Structural Characteristics and Immunomodulatory Effects of Melanoidins from Black Garlic
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
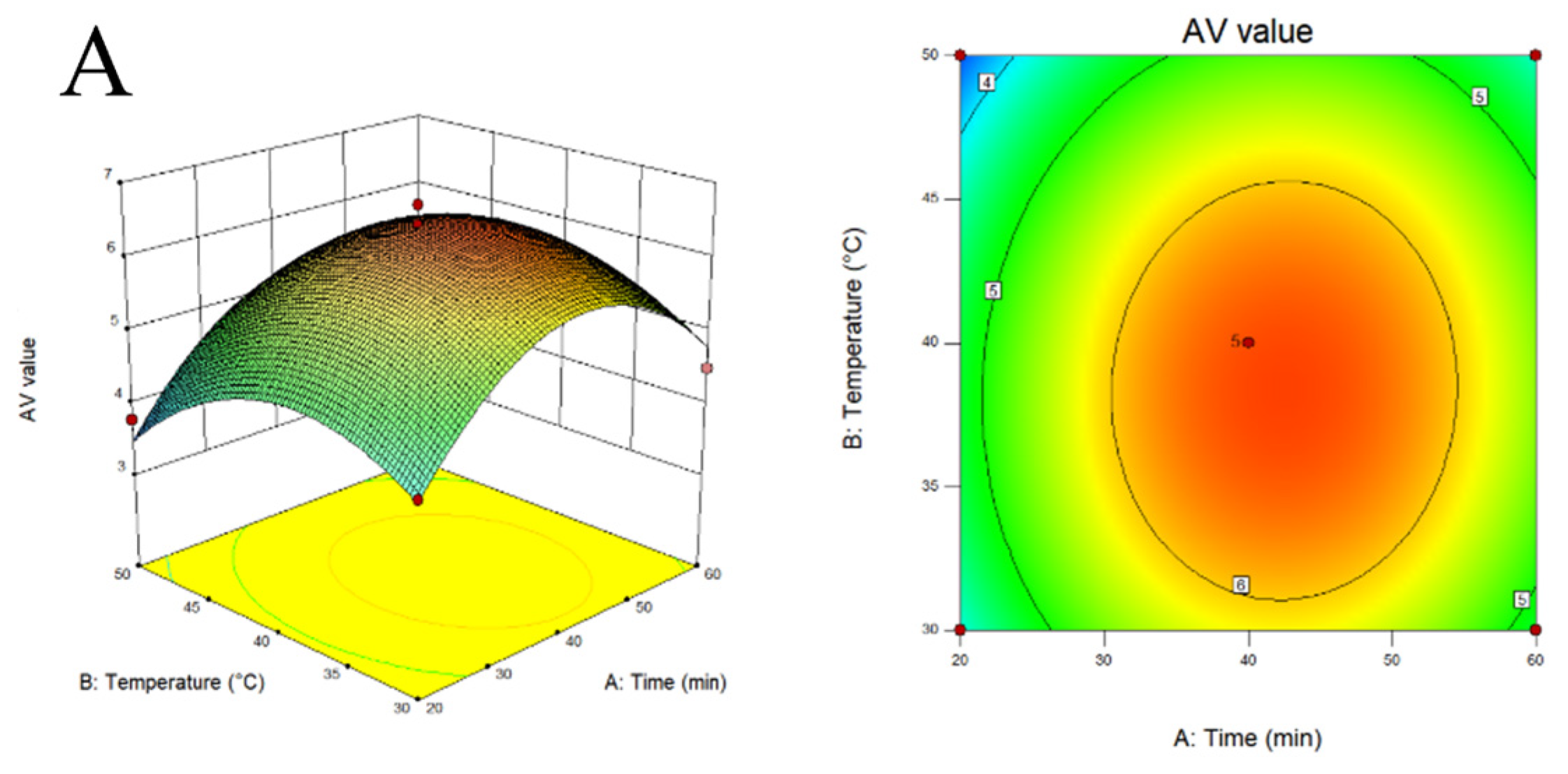
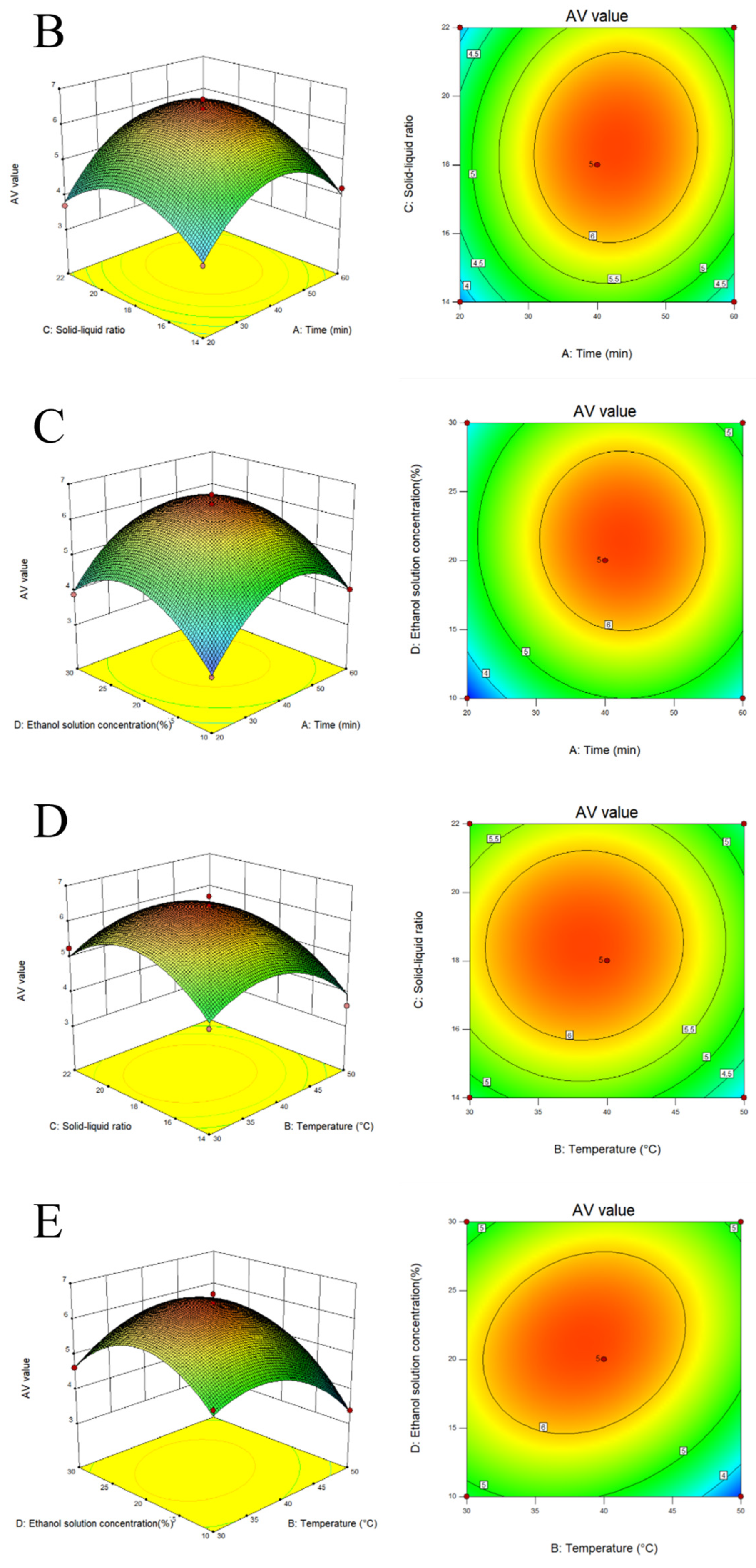
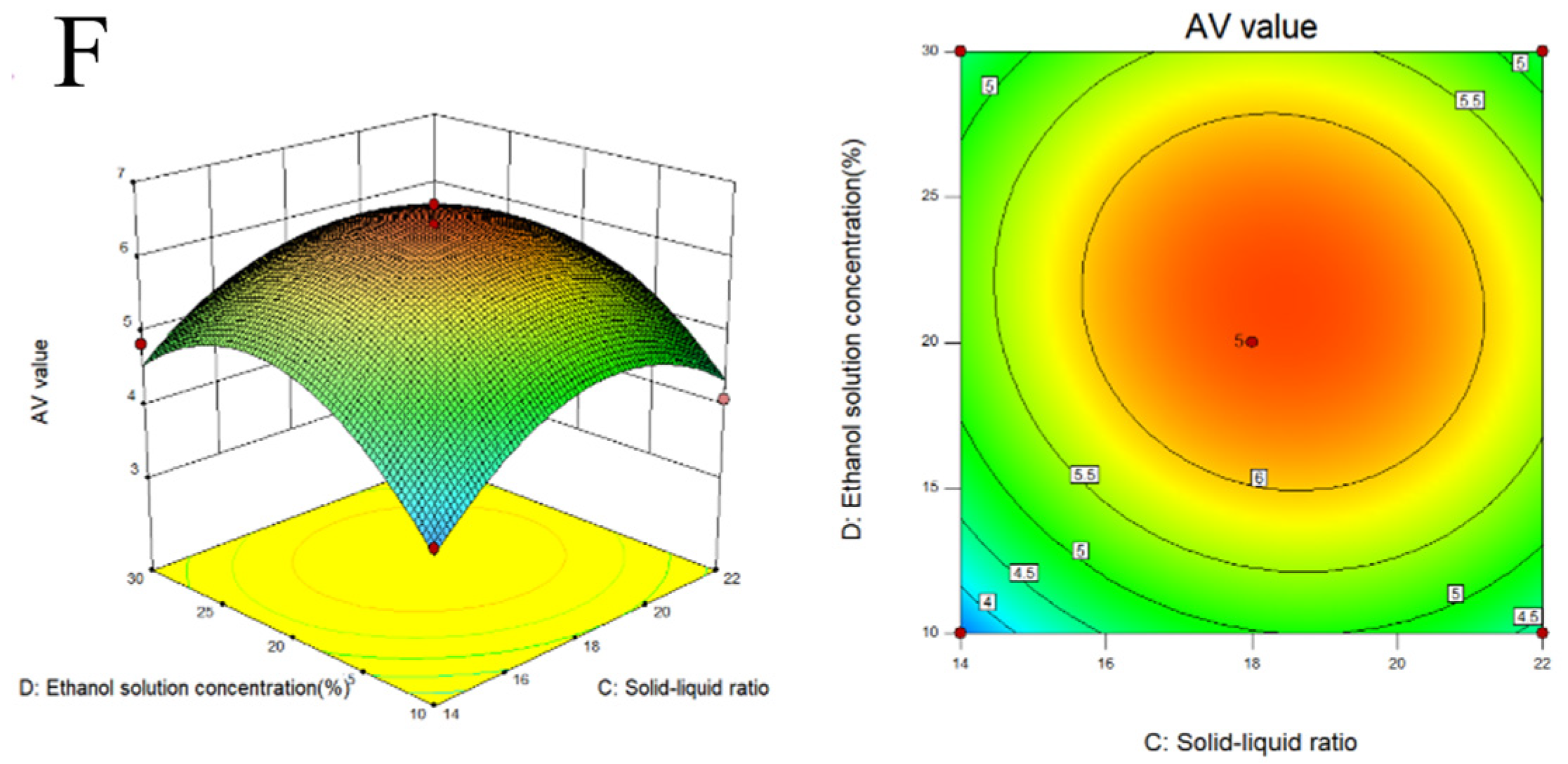
2.2. Response Surface Experiment on Extraction of Melanoidins from Black Garlic
2.3. Extraction of Melanoidins Samples
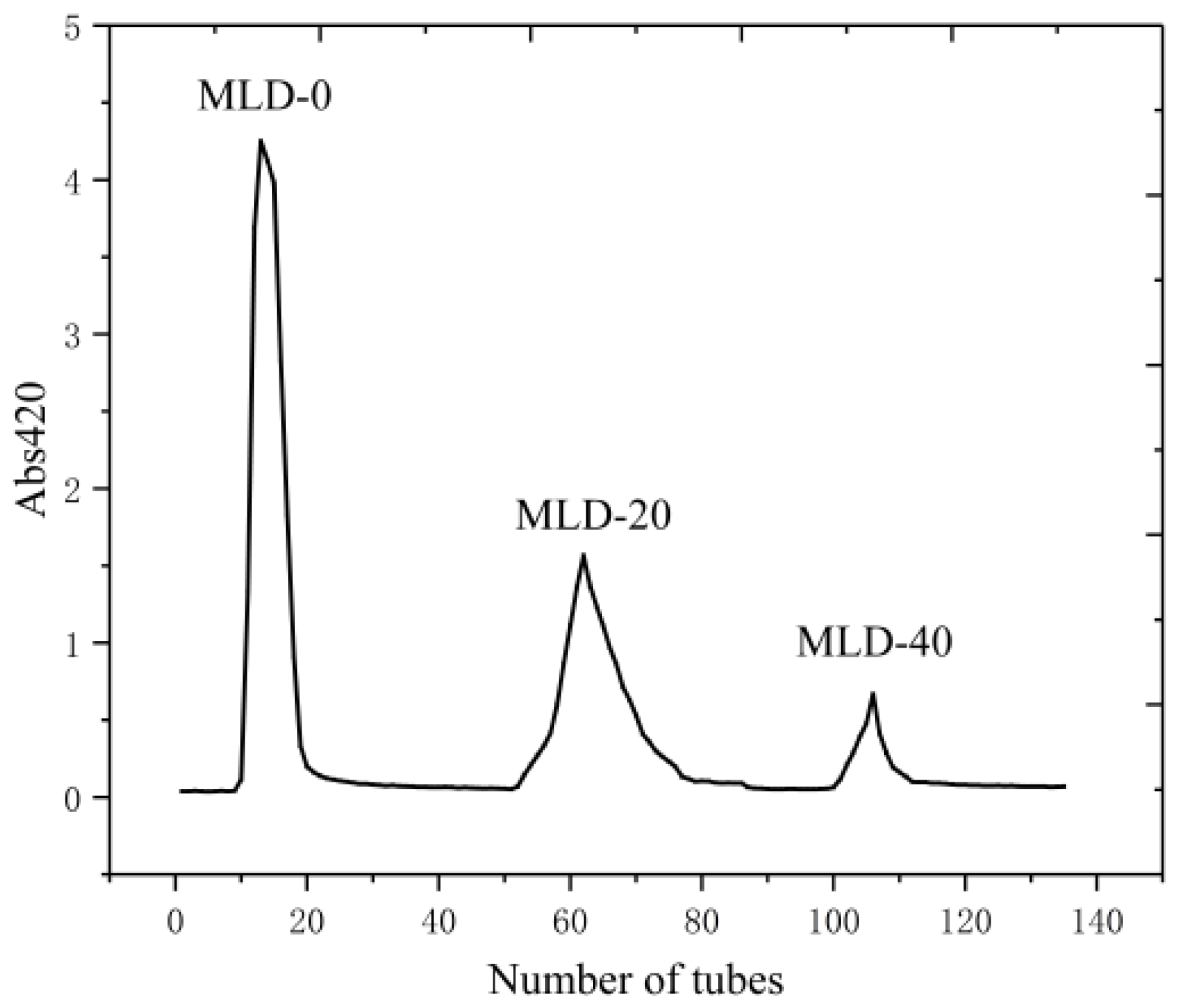
2.4. Purification of Melanoidins Samples
2.5. Determination of the Molecular Weight of Melanoidin Samples
2.6. Infrared Analysis
2.7. Microstructure Observation
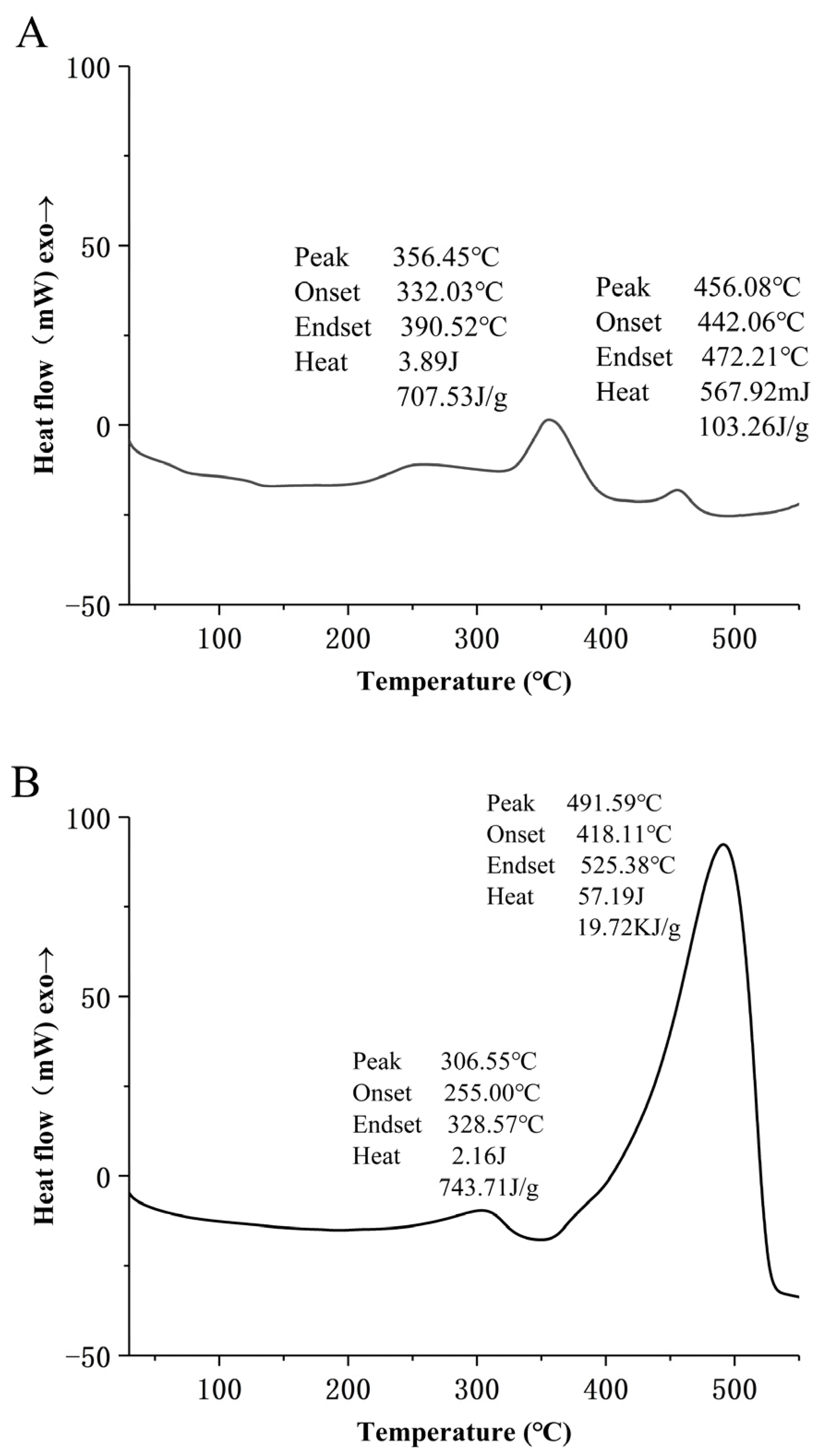
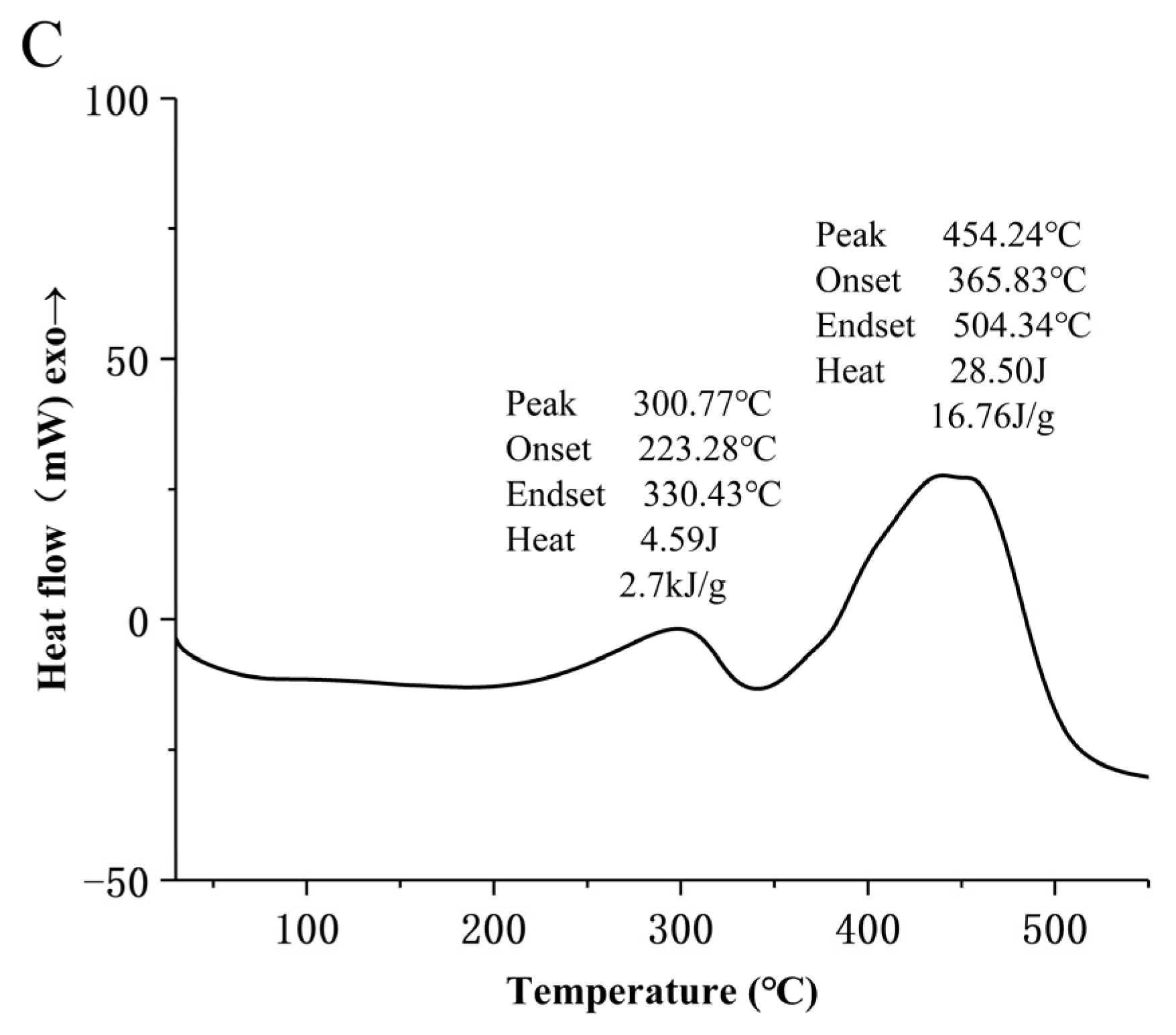
2.8. DSC Analysis
2.9. Design of Experimental Animals
2.10. Measurement of Organ Index
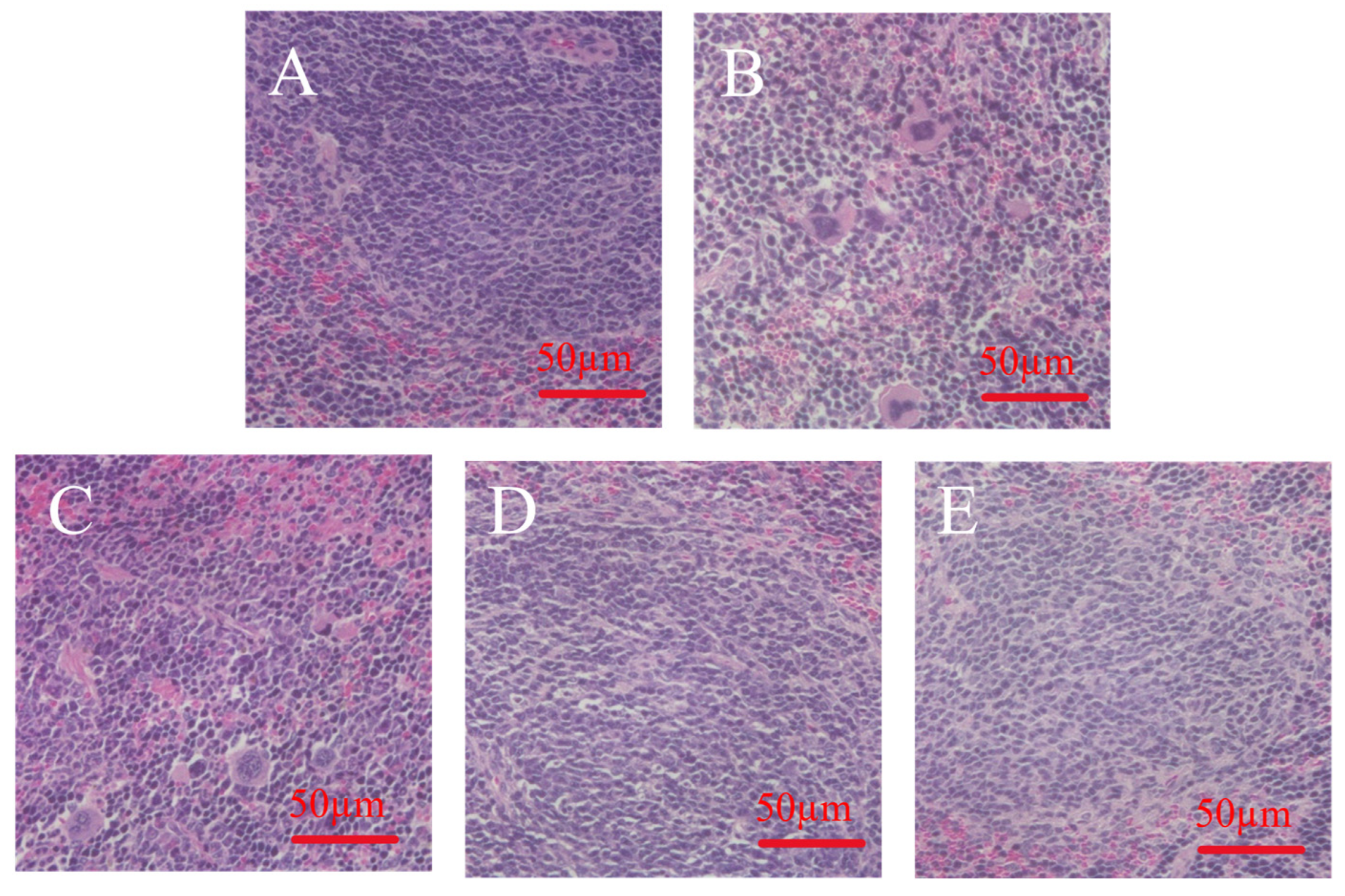
2.11. H&E Staining of Organs
2.12. Determination of the Activity of Mouse Peritoneal Macrophages
2.12.1. Determination of the Proliferative Activity of Macrophages
2.12.2. Determination of the Phagocytosis Ability of Macrophages
2.13. Determination of the Activity of Mouse Spleen Lymphocytes
2.13.1. Proliferative Activity of Mouse Spleen Lymphocytes
2.13.2. Determination of the NK Cell-Killing Activity of Spleen Lymphocytes in Mice
2.14. Determination of Related Immune Factors in the Serum of Mice
2.15. Determination of SCFA in the Mouse Intestinal Short-Chain Fatty Acids
2.16. Determination of Intestinal Flora in Mice
2.17. Data Analysis
3. Results and Discussion
3.1. Extraction and Optimization of MLDs
3.2. Separation and Purification of Melanoidins
3.3. Melanoidin Molecular Weight
3.4. MLD Infrared Analysis
3.5. SEM Observation
3.6. Thermal Analysis of MLD
3.7. Effects of MLDs on Body Weight and Organ Index in Mice
3.8. Effect of MLDs on Spleen Morphology in Immunocompromised Mice
3.9. The Effect of Black Garlic Melanoidins on the Abdominal Macrophage in Mice
