Characterization of Physicochemical, Biological, and Chemical Changes Associated with Coconut Milk Fermentation and Correlation Revealed by 1H NMR-Based Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Preparation of Fermented Coconut Milk
2.3. pH and Total Titratable Acidity (TTA) during Storage
2.4. Microbial Analysis
2.5. Proximate Analysis of Fermented and Fresh Coconut Milk
2.6. Extraction of Bioactive Compounds from Fermented and Nonfermented Coconut Milks
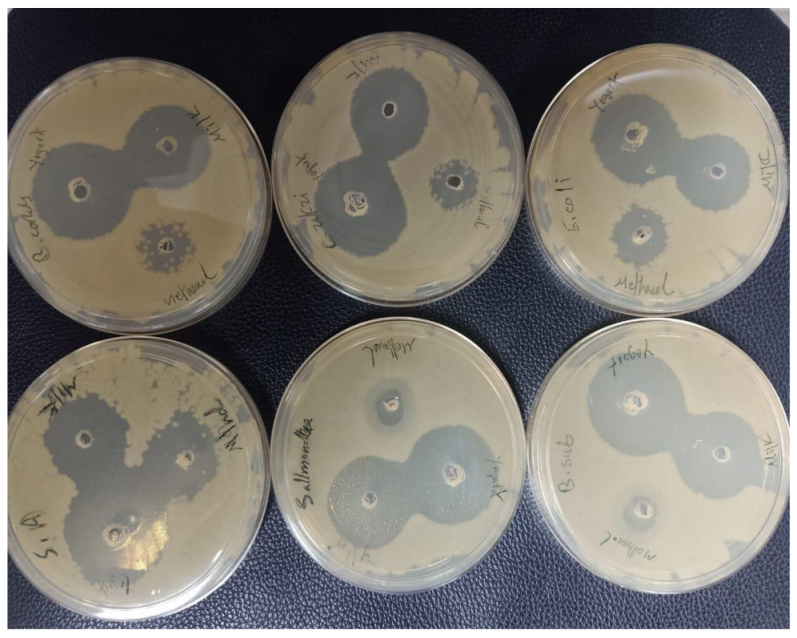
2.7. Antibacterial Activity of Fermented Coconut Milk
2.7.1. Determination of Antibacterial Activity by Microtiter Plate Assay
2.7.2. Antibacterial Activity by Well Diffusion Method
2.8. Antioxidant Activity of Fermented Coconut Milk
2.8.1. DPPH Radical Scavenging Activity Assay
2.8.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.9. NMR Measurement and Data Preprocessing
2.10. Statistical Analyses
3. Results and Discussion
3.1. pH and Total Titratable Acidity (TTA) during Cold Storage
3.2. Microbial Analysis during Cold Storage
3.3. Proximate Analysis of Fermented Coconut Milk and Fresh Coconut Milk
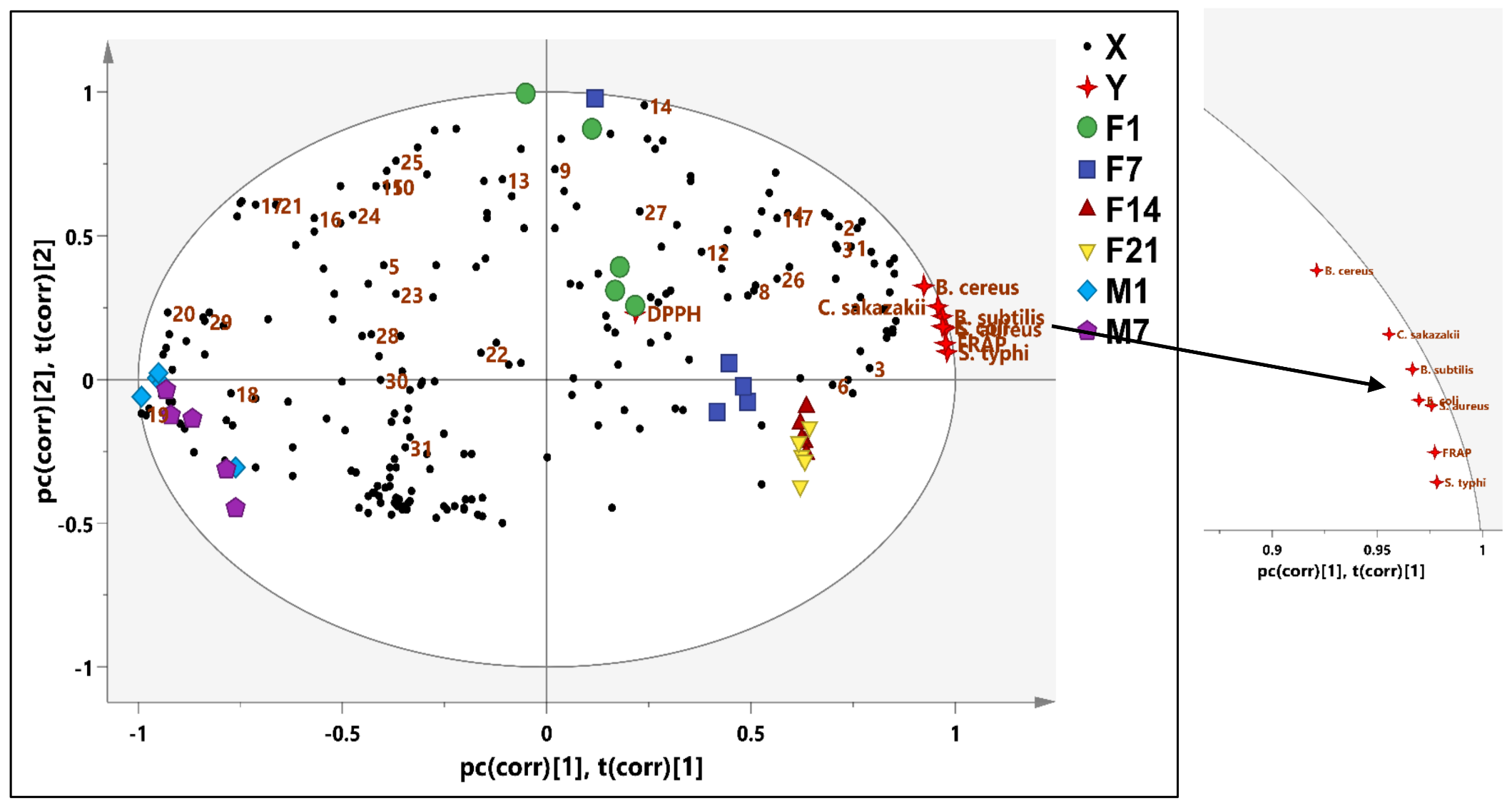
3.4. Antibacterial Activity of Fermented and Nonfermented Coconut Milk
3.5. Antioxidant Activity of Fermented and Pasteurized Coconut Milk
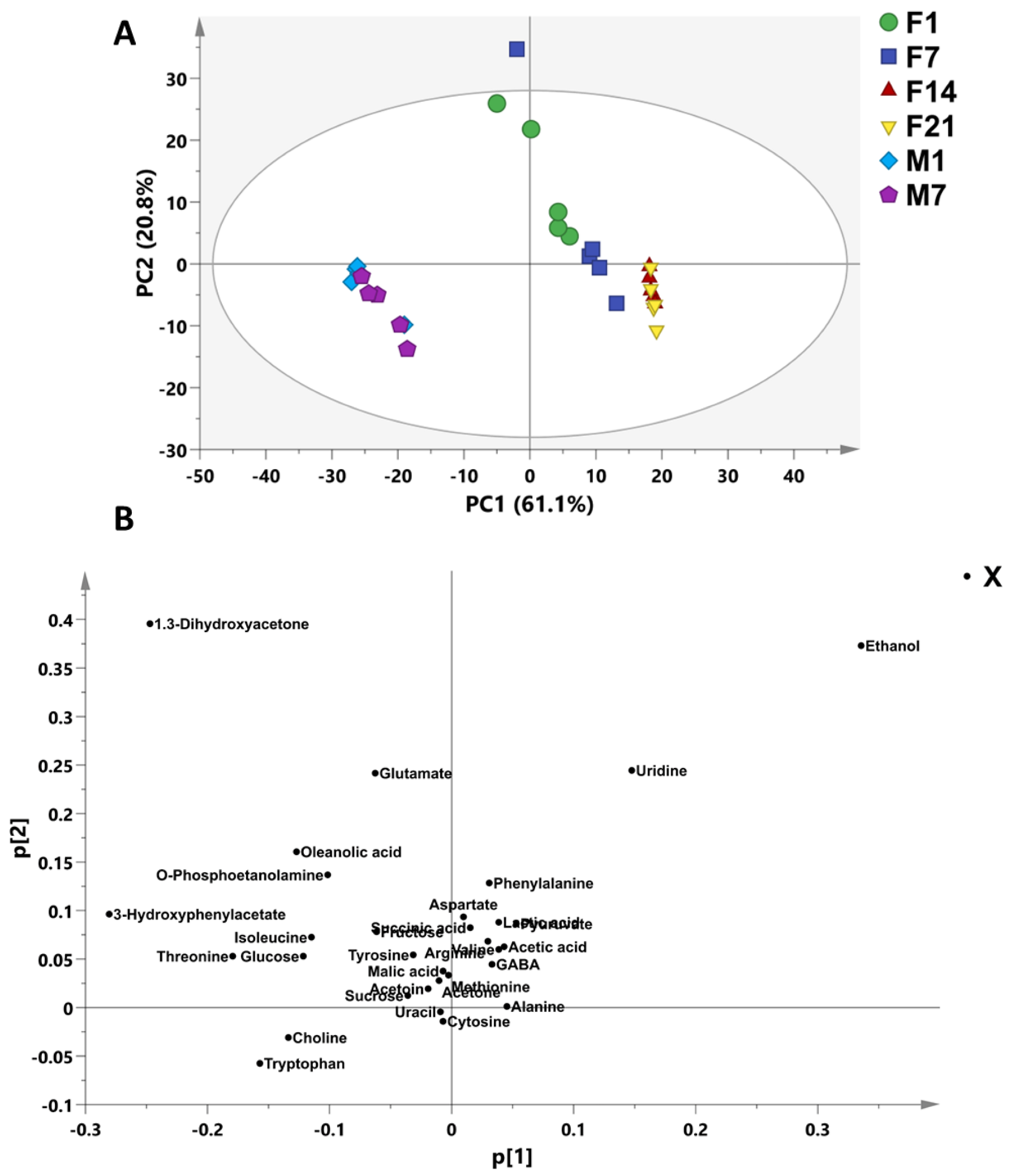
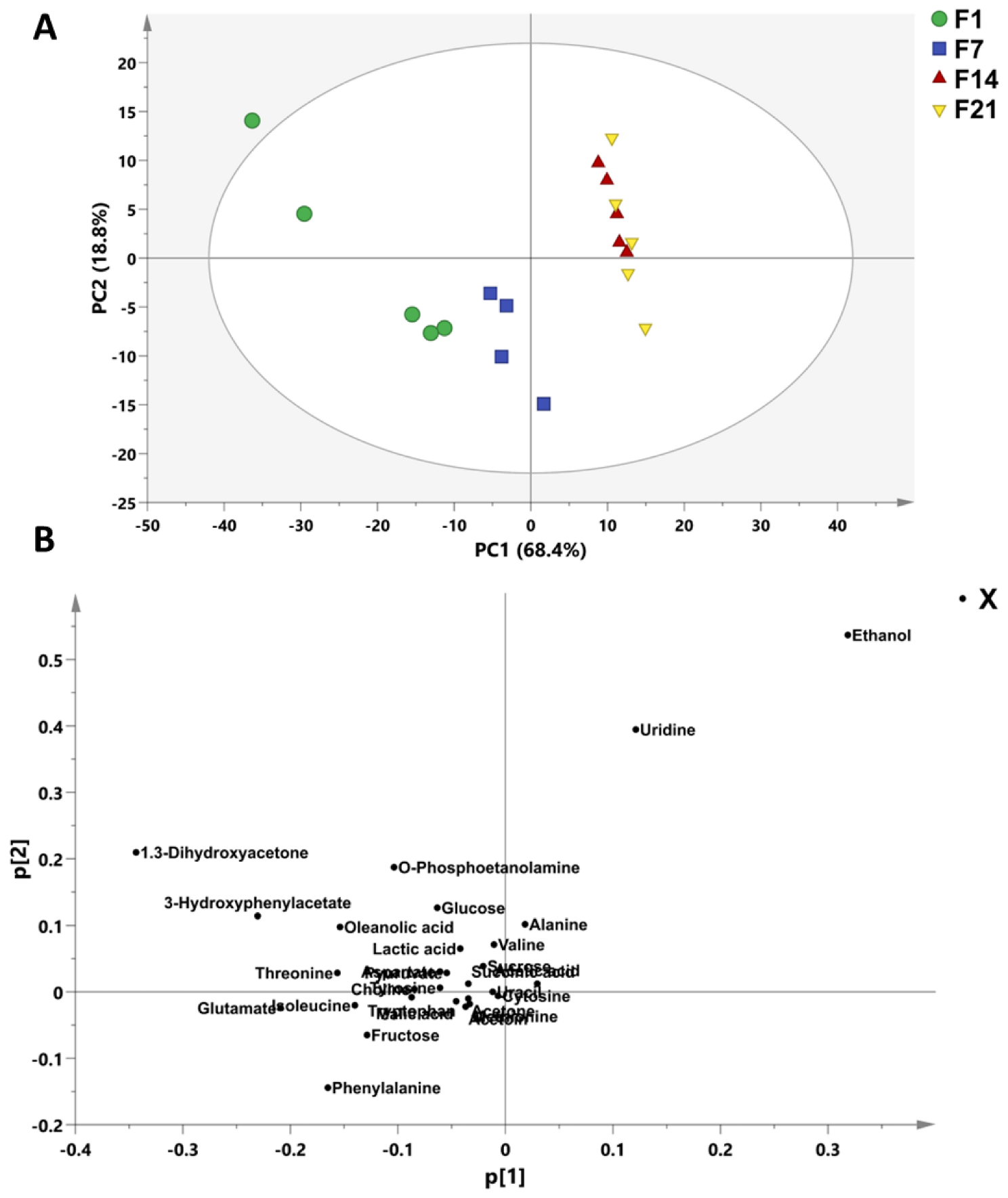
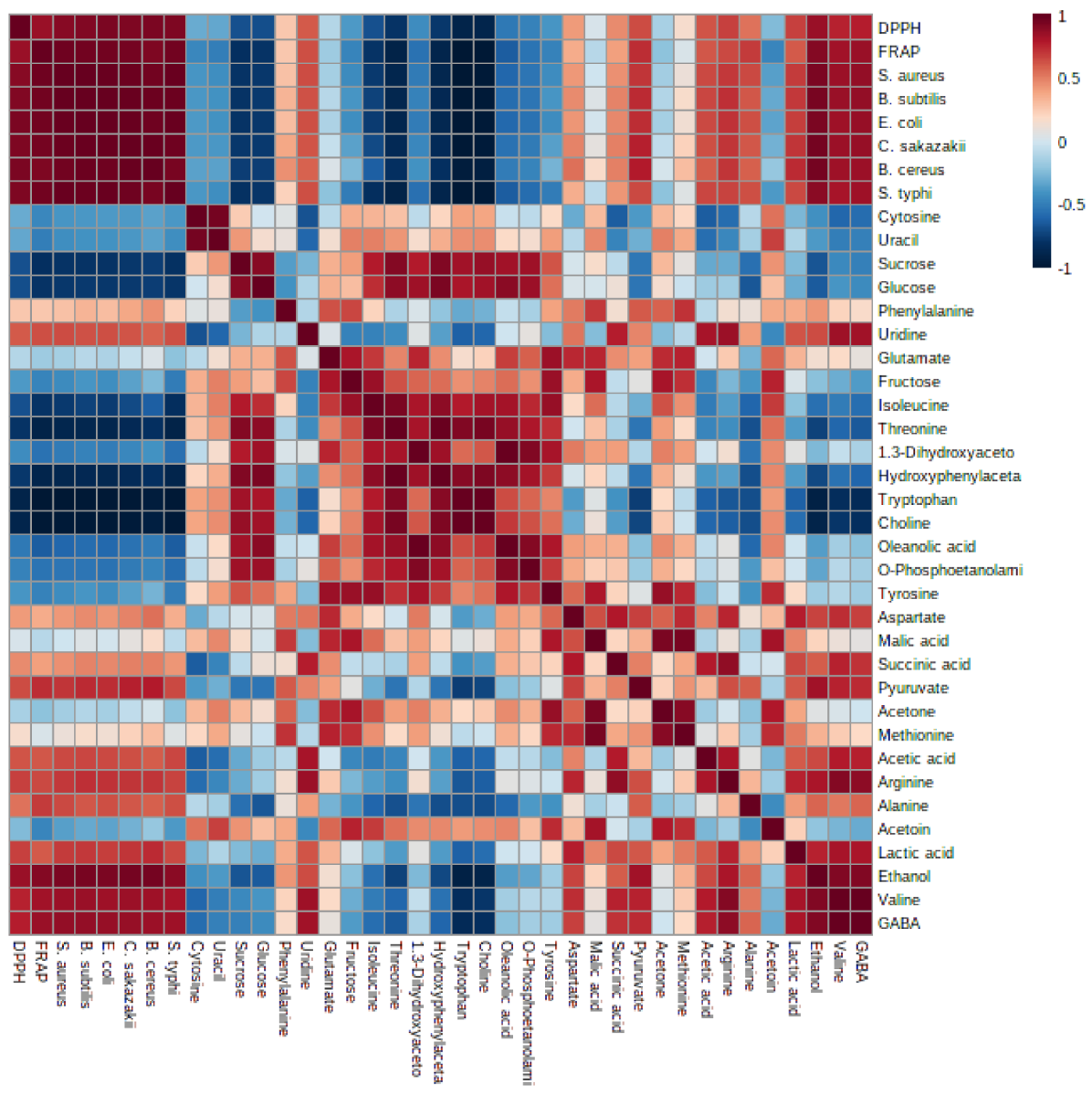
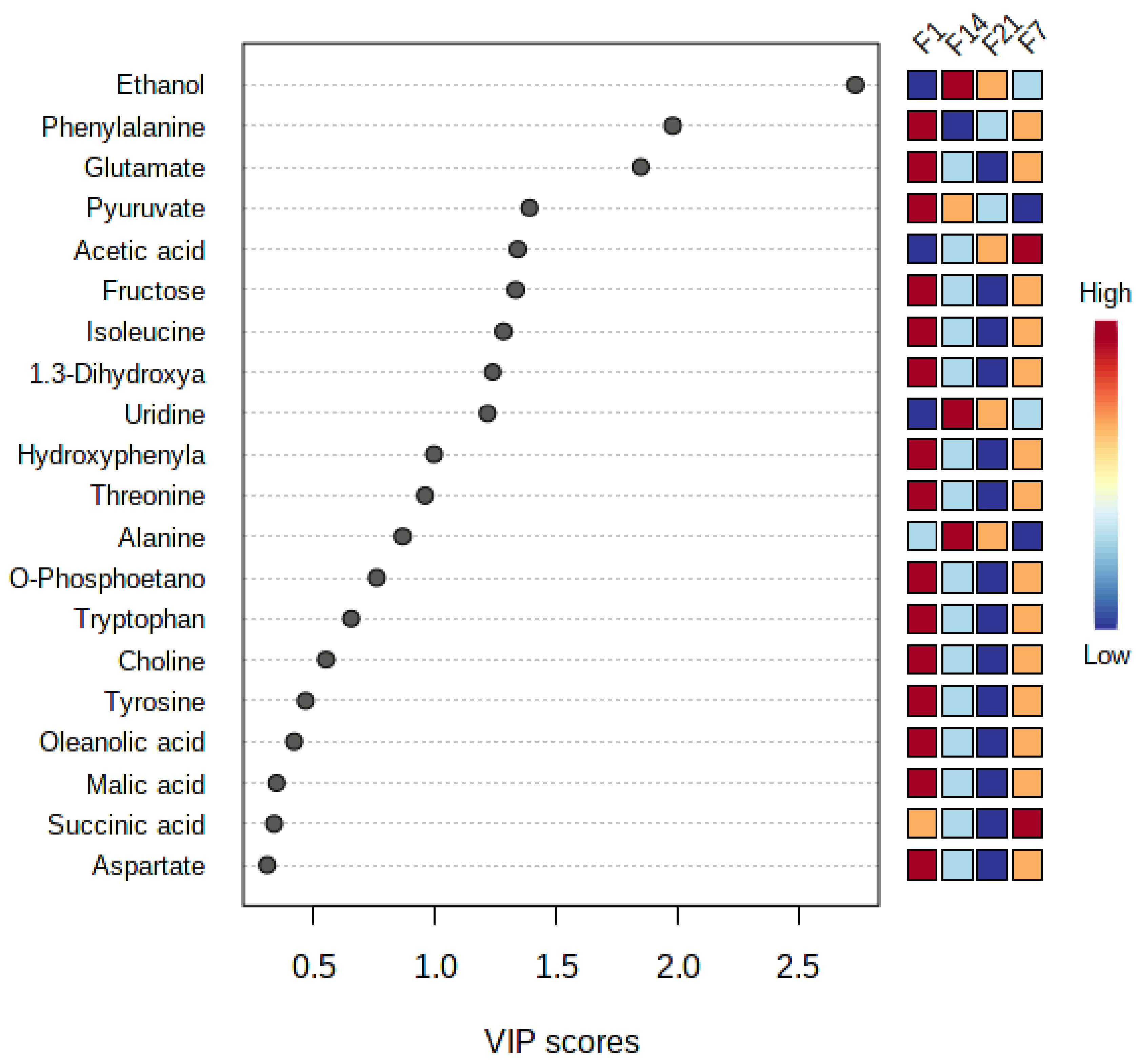
