Supplemental C Addressed the pH Conundrum in Sustainable Marine Aquaponic Food Production Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Aquaponic System Design
2.2. Biological Material
2.2.1. Shrimp
2.2.2. Plant Material and Germination Conditions
2.3. Experimental Design and System Management
2.4. Measurement of Water Quality
2.5. Growth Measurements
2.5.1. Shrimp
2.5.2. Plants
2.6. Statistical Analysis
3. Results
3.1. Shrimp
3.2. Plants
3.2.1. Growth and Yield of Plant
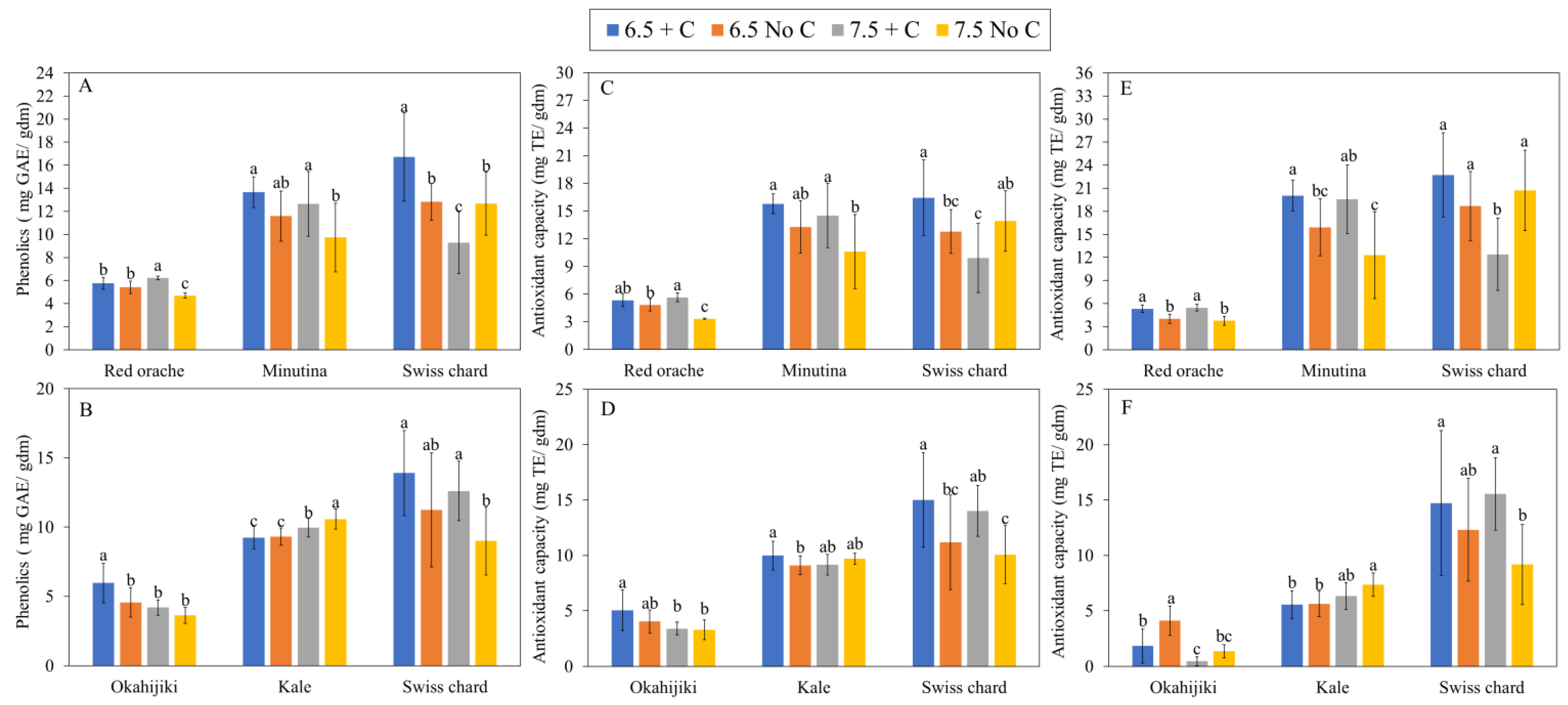
3.2.2. Mineral Nutrients, Total Phenolics Content, and Antioxidant Capacity
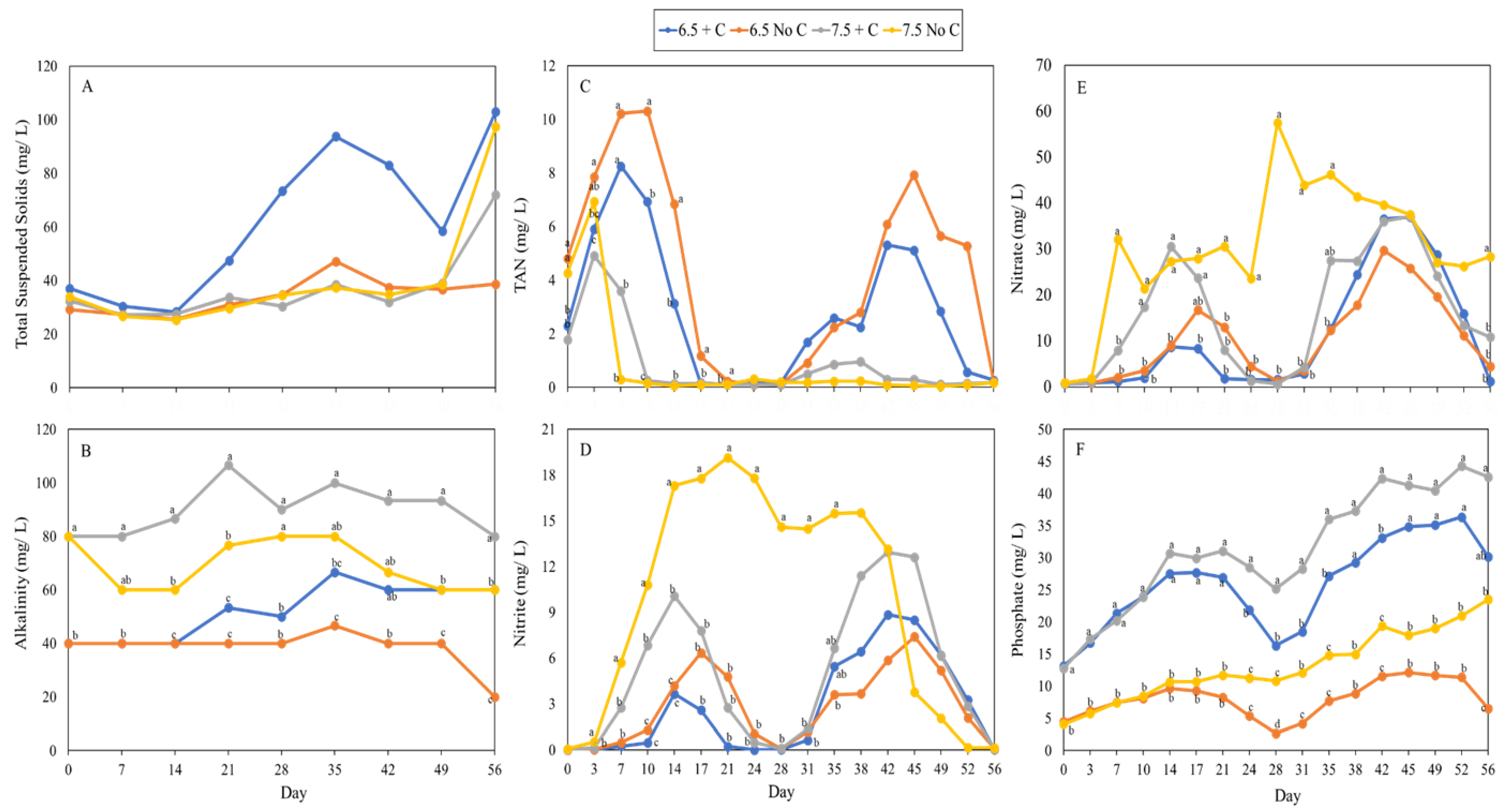
3.3. Water Quality
4. Discussion
4.1. Shrimp Growth
4.2. Plants
4.3. Water Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalmers, G.A. Aquaponics and Food Safety. 2004. Available online: http://www.backyardaquaponics.com/Travis/Aquaponics-andFood-Safety.pdf (accessed on 23 June 2019).
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; Food & Agriculture Organization: Rome, Italy, 2016; ISBN 978-9251091852. [Google Scholar]
- Pinho, S.M.; de Lima, J.P.; David, L.H.; Emerenciano, M.G.C.; Goddek, S.; Verdegem, M.C.J.; Keesman, K.J.; Portella, M.C. FLOCponics: The integration of biofloc technology with plant production. Rev. Aquac. 2021, 14, 647–675. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production. Integrated Fish and Plant Farming; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2014; ISBN 978-9251085325. [Google Scholar]
- Alshrouf, A. Hydroponics, aeroponic and aquaponic as compared with conventional farming. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar]
- Goddek, S.; Joyce, A.; Kotzen, B.; Butnell, G.M. Aquaponics Food Production Systems, 1st ed.; Goddek, S., Joyce, A., Kotzen, B., Butnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; ISBN 9783030159429. [Google Scholar]
- Fronte, B.; Galliano, G.; Bibbiani, C. From freshwater to marine aquaponic: New opportunities for marine fish species production. In Proceedings of the Conference VIVUS—On Agriculture, Environmentalism, Horticulture and Floristics, Food Production and Processing and Nutrition. With Knowledge and Experience to New Entrepreneurial Opportunities, Paris, France, 30 November–12 December 2015; Volume 4, pp. 514–521. [Google Scholar]
- Gunning, D.; Maguire, J.; Burnell, G. The development of sustainable saltwater-based food production systems: A review of established and novel concepts. Water 2016, 8, 598. [Google Scholar] [CrossRef]
- Chu, Y.-T.; Brown, P.B. Sustainable marine aquaponics: Effects of shrimp to plant ratio and C/N ratios. Front. Mar. Sci. 2021, 8, 771630. [Google Scholar] [CrossRef]
- Lam, S.S.; Ma, N.L.; Jusoh, A.; Ambak, M.A. Biological nutrient removal by recirculating aquaponic system: Optimization of the dimension ratio between the hydroponic & rearing tank components. Int. Biodeterior. Biodegrad. 2015, 102, 107–115. [Google Scholar] [CrossRef]
- Liang, J.Y.; Chien, Y.H. Effects of feeding frequency and photoperiod on water quality and crop production in a tilapia-water spinach raft aquaponics system. Int. Biodeterior. Biodegrad. 2013, 85, 693–700. [Google Scholar] [CrossRef]
