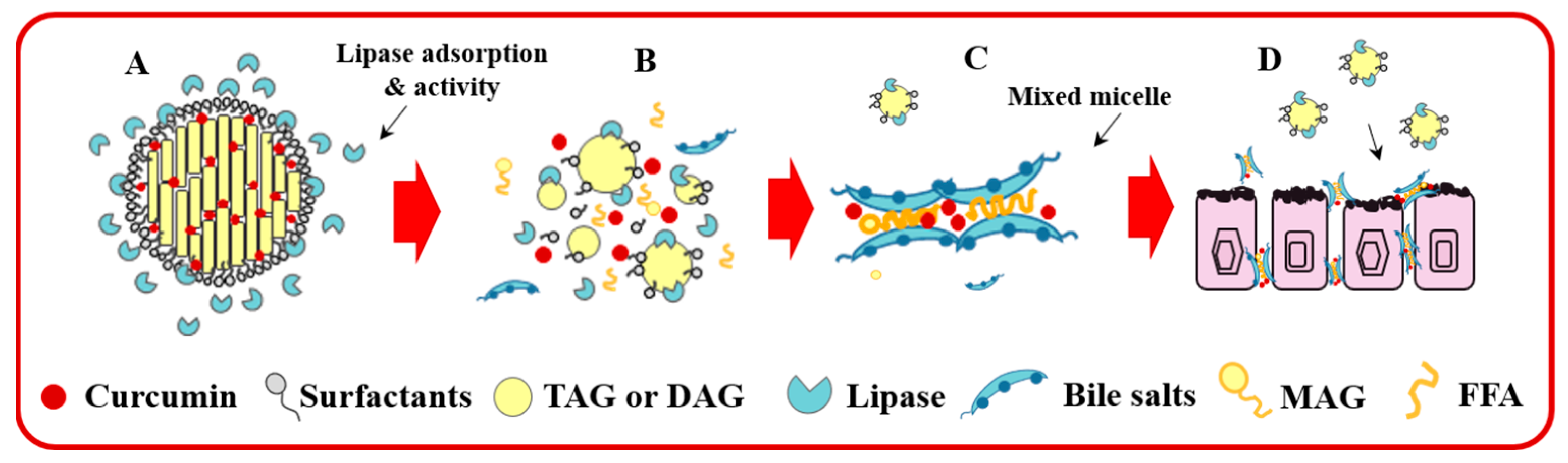
3.6. In Vitro Digestion of Cur-SLNs
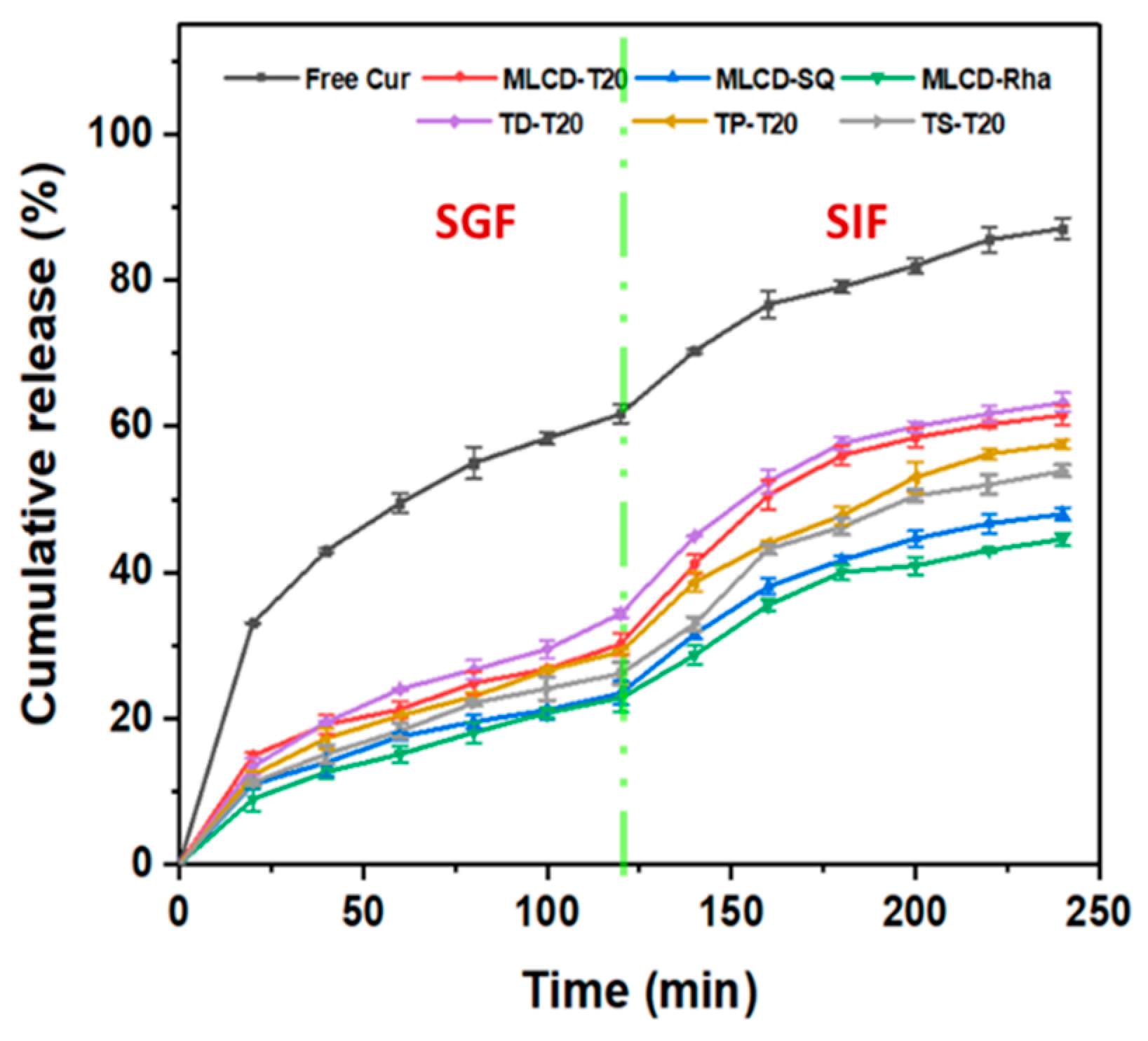
3.7. In Vitro Release Kinetics of Cur
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Liu, D.; Huang, M.; Huang, W.; Li, Y.; Feng, J. Interfacial engineering strategy to improve the stabilizing effect of curcumin-loaded nanostructured lipid carriers. Food Hydrocoll. 2022, 127, 107552. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Sripetthong, S.; Eze, F.N.; Sajomsang, W.; Ovatlarnporn, C. Development of pH-Responsive N-benzyl-N-O-succinyl Chitosan Micelles Loaded with a Curcumin Analog (Cyqualone) for Treatment of Colon Cancer. Molecules 2023, 28, 2693. [Google Scholar] [CrossRef]
- Honarvari, B.; Karimifard, S.; Akhtari, N.; Mehrarya, M.; Moghaddam, Z.S.; Ansari, M.J.; Jalil, A.T.; Matencio, A.; Trotta, F.; Yeganeh, F.E.; et al. Folate-Targeted Curcumin-Loaded Niosomes for Site-Specific Delivery in Breast Cancer Treatment: In Silico and In Vitro Study. Molecules 2022, 27, 4634. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, M.; Na, Y.G.; Kim, S.J.; Lee, H.K.; Cho, C.W. An EGF-and curcumin-co-encapsulated nanostructured lipid carrier accelerates chronic-wound healing in diabetic rats. Molecules 2020, 25, 4610. [Google Scholar] [CrossRef]
- Corrie, L.; Kaur, J.; Awasthi, A.; Vishwas, S.; Gulati, M.; Saini, S.; Kumar, B.; Pandey, N.K.; Gupta, G.; Dureja, H.; et al. Multivariate Data Analysis and Central Composite Design-Oriented Optimization of Solid Carriers for Formulation of Curcumin-Loaded Solid SNEDDS: Dissolution and Bioavailability Assessment. Pharmaceutics 2022, 14, 2395. [Google Scholar] [CrossRef]
- Li, J.; Chang, C.; Chen, W.; Su, Y.; Gu, L.; Yang, Y.; Zhai, J. Hybrid liposomes composed of hydrophilic emulsifiers and lecithin: Physicochemical, interaction and curcumin loading properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130210. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Drechsler, M.; Nicolas, V.; Bizien, T.; Gorshkova, Y.E.; Deng, Y.; Angelova, A. Liquid crystalline lipid nanoparticles for combined delivery of curcumin, fish oil and BDNF: In vitro neuroprotective potential in a cellular model of tunicamycin-induced endoplasmic reticulum stress. Smart Mater. Struct. 2022, 3, 274–288. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Yuan, F. Stability and release performance of curcumin-loaded liposomes with varying content of hydrogenated phospholipids. Food Chem. 2020, 326, 126973. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.; Villaseñor, M.; Ríos, Á. Analytical control of nanodelivery lipid-based systems for encapsulation of nutraceuticals: Achievements and challenges. Trends Food Sci. Technol. 2019, 90, 47–62. [Google Scholar] [CrossRef]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Sato, K.; Ueno, S. Crystallization, transformation and microstructures of polymorphic fats in colloidal dispersion states. Curr. Opin. Colloid Interface Sci. 2011, 16, 384–390. [Google Scholar] [CrossRef]