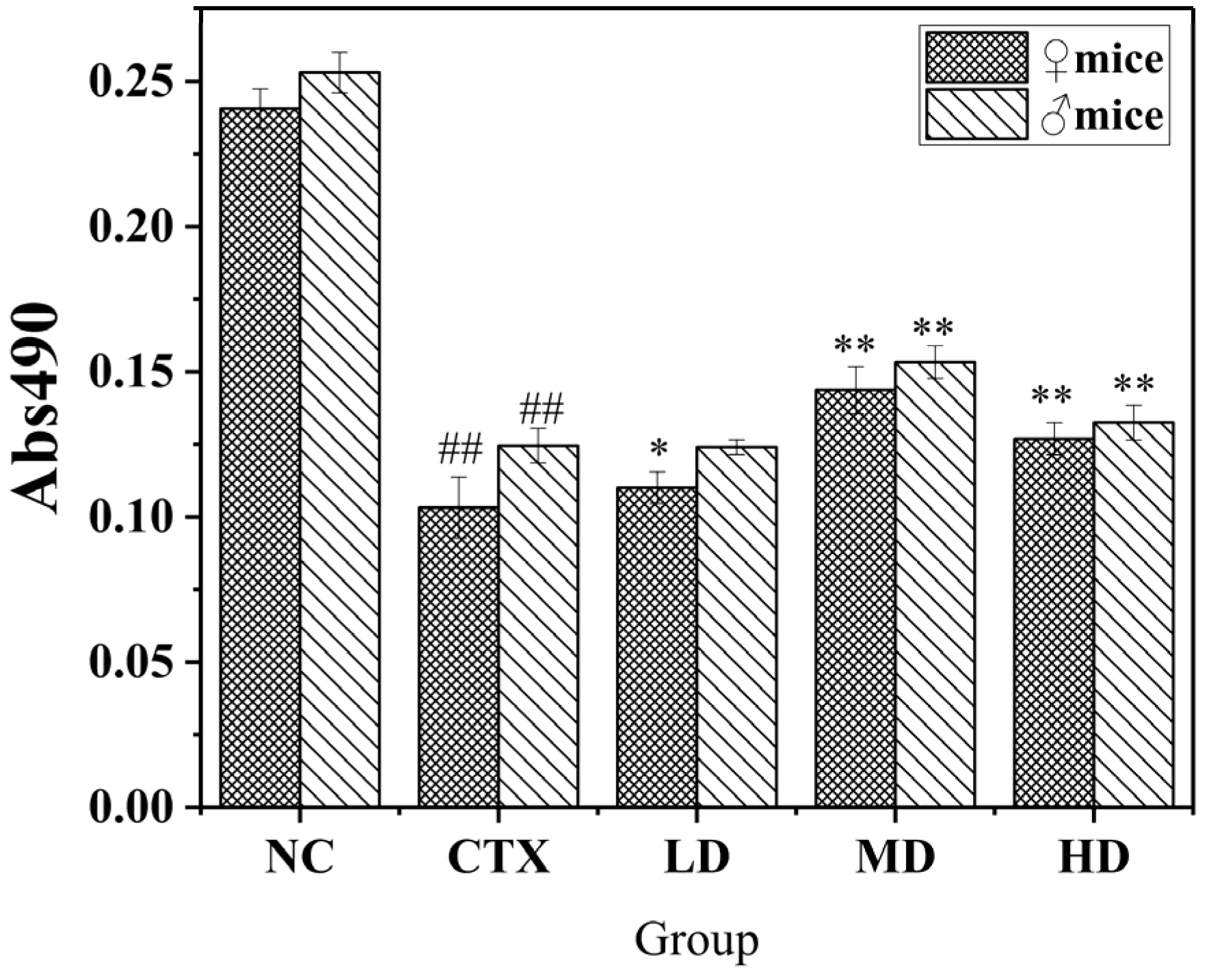
3.9.1. The Effect of MLDs on the Proliferation of Macrophages in Mice
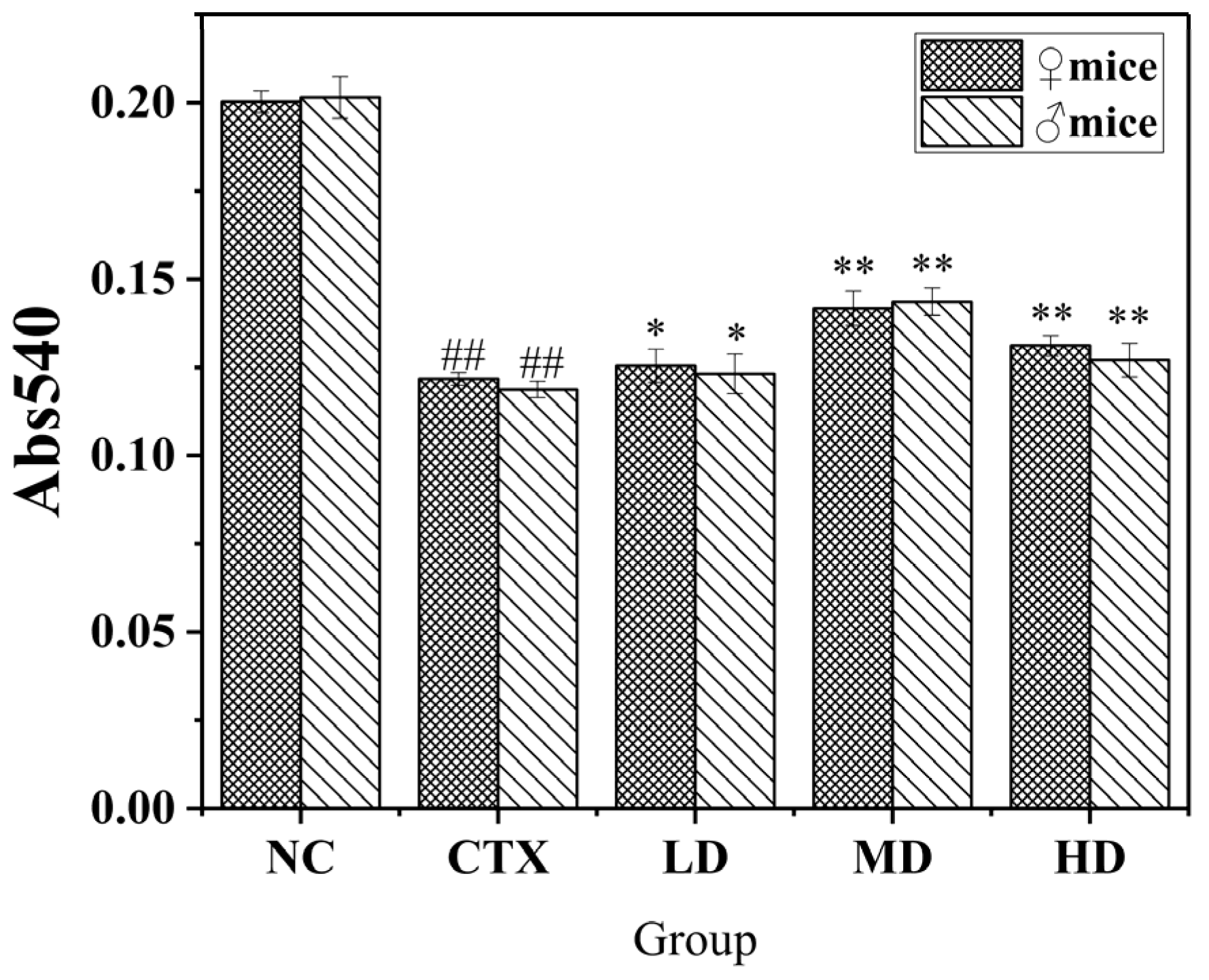
3.9.2. Effect of MLDs on the Phagocytic Ability of Mouse Peritoneal Macrophages
3.10. Effect of MLDs on Spleen Cells in Mice
3.10.1. Effect of MLDs on the Proliferative Activity of Spleen Lymphocytes in Mice
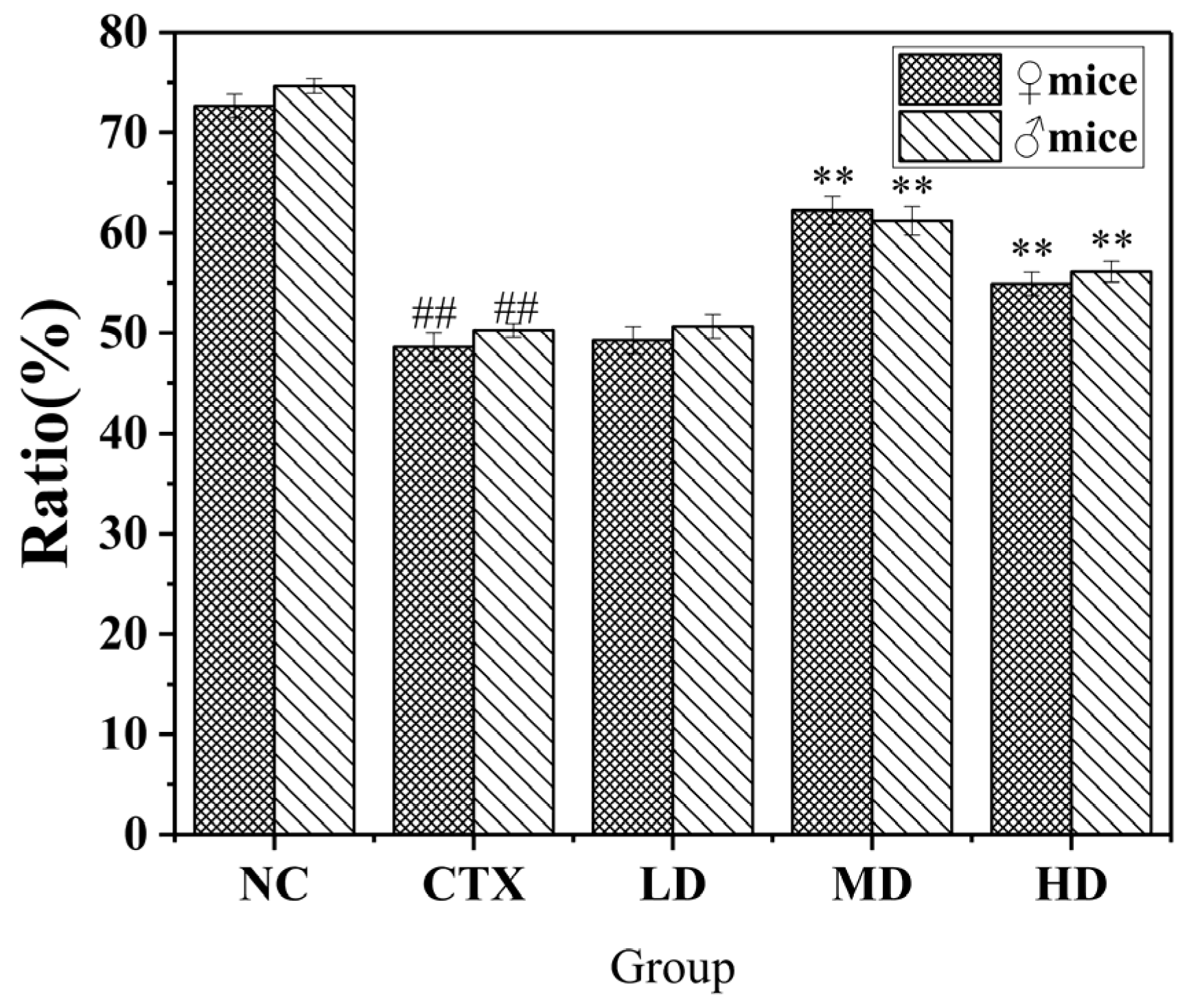
3.10.2. Effect of MLDs on the NK Cell-Killing Activity in Mice
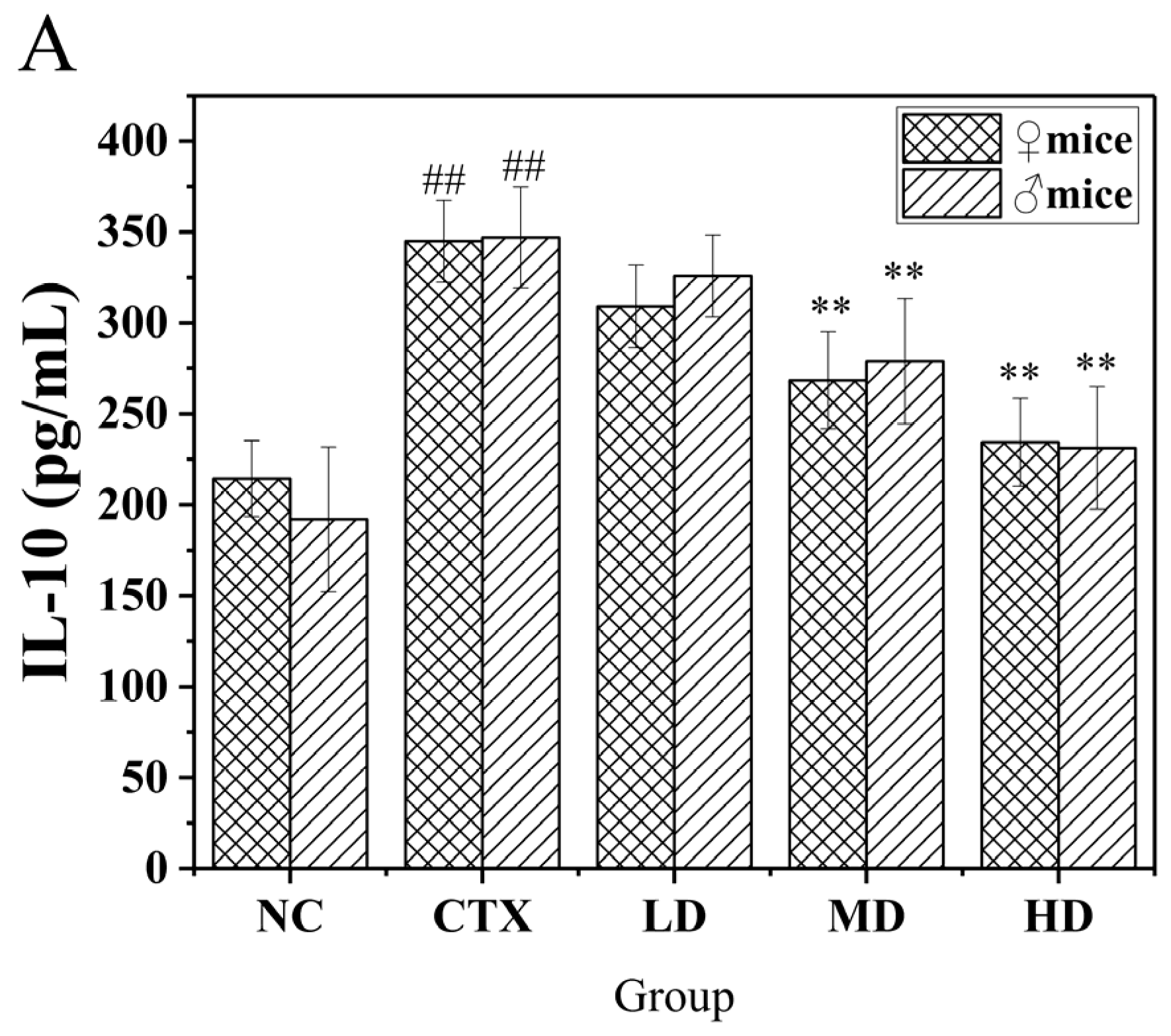
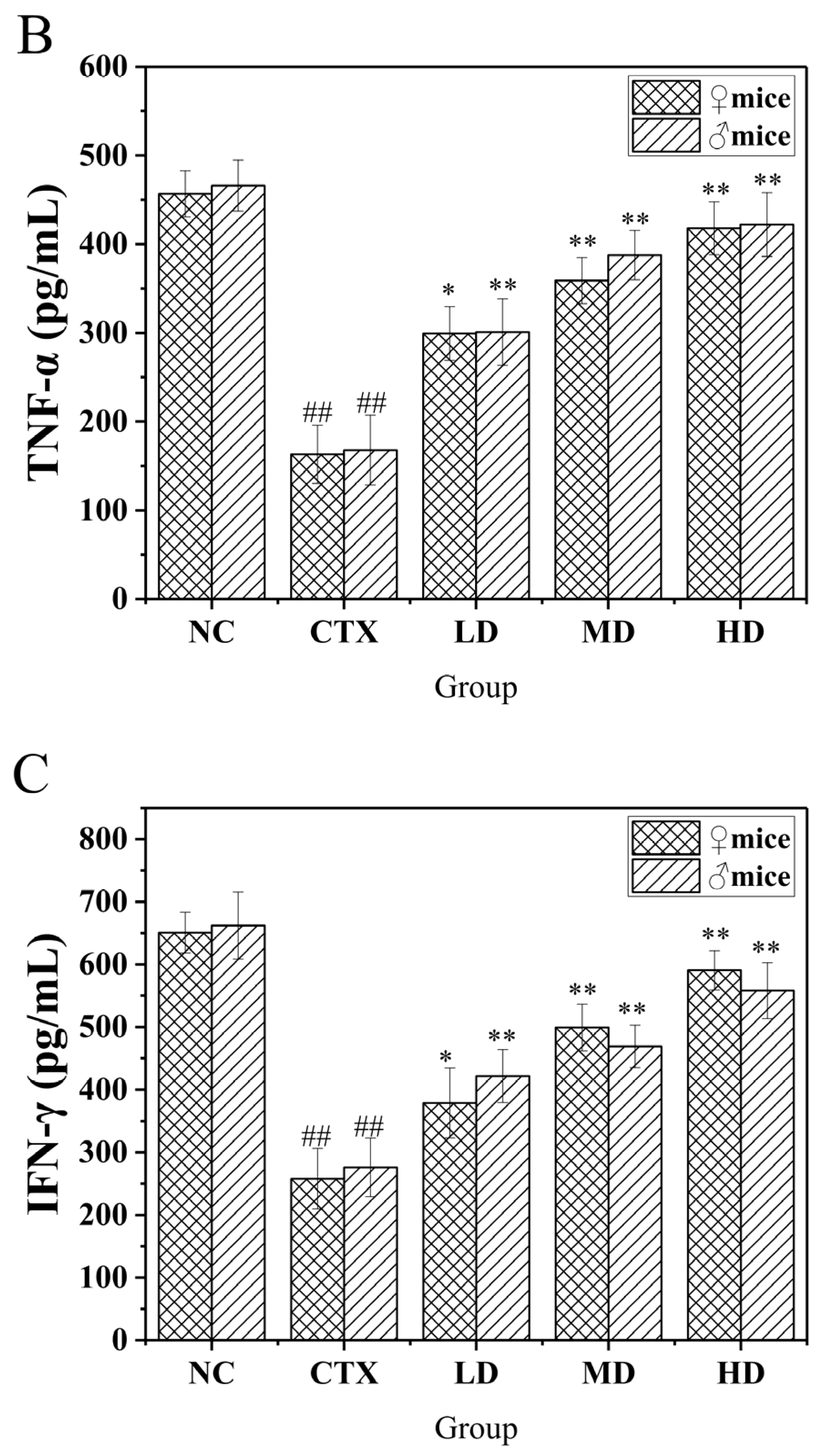
3.11. Determination of Related Immune Factors in the Serum of Mice
3.12. Determination of Short Chain Fatty Acids in Mouse Intestine
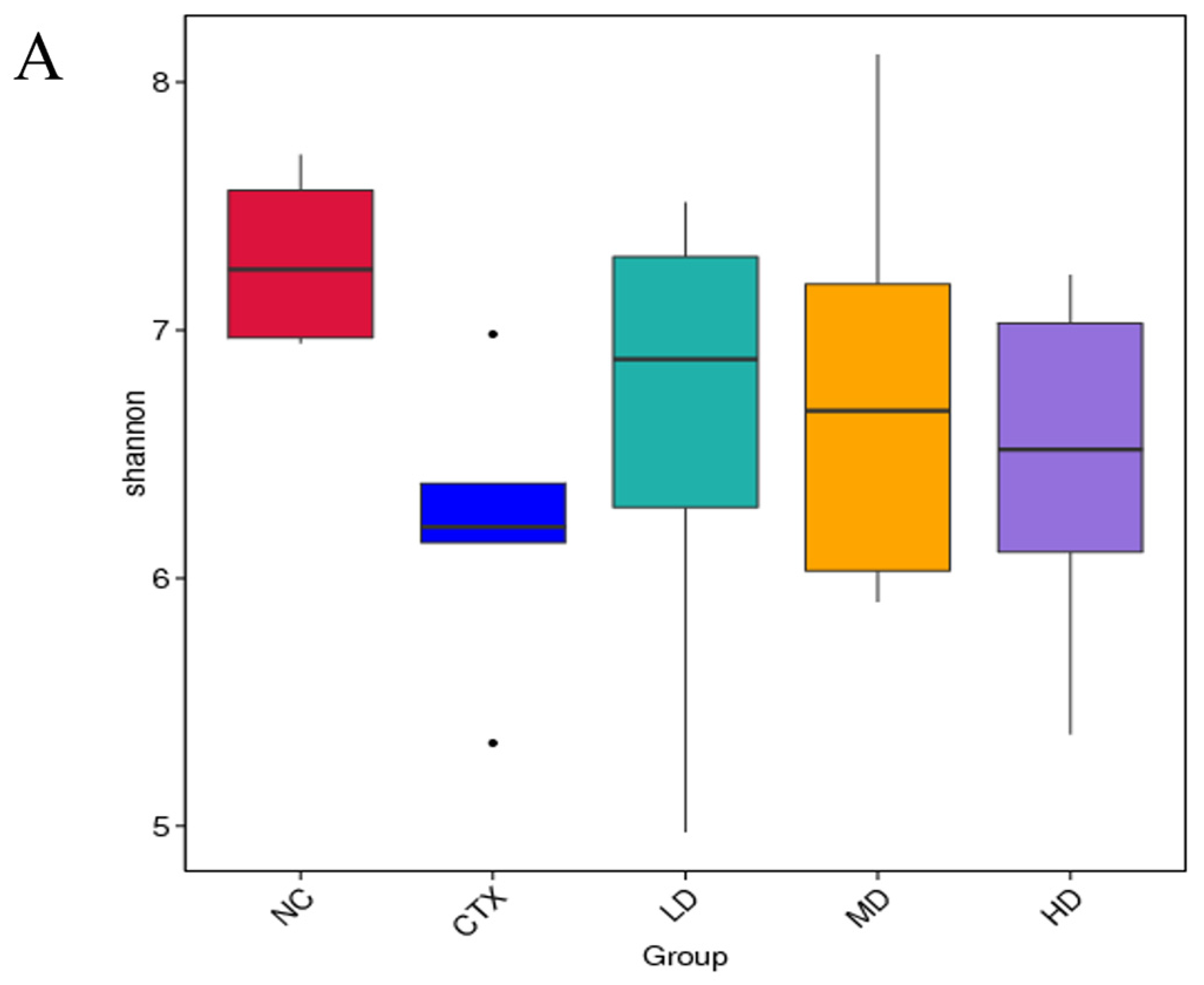
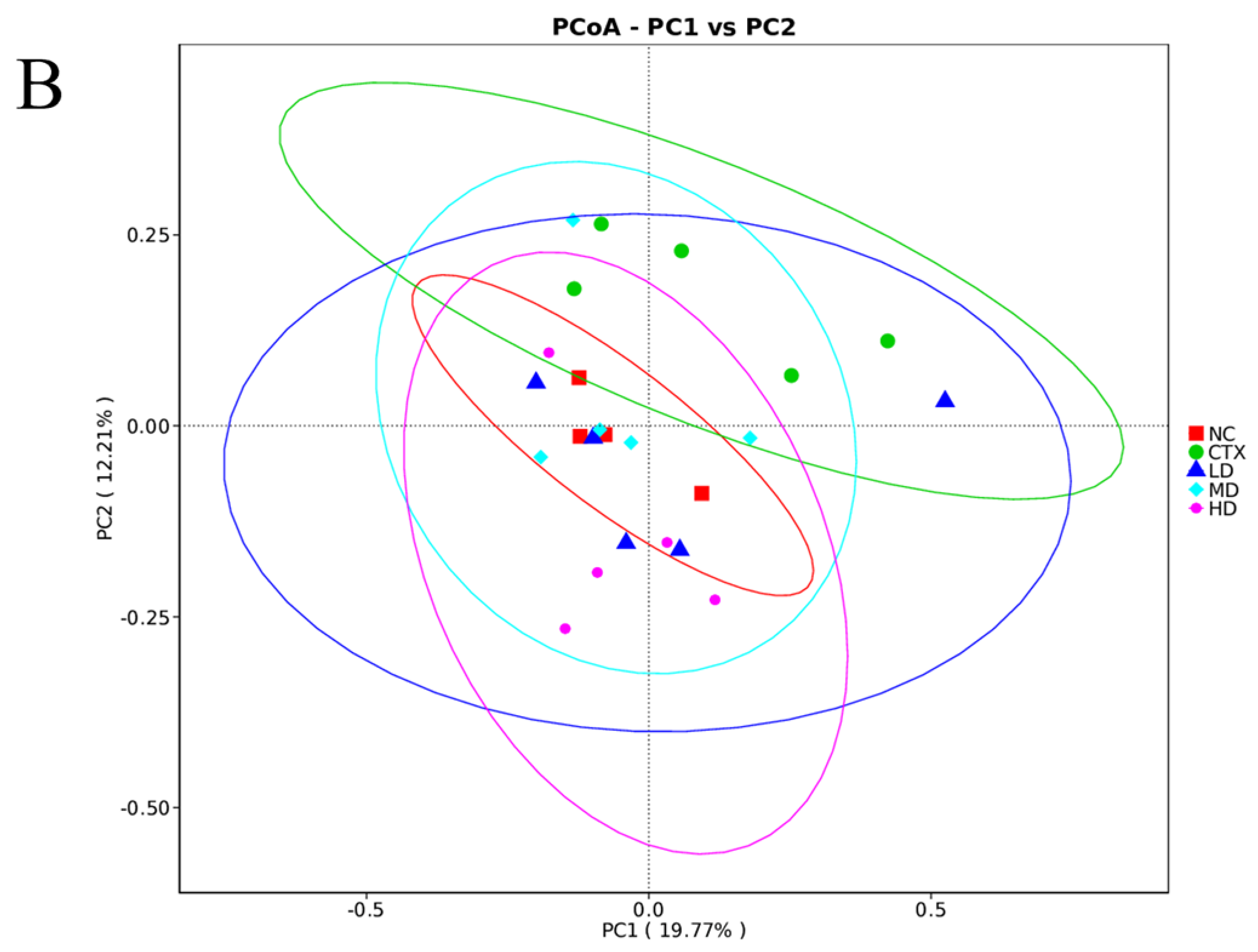
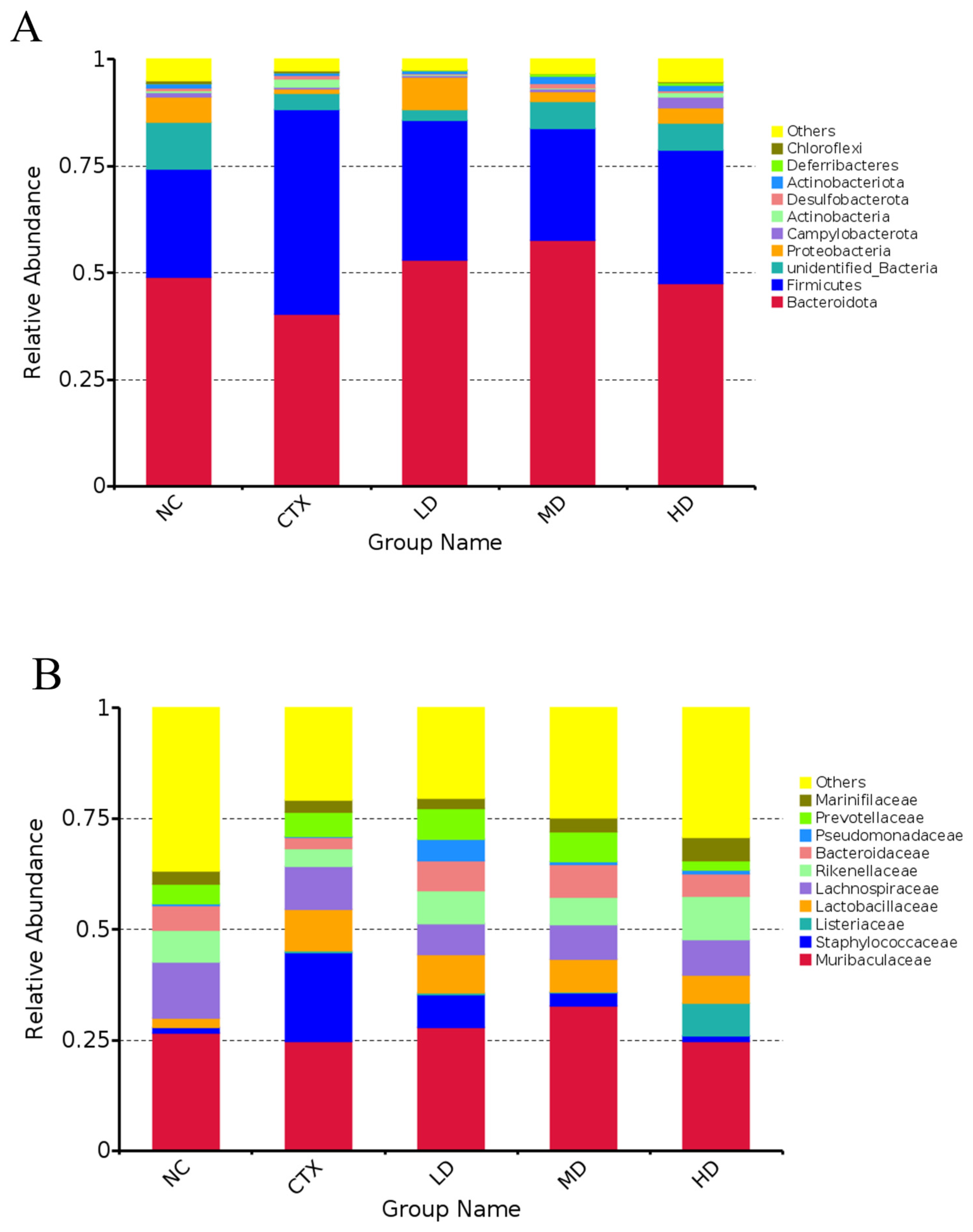
3.13. Determination of Intestinal Flora in Mice
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pang, X.; Zhang, H.; Ji, P. The cGAS-STING pathway in bacterial infection and bacterial immunity. Front. Immunol. 2022, 12, 814709. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.J.; Hwang, I.G.; Song, J.; Kim, Y.J. Low temperature-aged garlic extract suppresses psychological stress by modulation of stress hormones and oxidative stress response in brain. J. Chin. Med. Assoc. JCMA 2019, 82, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Oktari, K.; Azizah, Z.; Chandra, B.; Asra, R. A review: Antioxidant and immunomodulator effects of black garlic. EAS J. Pharm. Pharm. 2020, 2, 193–198. [Google Scholar] [CrossRef]
- Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The influence of dietary factors on the gut microbiota. Microorganisms 2022, 10, 1368. [Google Scholar] [CrossRef]