3.6. Bioactive Metabolites of Fermented Coconut Milk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bibra, M.; Krishnaraj, R.N.; Sani, R.K. Fermentation Strategies in the Food and Beverage Industry. In Biomolecular Engineering Solutions for Renewable Specialty Chemicals: Microorganisms, Products, and Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 141–164. [Google Scholar] [CrossRef]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimi-crobial and antifungal properties evaluation. Microorganisms 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Teshome, G. Review on lactic acid bacteria function in milk fermentation and preservation. Afr. J. Food Sci. 2015, 9, 170–175. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Amenakpor, J.; Atisey, S.; Odai, R.; Akpari, E.E.A. Production of coconut milk: A sustainable alternative plant-based milk. Case Stud. Chem. Environ. Eng. 2022, 6, 100206. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Samir, Z.T.; Jassim, M.A.; Saeed, S.K. Effect of different extraction methods on physicochemical properties, antioxidant activity, of virgin coconut oil. Mater. Today Proc. 2021, 42, 2000–2005. [Google Scholar] [CrossRef]
- Pachekrepapol, U.; Kokhuenkhan, Y.; Ongsawat, J. Formulation of yogurt-like product from coconut milk and evaluation of physicochemical, rheological, and sensory properties. Int. J. Gastron. Food Sci. 2021, 25, 100393. [Google Scholar] [CrossRef]
- Abdusalam, K.B.; Yee, L.S.; Mediani, A.; Akhtar, M.T.; Buzgaia, N.; Rukayadi, Y.; Ismail, I.S.; Shaari, K. 1H NMR-based metabolomics profiling of Syzygium grande and Oenanthe javanica and relationship between their metabolite compositions and antimicrobial activity against Bacillus species. Rec. Nat. Prod. 2022, 16, 128–143. [Google Scholar]