- Salminen, H.; Stübler, A.S.; Weiss, J. Preparation, characterization, and physical stability of cocoa butter and tristearin nanoparticles containing β-carotene. Eur. Food Res. Technol. 2020, 246, 599–608. [Google Scholar] [CrossRef]
- Li, G.; Lee, W.J.; Tan, C.P.; Lai, O.M.; Wang, Y.; Qiu, C. Tailored rigidity of W/O Pickering emulsions using diacylglycerol-based surface-active solid lipid nanoparticles. Food Funct. 2021, 12, 11732–11746. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Drechsler, M.; Angelova, A. Composition-switchable liquid crystalline nanostructures as green formulations of curcumin and fish oil. ACS Sustain. Chem. Eng. 2021, 9, 14821–14835. [Google Scholar] [CrossRef]
- Golemanov, K.; Tcholakova, S.; Denkov, N.; Pelan, E.; Stoyanov, S.D. Remarkably high surface visco-elasticity of adsorption layers of triterpenoid saponins. Soft Matter 2013, 9, 5738–5752. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Cerqueira, M.A.; Fuciños, P.; Silva, B.F.; Teixeira, J.A.; Pastrana, L. Rhamnolipids-based nanostructured lipid carriers: Effect of lipid phase on physicochemical properties and stability. Food Chem. 2021, 344, 128670. [Google Scholar] [CrossRef]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Luo, Y. Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. J. Colloid Interface Sci. 2017, 507, 119–130. [Google Scholar] [CrossRef]
- Kondo, H.; Hase, T.; Murase, T.; Tokimitsu, I. Digestion and assimilation features of dietary DAG in the rat small intestine. Lipids 2003, 38, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Huang, S.; He, J.; Han, L.; Zhang, W.; Zhong, Q. 1-Laurin-3-Palmitin as a Novel Matrix of Solid Lipid Particles: Higher Loading Capacity of Thymol and Better Stability of Dispersions Than Those of Glyceryl Monostearate and Glyceryl Tripalmitate. Nanomaterials 2019, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yin, W.; Liu, G. Development and characterisation of a novel krill oil nanostructured lipid carrier based on 1, 3-glycerol distearate. Int. J. Food Sci. Technol. 2020, 55, 3493–3502. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef]