- Qiu, Z.; Zheng, Z.; Zhang, B.; Sun-Waterhouse, D.; Qiao, X. Formation, nutritional value, and enhancement of characteristic components in black garlic: A review for maximizing the goodness to humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 801–834. [Google Scholar] [CrossRef]
- Ro, K.-S.; Chen, Y.; Du, L.; Wang, L.; Zhao, L.; Xie, J.; Wei, D. Improvement of S-allyl-L-cysteine content, probiotic properties and constipation prevention effect of black garlic by the lactic acid bacteria fermentation. Process Biochem. 2022, 115, 110–117. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, H.J.; Yoon, D.K.; Ji, D.S.; Kim, J.H.; Lee, C.H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2018, 27, 219–225. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: A randomised controlled trial. Maturitas 2010, 67, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Eun Lee, K.; Ambati, R.R.; Gundamaraju, R.; Fawzy Ramadan, M.; Gu Kang, S. Recent development in black garlic: Nutraceutical applications and health-promoting phytoconstituents. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
- Jung, E.-S.; Park, S.-H.; Choi, E.-K.; Ryu, B.-H.; Park, B.-H.; Kim, D.-S.; Kim, Y.-G.; Chae, S.-W. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: A randomized controlled trial. Nutrition 2014, 30, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Y.; Dou, Z.; Wu, T.; Liu, R.; Sui, W.; Jin, Y.; Zhang, M. Black garlic melanoidins prevent obesity, reduce serum LPS levels and modulate the gut microbiota composition in high-fat diet-induced obese C57BL/6J mice. Food Funct. 2020, 11, 9585–9598. [Google Scholar] [CrossRef]
- Wang, D.; Feng, Y.; Liu, J.; Yan, J.; Wang, M.; Sasaki, J.-i.; Lu, C. Black garlic (Allium sativum) extracts enhance the immune system. Med. Aromat. Plant Sci. Biotechnol. 2010, 4, 37–40. [Google Scholar]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomed. Rep. 2014, 2, 250–254. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Wang, Z.; Chen, S.; Luo, Y. Production and identification of antioxidant and angiotensin-converting enzyme inhibition and dipeptidyl peptidase IV inhibitory peptides from bighead carp (Hypophthalmichthys nobilis) muscle hydrolysate. J. Funct. Foods 2017, 35, 224–235. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective effect of bergenin against cyclophosphamide-induced immunosuppression by immunomodulatory effect and antioxidation in Balb/c mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264. 7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Peng, X.; Sun, P.; Shi, J.; Yuan, X.; Zhu, J.; Tai, G.; Zhou, Y. Structural characterization and immunostimulatory activity of a novel linear α-(1→ 6)-D-glucan isolated from Panax ginseng CA Meyer. Glycoconj. J. 2012, 29, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, L.; Bai, R.; Zheng, X.; Ma, Y.; Gao, X.; Sun, B.; Hu, F. Structural characterization of a pectic polysaccharide from Codonopsis pilosula and its immunomodulatory activities in vivo and in vitro. Int. J. Biol. Macromol. 2017, 104, 1359–1369. [Google Scholar] [CrossRef]
- Yu, X.-H.; Liu, Y.; Wu, X.-L.; Liu, L.-Z.; Fu, W.; Song, D.-D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pei, F.; Shi, Y.; Zhao, L.; Fang, Y.; Hu, Q. Purification, characterization and anti-proliferation activity of polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2012, 88, 474–480. [Google Scholar] [CrossRef]
- Hwang, I.; Ahn, G.; Park, E.; Ha, D.; Song, J.-Y.; Jee, Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol. Lett. 2011, 138, 169–178. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, J.; Meng, Y.; Kang, Y.; Han, Z.; Tai, G.; Zhou, Y.; Cheng, H. Immunomodulatory effects of Hericium erinaceus derived polysaccharides are mediated by intestinal immunology. Food Funct. 