- Kantachote, D.; Ratanaburee, A.; Hayisama-Ae, W.; Sukhoom, A.; Nunkaew, T. The use of potential probiotic Lactobacillus plantarum DW12 for producing a novel functional beverage from mature coconut water. J. Funct. Foods 2017, 32, 401–408. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis, Vol. 222; Association of Official Analytical Chemists: Washington, DC, USA, 1975. [Google Scholar]
- de Pasquale, I.; di Cagno, R.; Buchin, S.; de Angelis, M.; Gobbetti, M. Use of autochthonous mesophilic lactic acid bacteria as starter cultures for making Pecorino Crotonese cheese: Effect on compositional, microbiological and biochemical attributes. Food Res. Int. 2019, 116, 1344–1356. [Google Scholar] [CrossRef]
- Arulrajah, B.; Muhialdin, B.J.; Zarei, M.; Hasan, H.; Saari, N. Lacto-fermented Kenaf (Hibiscus cannabinus L.) seed protein as a source of bioactive peptides and their applications as natural preservatives. Food Control 2019, 110, 106969. [Google Scholar] [CrossRef]
- Awin, T.; Mediani, A.; Maulidiani; Shaari, K.; Faudzi, S.M.M.; Sukari, M.A.H.; Lajis, N.; Abas, F. Phytochemical profiles and biological activities of Curcuma species subjected to different drying methods and solvent systems: NMR-based metabolomics approach. Ind. Crop. Prod. 2016, 94, 342–352. [Google Scholar] [CrossRef]
- Gueimonde, M.; Delgado, S.; Mayo, B.; Ruas-Madiedo, P.; Margolles, A.; de los Reyes-Gavilán, C.G. Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res. Int. 2004, 37, 839–850. [Google Scholar] [CrossRef]
- Yaakob, H.; Ahmed, N.R.; Daud, S.K.; Malek, R.A.; Rahman, R.A. Optimization of ingredient and processing levels for the production of coconut yogurt using response surface methodology. Food Sci. Biotechnol. 2012, 21, 933–940. [Google Scholar] [CrossRef]