- Rakocy, J.E. Aquaponics—Integrating fish and plant culture. In Aquaculture Production Systems; Tidwell, J., Ed.; Johy Wiley & Sons, Inc.: Oxford, UK, 2012; pp. 343–386. ISBN 9781118250105. [Google Scholar]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Nutrient management regime affects water quality, crop growth, and nitrogen use efficiency of aquaponic systems. Sci. Hortic. 2019, 256, 108619. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Effects of hydraulic loading rate on spatial and temporal water quality characteristics and crop growth and yield in aquaponic systems. Horticulturae 2020, 6, 9. [Google Scholar] [CrossRef]
- Shete, A.P.; Verma, A.K.; Chadha, N.K.; Prakash, C.; Chandrakant, M.H. A comparative study on fish to plant component ratio in recirculating aquaponic system with common carp and mint. J. Environ. Biol. Sci. 2015, 29, 323–329. [Google Scholar]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Zanotto, F.P.; Wheatly, M.G. The effect of water pH on postmolt fluxes of calcium and associated electrolytes in the freshwater crayfish (Procambarus clarkii). Freshw. Crayfish 1995, 8, 437–450. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, L.; Chen, W.; Wang, C. Effect of using sodium bicarbonate to adjust the pH to different levels on water quality, the growth and the immune response of shrimp Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquac. Res. 2017, 48, 1194–1208. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Chu, Y.; Brown, P.B. Optimal dietary crude protein in commercial feeds for shrimp and halophytes in marine aquaponic biofloc systems. Front. Mar. Sci. 2022, 9, 824973. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Ye, X.; Zhang, L.; Shi, L.; Xu, F.; Ding, G. The effects of condensed molasses soluble on the growth and development of rapeseed through seed germination, hydroponics and field trials. Agriculture 2020, 10, 260. [Google Scholar] [CrossRef]
- Pyakurel, A.; Dahal, B.R.; Rijal, S. Effect of molasses and organic fertilizer in soil fertility and yield of spinach in Khotang, Nepal. Int. J. Appl. Sci. Biotechnol. 2019, 7, 49–53. [Google Scholar] [CrossRef]
- Browdy, C.L.; Ray, A.J.; Leffler, J.W.; Avnimelech, Y. Biofloc-based aquaculture systems. In Aquaculture Production Systems; Tidwell, J., Ed.; Johy Wiley & Sons, Inc.: Oxford, UK, 2012; pp. 278–307. ISBN 9780813801261. [Google Scholar]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Panigrahi, A.; Saranya, C.; Sundaram, M.; Vinoth Kannan, S.R.; Das, R.R.; Satish Kumar, R.; Rajesh, P.; Otta, S.K. Carbon: Nitrogen (C:N) ratio level variation influences microbial community of the system and growth as well as immunity of shrimp (Litopenaeus vannamei) in biofloc based culture system. Fish Shellfish. Immunol. 2018, 81, 329–337. [Google Scholar] [CrossRef]
- Xu, W.J.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Xu, W.J.; Morris, T.C.; Samocha, T.M. Effects of rwo commercial feeds for semi-intensive and hyper-intensive culture and four C/N ratios on water quality and performance of Litopenaeus vannamei juveniles at high density in biofloc-based, zero-exchange outdoor tanks. Aquaculture 2018, 490, 194–202. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; Food & Agriculture Organization: Rome, Italy, 2020; ISBN 978-9251326923. [Google Scholar]
- Quagrainie, K.K.; Flores, R.M.V.; Kim, H.J.; McClain, V. Economic analysis of aquaponics and hydroponics production in the U.S. midwest. J. Appl. Aquac. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Chu, Y.-T.; Brown, P.B. Evaluation of Pacific whiteleg shrimp and three halophytic plants in marine aquaponic systems under three salinities. Sustainability 2021, 13, 269. [Google Scholar] [CrossRef]