2017, 8, 1020–1027. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, N.; Xu, G.; Jiang, P.; Huang, S.; Zhao, Q.; He, Y. Anti-tumor and immunomodulation activity of polysaccharides from Dendrobium officinale in S180 tumor-bearing mice. J. Funct. Foods 2022, 94, 105105. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, J.-H.; Yang, S.-B.; Hong, S.-G.; Lee, S.-A.; Hwang, S.-J.; Shin, K.-S.; Suh, H.-J.; Park, M.-H. A polysaccharide extracted from rice bran fermented with Lentinus edodes enhances natural killer cell activity and exhibits anticancer effects. J. Med. Food 2007, 10, 25–31. [Google Scholar] [CrossRef]
- Guo, W.; Xiang, Q.; Mao, B.; Tang, X.; Cui, S.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Protective effects of microbiome-derived inosine on lipopolysaccharide-induced acute liver damage and inflammation in mice via mediating the TLR4/NF-κB pathway. J. Agric. Food Chem. 2021, 69, 7619–7628. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, X.; Dou, Z.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Different molecular weight black garlic melanoidins alleviate high fat diet induced circadian intestinal microbes dysbiosis. J. Agric. Food Chem. 2021, 69, 3069–3081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, M.; Coldea, T.E.; Yang, H.; Zhao, H. Structure, chemical stability and antioxidant activity of melanoidins extracted from dark beer by acetone precipitation and macroporous resin adsorption. Food Res. Int. 2023, 164, 112045. [Google Scholar] [CrossRef]
- Zhu, G.; Luo, J.; Du, H.; Jiang, Y.; Tu, Y.; Yao, Y.; Xu, M. Ovotransferrin enhances intestinal immune response in cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2018, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Immunoenhancement effects of pentadecapeptide derived from Cyclina sinensis on immune-deficient mice induced by Cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex affects immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef]