- Han, C.E.; Ewe, J.-A.; Kuan, C.-S.; Yeo, S.K. Growth characteristic of probiotic in fermented coconut milk and the antibacterial properties against Streptococcus pyogenes. J. Food Sci. Technol. 2021, 59, 3379–3386. [Google Scholar] [CrossRef]
- Fauziah, R.; Malaka, R.; Yuliati, F.N. Titratable acidity and pH changes of pasteurized milk by addition of Roselle flower extract in the refrigerator storage. IOP Conf. Ser. Earth Environ. Sci. 2020, 492. [Google Scholar] [CrossRef]
- Obi, T.E.; Henshaw, F.O.; Atanda, O.O. Quality evaluation of plain-stirred probiotic yoghurt produced from skim and whole milk powder during refrigerated storage. Electron. J. Environ. Agric. Food Chem. 2010, 9, 1203–1213. [Google Scholar]
- da Costa, M.P.; Frasao, B.D.S.; Lima, B.R.C.D.C.; Rodrigues, B.L.; Junior, C.A.C. Simultaneous analysis of carbohydrates and organic acids by HPLC-DAD-RI for monitoring goat’s milk yogurts fermentation. Talanta 2016, 152, 162–170. [Google Scholar] [CrossRef]
- Donkor, O.; Henriksson, A.; Vasiljevic, T.; Shah, N. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int. Dairy J. 2006, 16, 1181–1189. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (EPS) producing lactic acid bacteria strains. LWT-Food Sci. Technol. 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Stijepic, D.; Glusac, M.; Durdevic, J.; Pesic, D. Physicochemical characteristics of soy probiotic yoghurt with inulin additon during the refrigerated storage. Rom. Biotechnol. Lett. 2013, 18, 8077–8085. [Google Scholar]
- Hekmat, S.; Soltani, H.; Reid, G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov. Food Sci. Emerg. Technol. 2009, 10, 293–296. [Google Scholar] [CrossRef]