- Carlsson, R.; Clarke, E.M.W. Atriplex hortensis L. as a leafy vegetable, and as a leaf protein concentrate plant. Plant Foods Hum. Nutr. 1983, 33, 127–133. [Google Scholar] [CrossRef]
- Zhao, K.; Feng, L. Chinese Halophyte Resources, 1st ed.; China Science Publishing & Media Ltd.: Beijing, China, 2001; ISBN 7-03-009474-3. [Google Scholar]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.S.; Chuang, L.T. Nutrient contents of kale (Brassica oleraceae L. var. Acephala DC.). Food Chem. 2006, 96, 572–579. [Google Scholar] [CrossRef]
- Ivanovi, L.; Mila, I.; Topalovi, A. Nutritional and phytochemical content of Swiss chard from Montenegro, under different fertilization and irrigation treatments. Br. Food J. 2019, 121, 411–425. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance. In Agricultural Salinity. Assessment and Management; Wallender, W.W., Tanji, K.K., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2012; pp. 405–459. [Google Scholar]
- Slinkard, K.; Singleton, V. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, P.; Scarpa, J. Water quality and management. In Farming Marine Shrimp in Recirculating Freshwater Systems; Van Wyk, P., Davis-Hodgkins, M., Laramore, R., Main, K., Mountain, J., Scarpa, J., Eds.; Harbor Branch Oceanographic Institution: Fort Pierce, FL, USA, 1999; pp. 141–161. [Google Scholar]
- Chen, S.; Ling, J.; Blancheton, J. Nitrification kinetics of biofilm as affected by water quality factors. Aquac. Eng. 2006, 34, 179–197. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, J.; Huang, G. The effects of body weight, temperature, salinity, pH, light intensity and feeding condition on lethal DO levels of whiteleg shrimp, Litopenaeus vannamei (Boone, 1931). Aquaculture 2006, 256, 579–587. [Google Scholar] [CrossRef]
- Lemonnier, H.; Bernard, E.; Boglio, E.; Goarant, C.; Cochard, J.C. Influence of sediment characteristics on shrimp physiology: pH as principal effect. Aquaculture 2004, 240, 297–312. [Google Scholar] [CrossRef]
- Pan, L.Q.; Jiang, L.X.; Miao, J.J. Effects of salinity and pH on immune parameters of the white shrimp Litopenaeus vannamei. J. Shellfish Res. 2005, 24, 1223–1227. [Google Scholar] [CrossRef]
- Kathyayani, S.A.; Poornima, M.; Sukumaran, S.; Nagavel, A.; Muralidhar, M. Effect of ammonia stress on immune variables of Pacific white shrimp Penaeus vannamei under varying levels of pH and susceptibility to white spot syndrome virus. Ecotoxicol. Environ. Saf. 2019, 184, 109626. [Google Scholar] [CrossRef]
- Wright, B.Y.P.A.; Wood, C.M. An analysis of branchial ammonia excretion in the freshwater rainbow trout: Effects of environmental pH change and sodium uptake blockade. J. Exp. Biol. 1985, 114, 329–353. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C. Acute toxicity of ammonia on Litopenaeus vannamei (Boone) juveniles at different salinity levels. J. Exp. Mar. Bio. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C. Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 2003, 224, 193–201. [Google Scholar] [CrossRef]
- Jensen, F.B. Uptake, Elimination and effects of nitrite and nitrate in freshwater crayfish (Astacus astacus). Aquat. Toxiclol. 1996, 34, 95–104. [Google Scholar] [CrossRef]
- Boyd, C.E. Mineral salts correct ionic imbalances in cultute water. 2003. Available online: www.globalseafood.org/advocate/mineral-salts-correct-ionic-imbalances-in-culture-water (accessed on 6 September 2021).