- vom Steeg, L.G.; Klein, S.L. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef]
- Gunduz, M.; Ataca Atilla, P.; Atilla, E. New orders to an old soldier: Optimizing NK cells for adoptive immunotherapy in hematology. Biomedicines 2021, 9, 1201. [Google Scholar] [CrossRef]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell. Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Kang, M.; Oh, N.S.; Kim, M.; Ahn, H.Y.; Yoo, H.J.; Sun, M.; Kang, S.-H.; Yang, H.J.; Kwon, D.Y.; Lee, J.H. Supplementation of fermented Maillard-reactive whey protein enhances immunity by increasing NK cell activity. Food Funct. 2017, 8, 1718–1725. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef]
- Rajakaruna, S.; Pérez-Burillo, S.; Kramer, D.L.; Rufián-Henares, J.Á.; Paliy, O. Dietary Melanoidins from Biscuits and Bread Crust Alter the Structure and Short-Chain Fatty Acid Production of Human Gut Microbiota. Microorganisms 2022, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Gałęcka, M.; Basińska, A.; Bartnicka, A. The importance of intestinal microbiota in shaping human health—Implications in the practice of the family physician. Forum Med. Rodz. 2018, 4, 50–59. [Google Scholar]
- Wołkowicz, T.; Januszkiewicz, A.; Szych, J. Gut microbiome and its dysbiosis as an important factor influencing the human health condition. Med. Dosw. Mikrobiol. 2014, 66, 223–235. [Google Scholar] [PubMed]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The dynamic interplay between the gut microbiota and autoimmune diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Turnbaugh, P.J. Deconstructing mechanisms of diet-microbiome-immune interactions. Immunity 2020, 53, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Li, Y.; Bittinger, K.; Friedman, E.S.; Zhao, C.; Li, H.; Wu, G.D.; Trabulsi, J.C. The macronutrient composition of infant formula produces differences in gut microbiota maturation that associate with weight gain velocity and weight status. Nutrients 2022, 14, 1241. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.-D.; Li, H. The gut microbiota: Emerging evidence in autoimmune diseases. Trends Mol. Med. 2020, 26, 862–873. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, X.; Chen, T.; Peng, W.; Su, W. Chemical profile, antioxidative, and gut microbiota modulatory properties of Ganpu tea: A derivative of Pu-erh tea. Nutrients 2020, 12, 224. [Google Scholar] [CrossRef]
- Cahenzli, J.; Köller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; Van der Gaast-de Jongh, C.; Savelkoul, H.F.; de Jonge, M.I.; Faas, M.M.; Boekschoten, M.V. The impact of gut microbiota on gender-specific differences in immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef] [PubMed]




| Level | Primer | |||
|---|---|---|---|---|
| Time (min) | Temperature (°C) | Solid–Liquid Ratio | Ethanol Solution Concentration (%) | |
| −1 | 20 | 30 | 1:22 | 10 |
| 0 | 40 | 40 | 1:18 | 20 |
| 1 | 60 | 50 | 1:14 | 30 |
| Group | MLD | MLD-0 | MLD-20 | MLD-40 |
|---|---|---|---|---|
| X = retention time (rt) | 17.024 | 17.149 | 17.099 | 17.144 |
| Y = LgMW | 3.899898 | 3.856245 | 3.873706 | 3.857991 |
| MW (kDa) | 7.9 | 7.2 | 7.5 | 7.2 |
| Gender | Group | Initial Body Weigh (g) | Final Body Weight (g) | Index of Spleen (mg/g BW) | Index of Thymus (mg/g BW) |
|---|---|---|---|---|---|
| Female mouse (♀) | NC | 19.33 ± 0.93 | 20.29 ± 1.56 | 11.12 ± 0.93 | 3 ± 0.87 |
| CTX | 19.27 ± 0.58 | 17.79 ± 1.38 ## | 7.17 ± 0.62 ## | 2.25 ± 0.62 | |
| LD | 19.21 ± 1.1 | 19.08 ± 1.11 * | 8.4 ± 0.91 | 2.62 ± 1.48 | |