- Madureira, A.R.; Amorim, M.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Protective effect of whey cheese matrix on probiotic strains exposed to simulated gastrointestinal conditions. Food Res. Int. 2011, 44, 465–470. [Google Scholar] [CrossRef]
- Adebanke, B.M.; Kemisola, A.A.; Lola, K.F.; Mayowa, I. Effect of Partial Substitution of Cow Milk With Bambara. Ann. Food Sci. Technol. 2017, 18, 92–99. [Google Scholar]
- Handayani, F.F.; Malaka, R.; Maruddin, F. Total bacteria and pH changes of matoa leaf-pasteurized milk in refrigerator storage. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012047. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of high pressure processing on the safety, shelf life and quality of raw milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Falade, K.O.; Ogundele, O.M.; Ogunshe, A.O.; Fayemi, O.; Ocloo, F. Physico-chemical, sensory and microbiological characteristics of plain yoghurt from bambara groundnut (Vigna subterranea) and soybeans (Glycine max). J. Food Sci. Technol. 2015, 52, 5858–5865. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.; Shaker, R.; Osaili, T.; Al-Taani, M.; Olaimat, A.; Awaisheh, S.; Abushelaibi, A.; Holley, R. Sensory evaluation of flavored soy milk-based yogurt: A comparison between Jordanian and Malaysian con-sumers. J. Food Sci. Eng. 2014, 4, 27–35. [Google Scholar]
- Bokulich, N.A.; Mills, D.A. Facility-Specific “House” Microbiome Drives Microbial Landscapes of Artisan Cheesemaking Plants. Appl. Environ. Microbiol. 2013, 79, 5214–5223. [Google Scholar] [CrossRef]
- Ali, A.A.; Abdelgadir, W.S. Incidence of Escherichia coli in raw cow’s milk in Khartoum State. Br. J. Dairy Sci. 2011, 2, 23–26. Available online: http://maxwellsci.com/print/bjds/v2-23-26.pdf (accessed on 14 July 2022).