- Davis, D.A.; Samocha, T.M.; Boyd, C.E. Acclimating PACIFIC White Shrimp, Litopenaeus vannamei, to Inland, Low-Salinity Waters; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2004; p. 2601. [Google Scholar]
- Shiau, S. Nutrient requirements of penaeid shrimps. Aquaculture 1998, 164, 77–93. [Google Scholar] [CrossRef]
- Zanotto, F.P.; Wheatly, M.G. The effect of ambient pH on electrolyte regulation during the postmoult period in freshwater crayfish Procambarus clarkii. J. Exp. Biol. 1993, 178, 1–19. [Google Scholar] [CrossRef]
- Wheatly, M.G. Calcium Homeostasis in crustacea: The rvolving role of branchial, renal, digestive and hypodermal epithelia. J. Exp. Zool. 1999, 283, 620–640. [Google Scholar] [CrossRef]
- Rodríguez-Kábana, R. Organic and inorganic nitrogen amendments to soil as nematode suppressants. J. Nematol. 1986, 18, 129–134. [Google Scholar]
- White, P.J. Ion uptake mechanisms of individual cells and roots: Short-distance transport. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 7–47. ISBN 9780123849052. [Google Scholar]
- Schenck, S. Molasses soil amendment for crop improvement and nematode management. Hawaii Agric. Res. Cent. 2001, 3, 1–7. [Google Scholar]
- Jarrell, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar] [CrossRef]
- Piper, C.S. Investigations on copper deficiency in plants. J. Agric. Sci. 1942, 32, 143–178. [Google Scholar] [CrossRef]
- Steenbjerg, F. Yield curves and chemical plant analyses. Plant Soil 1951, 3, 97–109. [Google Scholar] [CrossRef]
- Steenbjerg, F.; Jakobsen, S.T. Plant nutrition and yield curves. Soil Sci. 1963, 95, 69–88. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Pratt, D.E. Natural antioxidants from plant material. In Phenolic Compounds in Food and Their Effects on Health II: Antioxidants and Cancer Prevention; Huang, M.-T., Ho, C.-T., Lee, C.Y., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 54–71. [Google Scholar]
- Ho, C.-T. Phenolic compounds in food. In Phenolic Compounds in Food and Their Effects on Health I: Analysis, Occurrence, and Chemistry; Huang, M.-T., Ho, C.-T., Lee, C.Y., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 2–7. ISBN 978-1604563030. [Google Scholar]
- Alexopoulos, A.A.; Marandos, E.; Assimakopoulou, A.; Vidalis, N.; Petropoulos, S.A.; Karapanos, I.C. Effect of nutrient solution pH on the growth, yield and quality of Taraxacum officinale and Reichardia picroides in a floating hydroponic system. Agronomy 2021, 11, 1118. [Google Scholar] [CrossRef]
- Radić, S.; Vujčić, V.; Glogoški, M.; Radić-Stojković, M. Influence of pH and plant growth regulators on secondary metabolite production and antioxidant activity of Stevia rebaudiana (Bert). Period. Biol. 2016, 118, 9–19. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Chrysargyris, A.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.D.; Barros, L.; et al. Chemical composition and plant growth of Centaurea raphanina subsp. mixta plants cultivated under saline conditions. Molecules 2020, 25, 2204. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The rgulation of plant secondary metabolism in response to abiotic stress: Interactions between heat shock and elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Haida, Z.; Nakasha, J.J.; Hakiman, M. In vitro responses of plant growth factors on growth, yield, phenolics content and antioxidant activities of Clinacanthus nutans (Sabah Snake Grass). Plants 2020, 9, 1030. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant Sci. 2017, 8, 300. [Google Scholar] [CrossRef]