| MD | 19.27 ± 1.11 | 19.33 ± 0.68 * | 9.28 ± 1.62 ** | 2.87 ± 1.07 | |
| HD | 19.3 ± 0.88 | 18.67 ± 0.98 | 9.89 ± 1.21 ** | 2.72 ± 0.48 | |
| Male mouse (♂) | NC | 23.7 ± 0.66 | 24.28 ± 1.38 | 9.2 ± 1.58 | 1.51 ± 0.42 |
| CTX | 24.01 ± 1.24 | 20.85 ± 1.19 ## | 5.81 ± 0.34 ## | 1 ± 0.62 | |
| LD | 23.94 ± 1.18 | 22.79 ± 1.41 ** | 6.83 ± 0.7 | 1.46 ± 0.25 | |
| MD | 23.89 ± 1.31 | 23.96 ± 1.03 ** | 7.47 ± 1.39 * | 1.36 ± 0.38 | |
| HD | 23.36 ± 0.82 | 21.77 ± 1.14 | 7.71 ± 0.91 ** | 1.13 ± 0.46 |
| ug/g | NC | CTX | LD | MD | HD |
|---|---|---|---|---|---|
| Acetic Acid | 5.09 ± 0.04 | 4.1 ± 0.13 | 3.42 ± 0.2 | 3.64 ± 0.67 | 6.15 ± 1.37 * |
| Propanoic Acid | 8.12 ± 0.56 | 1.63 ± 0.1 ## | 2.14 ± 0.1 * | 2.92 ± 0.16 ** | 5.45 ± 0.13 ** |
| Butyric Acid | 2.03 ± 0.35 | 0.51 ± 0.04 ## | 0.72 ± 0.09 | 0.68 ± 0.06 | 1.66 ± 0.21 ## |
| Valeric Acid | 6.11 ± 0.47 | 0.75 ± 0.12 ## | 2.57 ± 0.16 ** | 3.03 ± 0.3 ** | 4.38 ± 0.89 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Xue, L.; Geng, X.; Wu, J.; Wu, T.; Zhang, M. Structural Characteristics and Immunomodulatory Effects of Melanoidins from Black Garlic. Foods 2023, 12, 2004. https://doi.org/10.3390/foods12102004
Song X, Xue L, Geng X, Wu J, Wu T, Zhang M. Structural Characteristics and Immunomodulatory Effects of Melanoidins from Black Garlic. Foods. 2023; 12(10):2004. https://doi.org/10.3390/foods12102004
Chicago/Turabian StyleSong, Xiwang, Liangyu Xue, Xiaoyuan Geng, Jianfu Wu, Tao Wu, and Min Zhang. 2023. "Structural Characteristics and Immunomodulatory Effects of Melanoidins from Black Garlic" Foods 12, no. 10: 2004. https://doi.org/10.3390/foods12102004
APA StyleSong, X., Xue, L., Geng, X., Wu, J., Wu, T., & Zhang, M. (2023). Structural Characteristics and Immunomodulatory Effects of Melanoidins from Black Garlic. Foods, 12(10), 2004. https://doi.org/10.3390/foods12102004