- Huang, S.; He, J.; Cao, L.; Lin, H.; Zhang, W.; Zhong, Q. Improved Physicochemical Properties of Curcumin-Loaded Solid Lipid Nanoparticles Stabilized by Sodium Caseinate-Lactose Maillard Conjugate. J. Agric. Food Chem. 2020, 68, 7072–7081. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T. Observation of chitosan coated lipid nanoparticles with different lipid compositions under simulated in vitro digestion system. Food Hydrocoll. 2018, 84, 146–153. [Google Scholar] [CrossRef]
- Zhang, J.; Chuesiang, P.; Kim, J.T.; Shin, G.H. The role of nanostructured lipid carriers and type of biopolymers on the lipid digestion and release rate of curcumin from curcumin-loaded oleogels. Food Chem. 2022, 392, 133306. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Zhang, T.; Liu, Q.; Zhu, J.; Huang, Q. Evaluation of Oral Bioaccessibility of Aged Citrus Peel Extracts Encapsulated in Different Lipid-Based Systems: A Comparison Study Using Different in Vitro Digestion Models. J. Agric. Food Chem. 2020, 68, 97–105. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Rui, L.; Fang, Q.; Li, P.; Zhang, C.; Yuan, Y.; Zhuang, H. Effects of Emulsifier Type and Post-Treatment on Stability, Curcumin Protection, and Sterilization Ability of Nanoemulsions. Foods 2021, 10, 149. [Google Scholar]
- Riquelme, N.; Zúñiga, R.N.; Arancibia, C. Physical stability of nanoemulsions with emulsifier mixtures: Replacement of tween 80 with quillaja saponin. LWT 2019, 111, 760–766. [Google Scholar] [CrossRef]
- Bai, L.; McClements, D.J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Liu, G.; Du, X.; Hu, M.; Zhang, S.; Jiang, L.; Zhu, H.; Qi, B.; Li, Y. Emulsions co-stabilized by soy protein nanoparticles and tea saponin: Physical stability, rheological properties, oxidative stability, and lipid digestion. Food Chem. 2022, 387, 132891. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, L.; Wang, D.; Mao, L.; Gao, Y. Stabilization and Rheology of Concentrated Emulsions Using the Natural Emulsifiers Quillaja Saponins and Rhamnolipids. J. Agric. Food Chem. 2018, 66, 3922–3929. [Google Scholar] [CrossRef] [PubMed]
- Uluata, S.; Mcclements, D.J.; Decker, E.A. Physical Stability, Autoxidation, and Photosensitized Oxidation of ω-3 Oils in Nanoemulsions Prepared with Natural and Synthetic Surfactants. J. Agric. Food Chem. 2015, 63, 9333–9340. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; Mcclements, D.J. Solid lipid nanoparticles: Effect of carrier oil and emulsifier type on phase behavior and physical stability. J. Am. Oil Chem. Soc. 2012, 89, 17–28. [Google Scholar] [CrossRef]
- Lee, W.J.; Zhang, Z.; Lai, O.; Tan, C.; Wang, Y. Diacylglycerol in food industry: Synthesis methods, functionalities, health benefits, potential risks and drawbacks. Trends Food Sci. Technol. 2020, 97, 114–125. [Google Scholar] [CrossRef]