- AWWA. Nitrification; AWWA: Singapore, 2002. [Google Scholar]
- Jadhav, G.G.; Salunkhe, D.S.; Nerkar, D.P.; Bhadekar, R.K. Isolation and characterization of salt-tolerant nitrogen-fixing microorganisms from food. EurAsian J. Biosci. 2010, 4, 33–40. [Google Scholar] [CrossRef]
- Satapute, P.; Shetti, A.; Kulkarni, A.G.; Hiremath, G. Isolation and characterization of nitrogen fixing Bacillus Subtilis strain as-4 from agricultural soil as-4 from agricultural soil. Int. J. Recent Sci. Res. 2012, 3, 762–765. [Google Scholar]
- Watson, S.W.; Valois, F.W.; Waterbury, J.B. The Family Nitrobacteraceae. In The Prokaryotes; Starr, M.P., Stolp, H., Trüper, H.G., Balows, A., Schlegel, H.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 1005–1022. ISBN 978-3-662-13187-9. [Google Scholar]
- Phatthongkleang, T.; Sangnoi, Y.; Thong, S.O.; Uppabullung, A.; Keawtawee, T. The efficiency of Bacillus spp. to remove ammonia. Wichcha J. 2019, 38, 1–15. [Google Scholar]
- Tyson, R.V.; Simonne, E.H.; White, J.M.; Lamb, E.M. Reconciling water quality parameters impacting nitrification in aquaponics: The pH levels. Proc. Fla. State Hortic. Soc. 2004, 117, 79–83. [Google Scholar]
- Wongkiew, S.; Park, M.R.; Chandran, K.; Khanal, S.K. Aquaponic systems for sustainable resource recovery: Linking nitrogen transformations to microbial communities. Environ. Sci. Technol. 2018, 52, 12728–12739. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Comparisons of nitrogen and phosphorus mass balance for tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Alexander, M. Nitrification. In Soil Nitrogen; American Society of Agronomy, Inc.: New York, NY, USA, 1965; Volume 10, pp. 307–343. ISBN 9781493990658. [Google Scholar]
- Spann, T.M.; Schumann, A.W. Mineral nutrition contributes to plant disease and pest. EDIS 2010, 4, 1–5. [Google Scholar] [CrossRef]



| Parameter | Treatment | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 6.5 | 7.5 | P | pH | Additional C | pH × Additional C | |||
| Additional C | +C | No C | +C | No C | |||||
| Initial number of shrimp per tank | 28 ± 0 | 28 ± 0 | 28 ± 0 | 28 ± 0 | ns | ns | ns | ns | |
| Initial Weight (g) per shrimp | 2.25 ± 0.55 | 2.25 ± 0.58 | 2.25 ± 0.55 | 2.24 ± 0.58 | ns | ns | ns | ns | |
| Final number of shrimp per tank | 12 ± 3 | 15 ± 5 | 5 ± 7 | 8 ± 4 | ns | * | ns | ns | |
| Final Weight (g) per shrimp | 8.29 ± 0.64 | 7.66 ± 1.17 | 7.35 ± 2.01 | 8.03 ± 0.38 | ns | ns | ns | ns | |
| WG (%) | 268.5 ± 28.4 | 241.1 ± 51.7 | 226.2 ± 88.2 | 258.3 ± 17.1 | ns | ns | ns | ns | |
| SGR (%) | 2.33 ± 0.14 | 2.18 ± 0.26 | 2.07 ± 0.45 | 2.28 ± 0.09 | ns | ns | ns | ns | |
| Survival (%) | 42.8 ± 9.5 | 54.7 ± 18.0 | 19.0 ± 23.8 | 27.4 ± 12.5 | ns | * | ns | ns | |
| Plant Species | Treatment | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | Yield (kg/m2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Additional C | Total | Shoot | Root | Total | Shoot | Root | ||
| Red orache | 6.5 | +C | 42.7 ± 10.2 a | 23.3 ± 3.2 a | 18.1 ± 7.4 a | 4.97 ± 0.85 a | 3.22 ± 0.49 a | 1.75 ± 0.50 a | 0.53 ± 0.02 a |
| No C | 24.8 ± 6.6 b | 19.3 ± 3.8 b | 4.0 ± 1.3 bc | 3.29 ± 0.82 b | 2.64 ± 0.56 b | 0.55 ± 0.18 bc | 0.46 ± 0.10 a | ||