- Chowdhury, N.A.; Kamruzzaman, P.; Zaman, W. Study on the quality assessment of curd (dahi), locally available in Bangladeshi market. World J. Dairy Food Sci. 2011, 6, 15–20. [Google Scholar]
- Ranieri, M.; Boor, K. Short communication: Bacterial ecology of high-temperature, short-time pasteurized milk processed in the United States. J. Dairy Sci. 2009, 92, 4833–4840. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Gharibi, I.; Al-Abri, A.; Al-Humaimi, A.; Al-Nabhani, F.; Al-Hashmi, H.; Al-Sarmi, K.; Al-Shibli, S. Evaluating the shelf-life of pasteurized milk in Oman. Heliyon 2021, 7, e06555. [Google Scholar] [CrossRef]
- Shrestha, S.L.; Yadav, R.S. Preparation and Quality Evaluation of Soy Corn Yoghurt. Himal. J. Sci. Technol. 2018, 2, 102–107. [Google Scholar] [CrossRef]
- Priyadarshani, W.M.; Muthumuniarachchi, M.A. Physico-chemical and sensory quality of mung bean (Vigna radiata) enriched stirred yoghurt. Int. Food Res. J. 2018, 25, 2051–2055. [Google Scholar]
- Rawat, S. Food Spoilage: Microorganisms and their prevention Microbial Food Spoilage-Losses and Cont rol St rat egies A Brief Review of t he Lit erat ure. Asian J. Plant Sci. Res. 2015, 5, 47–56. Available online: www.pelagiaresearchlibrary.com (accessed on 2 June 2022).
- Ladokun, O.; Oni, S. Fermented Milk Products from Different Milk Types. Food Nutr. Sci. 2014, 05, 1228–1233. [Google Scholar] [CrossRef]
- Obadina, A.; Akinola, O.; Shittu, T.; Bakare, H. Effect of Natural Fermentation on the Chemical and Nutritional Composition of Fermented Soymilk Nono. Niger. Food J. 2013, 31, 91–97. [Google Scholar] [CrossRef]
- Ojokoh, A.O.; Alade, R.A.; Ozabor, P.T.; Fadahunsi, I.F. Effect of fermentation on sorghum and cowpea flour blends. J. Agric. Biotechnol. Sustain. Dev. 2020, 12, 39–49. [Google Scholar]
- Terefe, Z.K.; Omwamba, M.N.; Nduko, J.M. Effect of solid state fermentation on proximate composition, antinutritional factors and in vitro protein digestibility of maize flour. Food Sci. Nutr. 2021, 9, 6343–6352. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Sinamo, K.N.; Hasan, F.; Hasanah, U. Effect of concentration starter and fermentation time on the quality of yoghurt drink from buffalo milk. J. Physics Conf. Ser. 2020, 1542, 012070. [Google Scholar] [CrossRef]
- Vandarkuzhali, P.; Sangeetha, N. Effect of Fermentation and Dehydration on the Nutritional and Functional Properties of Horse Gram (Macrotyloma uniflorum) Flour. Int. J. Food Ferment. Technol. 2016, 6, 129. [Google Scholar] [CrossRef]
- Rahmawati, H.; Purnomo, A.J.; Umniyatie, S.; Pramiadi, D.; Sari, N. Identification and Characterization of Chitinase Enzyme Producing Bacteria from Bat Guano and its Potential to Inhibit The Growth of Fungus Colletotrichum Sp. Cause Anthracnose on the Chili by In Vitro. Int. J. Adv. Agric. Environ. Eng. 2016, 3, 249. [Google Scholar] [CrossRef]
- Lakshmi, T.S.; MaryPramela, A.; Iyer, P. Anti-microbial, anti-fungal and anti-carcinogenic properties of coconut milk kefir. Int. J. Home Sci. 2017, 3, 365–369. Available online: www.homesciencejournal.com (accessed on 12 June 2022).
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef]
- Lima, M.D.S.F.D.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.M.P.B.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian Kefir-Fermented Sheep’s Milk, a Source of Antimicrobial and Antioxidant Peptides. Probiotics Antimicrob. Proteins 2018, 10, 446–455. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Channip, A.-A.; Hwa, P.C.H.; Ja’Afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Muzolf, M.; Szymusiak, H.; Gliszczyńska-Świgło, A.; Rietjens, I.M.; Tyrakowska, B. pH-Dependent Radical Scavenging Capacity of Green Tea Catechins. J. Agric. Food Chem. 2008, 56, 816–823. [Google Scholar] [CrossRef]
- Nebbia, S.; Lamberti, C.; Bianco, G.L.; Cirrincione, S.; Laroute, V.; Cocaign-Bousquet, M.; Cavallarin, L.; Giuffrida, M.G.; Pessione, E. Antimicrobial Potential of Food Lactic Acid Bacteria: Bioactive Peptide Decrypting from Caseins and Bacteriocin Production. Microorganisms 2021, 9, 65. [Google Scholar] [CrossRef]
- Maryam, A.A.; Zaiton, H.; Mohamed, M.A. Antioxidant activity of lactic acid bacteria (LAB) fermented skim milk as determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ferrous chelating activity (FCA). Afr. J. Microbiol. Res. 2012, 6, 6358–6364. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of Fermentation with Different Lactic Acid Bacteria and in Vitro Digestion on the Biotransformation of Phenolic Compounds in Fermented Pomegranate Juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; El-Hussein, A.; Mohamed, M.S.M. Enhancement of Labneh Quality by Laser-Induced Modulation of Lactocaseibacillus casei NRRL B-1922. Fermentation 2022, 8, 132. [Google Scholar] [CrossRef]