- Bunjes, H.; Steiniger, F.; Richter, W. Visualizing the structure of triglyceride nanoparticles in different crystal modifications. Langmuir 2007, 23, 4005–4011. [Google Scholar] [CrossRef]
- McClements, D.J. Crystals and crystallization in oil-in-water emulsions: Implications for emulsion-based delivery systems. Adv. Colloid Interface Sci. 2012, 174, 1–30. [Google Scholar] [CrossRef]
- Kharat, M.; Mcclements, D.J. Fabrication and characterization of nanostructured lipid carriers (NLC) using a plant-based emulsifier: Quillaja saponin. Food Res. Int. 2019, 126, 108601. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Nanostructured lipid carriers (NLCs) stabilized by natural or synthetic emulsifiers for lutein delivery: Improved physicochemical stability, antioxidant activity, and bioaccessibility. Food Chem. 2023, 403, 134465. [Google Scholar] [CrossRef] [PubMed]
- Salminen, H.; Gömmel, C.; Leuenberger, B.H.; Weiss, J. Influence of encapsulated functional lipids on crystal structure and chemical stability in solid lipid nanoparticles: Towards bioactive-based design of delivery systems. Food Chem. 2016, 190, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, W.J.; Wang, Y.; Tan, C.P.; Lai, O.M.; Wang, Y. Effect of Purification Methods on the Physicochemical and Thermodynamic Properties and Crystallization Kinetics of Medium-Chain, Medium-Long-Chain, and Long-Chain Diacylglycerols. J. Agric. Food Chem. 2020, 68, 8391–8403. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.; Liao, W.; Zhang, L.; Liu, J.; Gao, Y. Formation, Physicochemical Stability, and Redispersibility of Curcumin-Loaded Rhamnolipid Nanoparticles Using the pH-Driven Method. J. Agric. Food Chem. 2020, 68, 7103–7111. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884. [Google Scholar] [CrossRef]
- Ng, S.P.; Lai, O.M.; Abas, F.; Lim, H.K.; Beh, B.K.; Ling, T.C.; Tan, C.P. Compositional and thermal characteristics of palm olein-based diacylglycerol in blends with palm super olein. Food Res. Int. 2014, 55, 62–69. [Google Scholar] [CrossRef]
- Öztürk, A.A.; Aygül, A.; Şenel, B. Influence of glyceryl behenate, tripalmitin and stearic acid on the properties of clarithromycin incorporated solid lipid nanoparticles (SLNs): Formulation, characterization, antibacterial activity and cytotoxicity. J. Drug Deliv. Sci. Technol. 2019, 54, 101240. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Zheng, L.; Yu, L.; Zhang, Q.; Liu, W. Preparation and characterization of monocaprate nanostructured lipid carriers. Colloid Surface A 2007, 311, 106–111. [Google Scholar] [CrossRef]