| 7.5 | +C | 24.2 ± 7.1 b | 18.1 ± 3.9 b | 6.4 ± 3.7 b | 3.38 ± 1.01 b | 2.53 ± 0.61 b | 0.85 ± 0.44 b | 0.41 ± 0.09 a | |
| No C | 12.0 ± 3.2 c | 9.0 ± 2.2 c | 2.6 ± 0.6 c | 1.65 ± 0.46 c | 1.28 ± 0.39 c | 0.37 ± 0.07 c | 0.21 ± 0.06 b | ||
| p | *** | *** | *** | *** | *** | *** | ** | ||
| ANOVA | |||||||||
| pH | *** | *** | *** | *** | *** | *** | ** | ||
| Additional C | *** | *** | *** | *** | *** | *** | * | ||
| pH × Additional C | ns | ** | *** | ns | ** | *** | ns | ||
| Minutina | 6.5 | +C | 45.3 ± 12.7 ab | 37.0 ± 9.8 ab | 8.3 ± 5.3 | 3.83 ± 1.10 ab | 3.17 ± 0.92 ab | 0.66 ± 0.32 | 0.85 ± 0.16 |
| No C | 55.5 ± 10.9 a | 46.2 ± 9.9 a | 9.3 ± 4.7 | 4.56 ± 0.94 a | 3.85 ± 0.92 a | 0.67 ± 0.20 | 0.99 ± 0.09 | ||
| 7.5 | +C | 53.4 ± 19.1 ab | 43.3 ± 17.4 ab | 10.1 ± 5.3 | 4.19 ± 1.39 ab | 3.44 ± 1.27 ab | 0.76 ± 0.29 | 0.99 ± 0.17 | |
| No C | 41.2 ± 17.1 b | 32.1 ± 13.6 b | 9.1 ± 4.6 | 3.30 ± 1.41 b | 2.63 ± 1.18 b | 0.67 ± 0.24 | 0.73 ± 0.30 | ||
| p | * | * | ns | * | * | ns | ns | ||
| ANOVA | |||||||||
| pH | ns | ns | ns | ns | ns | ns | ns | ||
| Additional C | ns | ns | ns | ns | ns | ns | ns | ||
| pH × Additional C | ** | ** | ns | ** | ** | ns | ns | ||
| Swiss chard | 6.5 | +C | 50.5 ± 9.6 a | 36.5 ± 10.7 a | 15.3 ± 5.3 b | 5.25 ± 0.81 a | 3.88 ± 1.12 a | 1.38 ± 0.35 b | 0.83 ± 0.11 ab |
| No C | 59.6 ± 20.2 a | 40.3 ± 12.4 a | 17.8 ± 6.1 ab | 5.22 ± 1.46 a | 4.05 ± 1.22 a | 1.35 ± 0.36 b | 0.92 ± 0.08 ab | ||
| 7.5 | +C | 66.3 ± 26.5 a | 43.617.0± a | 23.5 ± 10.2 a | 6.13 ± 2.10 a | 4.35 ± 1.59 a | 1.78 ± 0.55 a | 0.99 ± 0.27 a | |
| No C | 26.3 ± 11.1 b | 22.4 ± 10.0 b | 3.9 ± 1.3 c | 2.91 ± 0.94 b | 2.42 ± 0.85 b | 0.49 ± 0.11 c | 0.51 ± 0.16 b | ||
| p | *** | *** | *** | *** | *** | *** | * | ||
| ANOVA | |||||||||
| pH | * | ns | ns | * | * | * | ns | ||
| Additional C | *** | ** | *** | *** | ** | *** | ns | ||
| pH × Additional C | *** | *** | *** | *** | *** | *** | * | ||
| Plant Species | Treatment | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | Yield (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Additional C | Total | Shoot | Root | Total | Shoot | Root | |||
| Okahijiki | 6.5 | +C | 30.3 ± 8.6 a | 26.3 ± 8.1 a | 4.0 ± 1.5 a | 2.91 ± 0.82 a | 2.44 ± 0.78 a | 0.49 ± 0.09 a | 0.64 ± 0.14 a | |
| No C | 22.1 ± 9.7 b | 17.9 ± 9.2 b | 4.2 ± 2.1 a | 1.82 ± 0.78 b | 1.47 ± 0.72 b | 0.33 ± 0.07 b | 0.41 ± 0.17 ab | |||
| 7.5 | +C | 10.6 ± 3.7 c | 8.2 ± 3.0 c | 2.7 ± 1.2 b | 1.07 ± 0.35 c | 0.69 ± 0.22 c | 0.32 ± 0.07 b | 0.22 ± 0.07 bc | ||
| No C | 2.9 ± 1.3 d | 1.9 ± 1.1 d | 1.1 ± 0.3 c | 0.43 ± 0.11 d | 0.20 ± 0.08 c | 0.23 ± 0.04 c | 0.05 ± 0.02 c | |||
| p | *** | *** | *** | *** | *** | *** | ** | |||
| ANOVA | ||||||||||
| pH | *** | *** | *** | *** | *** | *** | *** | |||
| Additional C | *** | *** | ns | *** | *** | *** | * | |||
| pH × Additional C | Ns | ns | * | ns | ns | * | ns | |||
| Kale | 6.5 | +C | 27.0 ± 5.0 ab | 21.1 ± 3.7 a | 5.9 ± 1.7 ab | 2.96 ± 0.46 a | 2.42 ± 0.37 a | 0.52 ± 0.10 a | 0.48 ± 0.06 a | |
| No C | 29.2 ± 5.3 a | 22.3 ± 3.9 a | 6.8 ± 2.0 a | 2.96 ± 0.46 a | 2.42 ± 0.37 a | 0.52 ± 0.10 a | 0.51 ± 0.06 a | |||
| 7.5 | +C | 25.2 ± 3.3 b | 19.9 ± 2.7 a | 5.2 ± 1.1 b | 2.72 ± 0.35 a | 2.21 ± 0.28 a | 0.51 ± 0.09 a | 0.46 ± 0.02 a | ||
| No C | 16.1 ± 3.0 c | 13.0 ± 2.5 b | 3.0 ± 0.5 c | 1.76 ± 0.32 b | 1.35 ± 0.27 b | 0.36 ± 0.03 b | 0.30 ± 0.02 b | |||