- Nematollahi, A.; Sohrabvandi, S.; Mortazavian, A.M.; Jazaeri, S. Viability of probiotic bacteria and some chemical and sensory characteristics in cornelian cherry juice during cold storage. Electron. J. Biotechnol. 2016, 21, 49–53. [Google Scholar] [CrossRef]
- Kurnia, Y.F.; Suharto, E.L.S.; Purwati, E. Quality of fermented goat milk with carrot juice during cold storage. IOP Conf. Ser. Earth Environ. Sci. 2021, 694, 012076. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Salmerón, I.; Loeza-Serrano, S.; Pérez-Vega, S.; Pandiella, S.S. Headspace gas chromatography (HS-GC) analysis of imperative flavor compounds in Lactobacilli-fermented barley and malt substrates. Food Sci. Biotechnol. 2015, 24, 1363–1371. [Google Scholar] [CrossRef]
- Hernandez-Valdes, J.A.; de Stegge, M.A.; Hermans, J.; Teunis, J.; van Tatenhove-Pel, R.J.; Teusink, B.; Bachmann, H.; Kuipers, O.P. Enhancement of amino acid production and secretion by Lactococcus lactis using a droplet-based biosensing and selection system. Metab. Eng. Commun. 2020, 11, e00133. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT-Food Sci. Technol. 2015, 61, 263–268. [Google Scholar] [CrossRef]
- Medoua, G.N.; Mbome, I.; Agbor-Egbe, T.; Mbofung, C. Influence of fermentation on some quality characteristics of trifoliate yam (Dioscorea dumetorum) hardened tubers. Food Chem. 2008, 107, 1180–1186. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Kadum, H.; Hussin, A.S.M. Metabolomics profiling of fermented cantaloupe juice and the potential application to extend the shelf life of fresh cantaloupe juice for six months at 8 °C. Food Control 2021, 120, 107555. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Antimicrobial properties of traditional kefir: An in vitro screening for antagonistic effect on Salmonella Typhimurium and Salmonella Arizonae. Int. Dairy J. 2022, 124, 105180. [Google Scholar] [CrossRef]
- Lu, H.J.; Breidt, F.; Pérez-Díaz, I.M.; Osborne, J.A.; Breidt, J.F. Antimicrobial Effects of Weak Acids on the Survival of Escherichia coli O157:H7 under Anaerobic Conditions†‡. J. Food Prot. 2011, 74, 893–898. [Google Scholar] [CrossRef]
- Brassica, M.; Huang, S.; Chen, X.; Yan, R.; Huang, M.; Chen, D. Isolation, identification and antibacterial mechanism the main antibacterial component from pickled and dried mustard (Brassica juncea Coss. Var. foliosa Bailey). Molecules 2022, 27, 2418. [Google Scholar]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Pramai, P.; Hamid, N.A.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Jiamyangyuen, S. Metabolite profiling, antioxidant, and α-glucosidase inhibitory activities of germinated rice: Nuclear-magnetic-resonance-based metabolomics study. J. Food Drug Anal. 2018, 26, 47–57. [Google Scholar] [CrossRef]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]

| Parameters | Fermented Coconut Milk | Pasteurized Coconut Milk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days of Storage | ||||||||||
| 1 | 7 | 14 | 21 | 28 | 1 | 7 | 14 | 21 | 28 | |
| pH | 4.26 a ± 0.10 | 4.20 a ± 0.11 | 4.17 a ± 0.12 | 4.13 a ± 0.32 | 3.92 b ± 0.02 | 6.04 c ± 0.08 | 5.92 c ± 0.07 | 5.65 d ± 0.05 | 5.39 e ± 0.03 | 5.09 f ± 0.04 |
| Acidity | 0.7 a ± 0.02 | 0.82 b ± 0.02 | 0.92 b ± 0.01 | 0.93 c ± 0.02 | 1.1 d ± 0.02 | 0.15 d ± 0.005 | 0.22 e ± 0.03 | 0.32 ef ± 0.02 | 0.35 f ± 0.01 | 0.41 g ± 0.03 |
| Lactic acid bacteria | 3.43 × 108 a ± 0.41 | 5.26 × 108 b ± 0.25 | 6.4 × 108 c ± 0.49 | 2.1 × 108 d ± 0.26 | 1.6 × 108 e ± 0.15 | Absent ≤1.0 × 101 | 4.6 × 102 f ± 0.17 | 4.3 × 103 g ± 0.25 | 3.1 × 104 h ± 0.20 | 6.2 × 10 4 i ± 0.25 |
| Total yeast and mold | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | 1.7 × 102 a ±0.30 | 1.2 × 104 b ±0.15 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 |
| Coliform | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | 2.7 × 101 a ± 0.15 | 3.7 × 102 b ± 0.28 | 4.7 × 102 c ± 0.35 |
| E. coli | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | Absent ≤1.0 × 101 | 1.4 × 101± 0.28 | 2.6 × 102 ± 0.30 | 3.4 × 102 ± 0.20 |
| Parameters | Fermented Coconut Milk (kcal/100 g) | Pasteurized Coconut Milk (kcal/100 g) |
|---|---|---|
| Total fat | 19.37 a ± 0.11 | 19.27 a ± 0.05 |
| Carbohydrate | 2.51 a ± 0.025 | 4.13 b± 0.034 |
| Energy | 193.3 a ± 0.57 | 197.3 b ± 0.57 |
| Protein | 2.31 a ± 0.155 | 1.86 b ± 0.096 |
| Moisture | 75.14 a ± 0.015 | 74.05 b ± 0.01 |
| Total Ash | 0.66 a ± 0.02 | 0.68 a ± 0.02 |