- Guo, S.J.; Ma, C.G.; Hu, Y.Y.; Bai, G.; Song, Z.J.; Cao, X.Q. Solid lipid nanoparticles for phytosterols delivery: The acyl chain number of the glyceride matrix affects the arrangement, stability, and release. Food Chem. 2022, 394, 133412. [Google Scholar] [CrossRef]
- Hajj Ali, H.; Michaux, F.; Bouelet Ntsama, I.S.; Durand, P.; Jasniewski, J.; Linder, M. Shea butter solid nanoparticles for curcumin encapsulation: Influence of nanoparticles size on drug loading. Eur. J. Lipid Sci. Technol. 2016, 118, 1168–1178. [Google Scholar] [CrossRef]
- Kharat, M.; Zhang, G.; McClements, D.J. Stability of curcumin in oil-in-water emulsions: Impact of emulsifier type and concentration on chemical degradation. Food Res. Int. 2018, 111, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Huang, M.; Chai, Z.; Li, C.; Huang, W.; Cui, L.; Li, Y. The influence of oil composition on the transformation, bioaccessibility, and intestinal absorption of curcumin in nanostructured lipid carriers. Food Funct. 2020, 11, 5223–5239. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, Z.; Mundo, J.M.; McClements, D.J. Factors impacting lipid digestion and nutraceutical bioaccessibility assessed by standardized gastrointestinal model (INFOGEST): Emulsifier type. Food Res. Int. 2020, 137, 109739. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, S.; Desroches, V.; Britten, M. Effect of milk proteins and food-grade surfactants on oxidation of linseed oil-in-water emulsions during in vitro digestion. Food Chem. 2019, 294, 130–137. [Google Scholar] [CrossRef]
- Ye, Z.; Cao, C.; Liu, Y.; Cao, P.; Li, Q. Triglyceride Structure Modulates Gastrointestinal Digestion Fates of Lipids: A Comparative Study between Typical Edible Oils and Triglycerides Using Fully Designed in Vitro Digestion Model. J. Agric. Food Chem. 2018, 66, 6227–6238. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin E encapsulation within oil-in-water emulsions: Impact of emulsifier type on physicochemical stability and bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef]
- Carpenter, J.; George, S.; Saharan, V.K. Curcumin Encapsulation in Multilayer Oil-in-Water Emulsion: Synthesis Using Ultrasonication and Studies on Stability and Antioxidant and Release Activities. Langmuir 2019, 35, 10866–10876. [Google Scholar] [CrossRef]
- Osaki, N.; Meguro, S.; Yajima, N.; Matsuo, N.; Tokimitsu, I.; Shimasaki, H. Metabolities of dietary triacylglycerol and diacylglycerol during the digestion process in rats. Lipids 2005, 40, 281–286. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Wang, J.; Li, Y.; Feng, F.; Zhao, M. Digestive behavior of unemulsified triglycerides with different chain lengths: In vitro dynamic and static simulated digestion models. LWT 2021, 149, 112006. [Google Scholar] [CrossRef]