| p | *** | *** | *** | *** | *** | *** | ** | |||
| ANOVA | ||||||||||
| pH | *** | *** | *** | *** | *** | *** | ** | |||
| Additional C | *** | *** | ns | *** | *** | *** | * | |||
| pH × Additional C | *** | *** | *** | *** | *** | *** | ** | |||
| Swiss chard | 6.5 | +C | 50.2 ± 13.4 a | 37.0 ± 10.3 a | 13.3 ± 3.7 a | 4.54 ± 1.22 a | 3.53 ± 1.04 a | 1.02 ± 0.24 a | 0.85 ± 0.09 ab | |
| No C | 54.4 ± 19.8 a | 38.6 ± 13.0 a | 13.7 ± 4.8 a | 4.78 ± 1.61 a | 3.82 ± 1.32 a | 1.00 ± 0.31 a | 0.93 ± 0.10 a | |||
| 7.5 | +C | 51.8 ± 20.2 a | 38.3 ± 13.8 a | 11.2 ± 4.2 a | 4.86 ± 1.73 a | 3.89 ± 1.43 a | 0.97 ± 0.34 a | 0.93 ± 0.26 a | ||
| No C | 26.0 ± 9.1 b | 21.5 ± 7.9 b | 4.5 ± 1.5 a | 2.26 ± 0.38 b | 1.80 ± 0.31 b | 0.50 ± 0.11 b | 0.49 ± 0.14 b | |||
| p | *** | *** | *** | *** | *** | *** | * | |||
| ANOVA | ||||||||||
| pH | *** | ** | *** | ** | ** | *** | ns | |||
| Additional C | ** | ** | *** | *** | ** | *** | ns | |||
| pH × Additional C | *** | ** | *** | *** | *** | ** | * | |||
| Plant Species | Treatment | Plant Height (cm) | Leaf Length (cm) | SPAD | Fv/Fm | RGR x (%) | |
|---|---|---|---|---|---|---|---|
| pH | Additional C | ||||||
| Red orache | 6.5 | +C | 49.7 a | - | 48.2 a | 0.83 a | 11.4 a |
| No C | 38.3 b | - | 46.7 ab | 0.82 a | 11.8 ab | ||
| 7.5 | +C | 37.6 b | - | 44.8 b | 0.82 a | 10.4 b | |
| No C | 16.4 c | - | 39.9 c | 0.76 b | 8.0 c | ||
| p | *** | - | *** | *** | *** | ||
| ANOVA | |||||||
| pH | *** | - | *** | *** | *** | ||
| Additional C | *** | - | *** | *** | *** | ||
| pH × Additional C | *** | - | * | *** | *** | ||
| Minutina | 6.5 | +C | - | - | - | 0.83 | 16.4 a |
| No C | - | - | - | 0.83 | 17.1 a | ||
| 7.5 | +C | - | - | - | 0.83 | 16.9 a | |
| No C | - | - | - | 0.83 | 12.3 b | ||
| p | - | - | - | ns | *** | ||
| ANOVA | |||||||
| pH | - | - | - | ns | *** | ||
| Additional C | - | - | - | ns | *** | ||
| pH × Additional C | - | - | - | ns | *** | ||
| Swiss chard | 6.5 | +C | - | 13.9 a | 46.1 a | 0.83 ab | 13.6 a |
| No C | - | 12.6 a | 45.9 a | 0.84 a | 13.8 a | ||
| 7.5 | +C | - | 14.1 a | 42.5 a | 0.84 a | 13.9 a | |
| No C | - | 9.1 b | 33.3 b | 0.82 b | 11.0 b | ||
| p | - | *** | *** | ** | *** | ||
| ANOVA | |||||||
| pH | - | *** | *** | ns | *** | ||
| Additional C | - | *** | ** | ns | *** | ||
| pH × Additional C | - | ** | ** | *** | *** | ||
| Plant Species | Treatment | Plant Height (cm) | Leaf Length (cm) | SPAD | Fv/Fm | RGR x (%) | |
|---|---|---|---|---|---|---|---|
| pH | Additional C | ||||||
| Okahijiki | 6.5 | +C | - | - | - | - | 15.5 a |
| No C | - | - | - | - | 13.5 b | ||
| 7.5 | +C | - | - | - | - | 11.5 c | |
| No C | - | - | - | - | 5.8 d | ||
| p | - | - | - | - | *** | ||
| ANOVA | |||||||
| pH | - | - | - | - | *** | ||
| Additional C | - | - | - | - | *** | ||
| pH × Additional C | - | - | - | - | *** | ||
| Kale | 6.5 | +C | 3.2 a | - | 49.5 ab | 0.83 | 10.2 a |
| No C | 2.8 b | - | 50.2 a | 0.84 | 10.4 a | ||
| 7.5 | +C | 3.1 ab | - | 45.8 b | 0.83 | 10.0 a | |
| No C | 2.5 c | - | 34.7 c | 0.83 | 8.5 b | ||
| p | *** | - | *** | ns | *** | ||
| ANOVA | |||||||
| pH | ** | - | *** | ns | *** | ||
| Additional C | *** | - | *** | ns | *** | ||
| pH × Additional C | ns | - | *** | ns | *** | ||
| Swiss chard | 6.5 | +C | - | 14.0 a | 45.5 a | 0.85 a | 13.5 a |
| No C | - | 14.1 a | 43.7 ab | 0.84 a | 13.9 a | ||
| 7.5 | +C | - | 13.7 a | 41.4 b | 0.85 a | 13.7 a | |