| Total Solid | 24.85 a ± 0.015 | 25.95 b ± 0.01 |
| Samples | S. aureus | B. subtilis | E. coli | C. sakazakii | B. cereus | S. Typhi |
|---|---|---|---|---|---|---|
| M1 | 41.86 a ± 1.56 | 41.36 a ± 1.26 | 40.2 a ±1.16 | 45.6 a ± 0.60 | 41.26 a ± 1.20 | 40.3 a ± 0.91 |
| M7 | 41.73 a ± 1.45 | 43.33 a ± 1.00 | 42.63 a ± 0.75 | 44.93 a ± 0.66 | 40.3 a ± 1.11 | 39.9 a ± 1.41 |
| F1 | 76.93 b ± 2.07 | 83.93 b ± 2.12 | 81.66 b ± 2.15 | 86.63 b ± 2.70 | 90.06 b ± 2.66 | 71.9 b ± 2.45 |
| F7 | 89.0 c ± 0.45 | 93.30 c ±1.37 | 90.70 c ± 2.05 | 92.6 c ±2.06 | 94.03 b ± 1.10 | 85.90 c ± 2.11 |
| F14 | 91.13 c ±0.72 | 93.7 c ±1.96 | 95.3 c ± 1.41 | 93.66 c ± 1.05 | 94.73 b ± 2.32 | 89.60 c ± 2.04 |
| F21 | 88.53 c ±1.40 | 88.63 bc ± 0.90 | 85.06 b ± 1.35 | 86.43 b ± 2.24 | 80.73 c ± 2.15 | 83.43 c ± 1.95 |
| Samples | S. aureus | B. subtilis | E. coli | C. sakazakii | B. cereus | S. Typhi |
|---|---|---|---|---|---|---|
| M1 | 9.33 a ± 0.57 | 10.66 a ± 1.52 | 9.33 a ± 0.57 | 10.66 a ± 0.57 | 8.33 a ± 1.15 | 8.66 a ± 1.52 |
| M7 | 8.66 a ± 0.57 | 9.66 a ± 0.57 | 8.66 a ± 0.57 | 8.33 b ± 0.57 | 8.66 a ± 1.15 | 8.33 a ± 0.57 |
| F1 | 13.33 b ± 1.52 | 14.33 b ± 1.15 | 14.0 b ± 1 | 15.33 c ± 1.52 | 13.66 b ± 1.15 | 14.33 b ± 0.57 |
| F7 | 16.33 c ± 0.57 | 18.66 c ± 1.52 | 21.33 c ± 2.08 | 20.66 d ± 1.52 | 15.33 bd ± 1.52 | 14 b ± 1.73 |
| F14 | 20 d ± 1 | 22.66 d ± 2.08 | 22.0 c ± 2 | 22.66 d ± 1.52 | 20.66 c ± 1.15 | 23 c ± 1.73 |
| F21 | 15.33 bc ± 1.52 | 16 bc ± 1.73 | 14.33 b ± 2.08 | 14.33 c ± 1.5 | 17.33 d ± 1.52 | 18.66 d ± 1.52 |
| Sample | DPPH (%) | FRAP (mmol TE/g) |
|---|---|---|
| M1 | 48.0 a ± 0.022 | 20.38 a ± 0.17 |
| M7 | 46.0 a ± 0.015 | 17.80 a ± 0.90 |
| F1 | 59 b ± 0.011 | 53.53 b ± 1.64 |
| F7 | 65.0 c ± 0.012 | 56.70 b ± 2.66 |
| F14 | 67.17 c ± 0.024 | 61.96 c ± 0.78 |
| F21 | 58.0b ± 0.014 | 55.11b ± 2.68 |
| Metabolites | 1H NMR Signals | Fermented Coconut Milk | Pasteurized Coconut Milk |
|---|---|---|---|
| Ethanol | 1.22 (t, J = 7.0 Hz) | + | - |
| Valine | 1.02 (m) | + | - |
| ϒ-Aminobutyric acid (GABA | 1.90 (m), 0.98 (t) | + | - |
| Arginine | 1.78 (m). 3.82 (t) | + | - |
| Lactic acid | 1.31 (d, J = 6.5) | + | - |
| Acetoin | δ 1.42 (s) | + | - |
| Alanine | 1.5 (d, J = 7.0 Hz) | + | - |
| Threonine | 3.62 (d, J = 7.0 Hz) | - | + |
| Phenylalanine | 3.9 (q) | + | - |
| Acetic acid | 1.96 (s) | + | - |
| Methionine | 2.13 (s) | + | - |
| Isoleucine | 3.72 (t) | - | + |
| Acetone | 2.21 (s) | + | - |
| Pyruvate | 2.39 (s) | + | - |
| Succinic acid | 2.63 (s) | + | - |
| Malic acid | 2.76 (dd, J = 13.5, 2.5 Hz | + | - |
| Aspartate | 2.82 (dd, J = 17.0 Hz, 8.5 Hz) | + | - |
| Tyrosine | 3.02 (dd) | + | - |
| O- Phosphoetanolamine | 3.18 (m) | - | + |
| Oleanolic acid | 3.21 (dd, J = 4.0, 3.5 Hz) | - | + |
| Choline | δ 3.27 (s) | - | + |
| Tryptophan | 3.31 (dd, J= 13.4, 5.0 Hz) | + | - |
| 3-Hydroxyphenylacetate | 3.5 (s) | - | + |
| 1.3-Dihydroxyacetone | 3.58 (d, J = 4.2 Hz) | - | + |
| Fructose | 3.78–3.82 (m) | - | + |
| Glutamate | 3.75(m) | - | + |
| Uridine | 3.92 (dd, J = 12.0, 3.8 Hz) | + | - |
| Glucose | 5.20 (d, J = 3.5 Hz) | - | + |
| Sucrose | 5.42 (d, J = 3.5 Hz) | - | + |
| Uracil | 5.78(d, J = 7.6 Hz) | + | - |
| Cytosine | 5.92(d, J = 8.0 Hz) | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadi, W.S.M.; Mediani, A.; Benchoula, K.; Wong, E.H.; Misnan, N.M.; Sani, N.A. Characterization of Physicochemical, Biological, and Chemical Changes Associated with Coconut Milk Fermentation and Correlation Revealed by 1H NMR-Based Metabolomics. Foods 2023, 12, 1971. https://doi.org/10.3390/foods12101971
Qadi WSM, Mediani A, Benchoula K, Wong EH, Misnan NM, Sani NA. Characterization of Physicochemical, Biological, and Chemical Changes Associated with Coconut Milk Fermentation and Correlation Revealed by 1H NMR-Based Metabolomics. Foods. 2023; 12(10):1971. https://doi.org/10.3390/foods12101971
Chicago/Turabian StyleQadi, Wasim S. M., Ahmed Mediani, Khaled Benchoula, Eng Hwa Wong, Norazlan Mohmad Misnan, and Norrakiah Abdullah Sani. 2023. "Characterization of Physicochemical, Biological, and Chemical Changes Associated with Coconut Milk Fermentation and Correlation Revealed by 1H NMR-Based Metabolomics" Foods 12, no. 10: 1971. https://doi.org/10.3390/foods12101971
APA StyleQadi, W. S. M., Mediani, A., Benchoula, K., Wong, E. H., Misnan, N. M., & Sani, N. A. (2023). Characterization of Physicochemical, Biological, and Chemical Changes Associated with Coconut Milk Fermentation and Correlation Revealed by 1H NMR-Based Metabolomics. Foods, 12(10), 1971. https://doi.org/10.3390/foods12101971