- Diao, X.; Guan, H.; Kong, B.; Liu, D.; Zhang, Y. In vitro digestion of emulsified lard-based diacylglycerols. J. Sci. Food Agric. 2021, 101, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Bribiesca, E.; Turgeon, S.L.; Britten, M. Effect of calcium on fatty acid bioaccessibility during in vitro digestion of Cheddar-type cheeses prepared with different milk fat fractions. J. Dairy Sci. 2017, 100, 2454–2470. [Google Scholar] [CrossRef]
- Wan, L.; Li, L.; Harro, J.M.; Hoag, S.W.; Li, B.; Zhang, X.; Shirtliff, M.E. In Vitro Gastrointestinal Digestion of Palm Olein and Palm Stearin-in-Water Emulsions with Different Physical States and Fat Contents. J. Agric. Food Chem. 2020, 68, 7062–7071. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Tong, Z.; Dai, L.; Wang, D.; Lv, P.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Influence of interfacial compositions on the microstructure, physiochemical stability, lipid digestion and β-carotene bioaccessibility of Pickering emulsions. Food Hydrocoll. 2020, 104, 105738. [Google Scholar] [CrossRef]
- Fahami, A.; Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin A delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Sangsen, Y.; Likhitwitayawuid, K.; Sritularak, B.; Wiwattanawongsa, K.; Wiwattanapatapee, R. Novel Solid Lipid Nanoparticles for Oral Delivery of Oxyresveratrol: Effect of the Formulation Parameters on the Physicochemical Properties and in vitro Release. Int. J. Pharm. 2013, 7, 877–884. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Chen, D.; Lee, Y.Y.; Chen, N.; Wang, Y.; Qiu, C. Physicochemical and In Vitro Digestion Properties of Curcumin-Loaded Solid Lipid Nanoparticles with Different Solid Lipids and Emulsifiers. Foods 2023, 12, 2045. https://doi.org/10.3390/foods12102045
Yu Y, Chen D, Lee YY, Chen N, Wang Y, Qiu C. Physicochemical and In Vitro Digestion Properties of Curcumin-Loaded Solid Lipid Nanoparticles with Different Solid Lipids and Emulsifiers. Foods. 2023; 12(10):2045. https://doi.org/10.3390/foods12102045
Chicago/Turabian StyleYu, Yasi, Dechu Chen, Yee Ying Lee, Nannan Chen, Yong Wang, and Chaoying Qiu. 2023. "Physicochemical and In Vitro Digestion Properties of Curcumin-Loaded Solid Lipid Nanoparticles with Different Solid Lipids and Emulsifiers" Foods 12, no. 10: 2045. https://doi.org/10.3390/foods12102045
APA StyleYu, Y., Chen, D., Lee, Y. Y., Chen, N., Wang, Y., & Qiu, C. (2023). Physicochemical and In Vitro Digestion Properties of Curcumin-Loaded Solid Lipid Nanoparticles with Different Solid Lipids and Emulsifiers. Foods, 12(10), 2045. https://doi.org/10.3390/foods12102045