| No C | - | 10.5 b | 34.5 c | 0.82 b | 11.5 b | ||
| p | - | *** | *** | *** | *** | ||
| ANOVA | |||||||
| pH | - | *** | *** | * | *** | ||
| Additional C | - | ** | *** | *** | ** | ||
| pH × Additional C | - | *** | ** | ** | *** | ||
| Plant Species | Treatment | N (%) | NUE x | P (%) | PUE y | |||
|---|---|---|---|---|---|---|---|---|
| pH | Additional C | Shoot | Root | Shoot | Root | |||
| Red orache | 6.5 | +C | 3.19 b | 2.69 | 14 | 0.91 a | 0.85 a | 2.4 a |
| No C | 3.55 b | 2.40 | 13.4 | 0.60 b | 0.48 b | 1.3 b | ||
| 7.5 | +C | 3.28 b | 2.28 | 11.4 | 0.62 b | 0.46 b | 1.2 b | |
| No C | 4.80 a | - | 9.2 | 0.59 b | - | 0.7 b | ||
| p | *** | ns | ns | *** | ** | *** | ||
| ANOVA | ||||||||
| pH | ** | - | ns | *** | - | *** | ||
| Additional C | *** | - | ns | *** | - | *** | ||
| pH × Additional C | * | - | ns | *** | - | ns | ||
| Minutina | 6.5 | +C | 2.42 b | 2.23 c | 10.5 | 0.76 ab | 2.03 ab | 2.1 ab |
| No C | 2.57 b | 2.91 ab | 12.8 | 0.61 ab | 1.80 bc | 1.7 ab | ||
| 7.5 | +C | 2.54 b | 2.54 bc | 12.0 | 0.92 a | 2.25 a | 2.5 a | |
| No C | 3.39 a | 3.45 a | 13.3 | 0.57 b | 1.51 c | 1.3 b | ||
| p | *** | *** | ns | * | ** | * | ||
| ANOVA | ||||||||
| pH | ** | ** | ns | ns | ns | ns | ||
| Additional C | ** | *** | ns | * | *** | * | ||
| pH × Additional C | * | ns | ns | ns | * | ns | ||
| Swiss chard | 6.5 | +C | 3.14 b | 2.73 | 16.7 | 1.31 a | 1.35 a | 4.1 a |
| No C | 3.63 ab | 3.37 | 20.1 | 0.55 b | 0.84 b | 1.8 b | ||
| 7.5 | +C | 3.13 b | 2.88 | 19.3 | 0.56 b | 0.67 b | 1.9 b | |
| No C | 4.23 a | - | 15.0 | 0.30 b | - | 0.7 c | ||
| p | ** | ns | ns | *** | ** | *** | ||
| ANOVA | ||||||||
| pH | ns | - | ns | *** | - | *** | ||
| Additional C | ** | - | ns | *** | - | *** | ||
| pH × Additional C | ns | - | * | * | - | ** | ||
| Plant Species | Treatment | N (%) | NUE x | P (%) | PUE y | |||
|---|---|---|---|---|---|---|---|---|
| pH | Additional C | Shoot | Root | Shoot | Root | |||
| Okahijiki | 6.5 | +C | 4.17 | 3.22 | 18.9 a | 1.29 a | 0.87 | 3.4 a |
| No C | 4.45 | 3.39 | 12.2 ab | 1.03 b | 0.65 | 1.6 b | ||
| 7.5 | +C | 4.88 | 3.29 | 8.3 bc | 1.19 ab | 0.67 | 1.2 bc | |
| No C | 4.87 | - | 2.1 c | 1.07 ab | - | 0.3 c | ||
| p | ns | ns | ** | * | ns | *** | ||
| ANOVA | ||||||||
| pH | ns | - | *** | ns | - | *** | ||
| Additional C | ns | - | ** | * | - | ** | ||
| pH × Additional C | ns | - | ns | ns | - | ns | ||
| Kale | 6.5 | +C | 5.13 ab | 3.28 | 23.9 a | 0.75 a | 0.79 | 2.0 a |
| No C | 5.20 a | 3.46 | 23.3 a | 0.74 a | 0.69 | 1.9 a | ||
| 7.5 | +C | 4.63 bc | 3.37 | 22.1 a | 0.74 a | 0.68 | 1.6 ab | |
| No C | 4.40 c | 3.52 | 12.3 b | 0.57 b | 0.66 | 1.0 b | ||
| p | ** | ns | ** | *** | ns | ** | ||
| ANOVA | ||||||||
| pH | *** | ns | ** | ** | ns | ** | ||
| Additional C | ns | ns | * | * | ns | ns | ||
| pH × Additional C | ns | ns | * | * | ns | ns | ||
| Swiss chard | 6.5 | +C | 4.58 a | 3.86 | 30.0 ab | 0.68 a | 0.97 a | 2.6 a |
| No C | 4.36 a | 3.96 | 30.9 ab | 0.61 a | 0.74 ab | 2.5 a | ||
| 7.5 | +C | 4.34 a | 4.16 | 36.4 a | 0.30 b | 0.71 b | 1.5 b | |
| No C | 3.45 b | 4.18 | 14.7 b | 0.29 b | 0.72 b | 0.7 b | ||
| p | *** | ns | * | ** | * | *** | ||
| ANOVA | ||||||||
| pH | *** | * | ns | *** | * | *** | ||
| Additional C | *** | ns | * | ns | ns | ns | ||
| pH × Additional C | * | ns | * | ns | ns | ns | ||
| Phenolics | ||||||
|---|---|---|---|---|---|---|
| ANOVA | First Harvest | Second Harvest | ||||
| Red Orache | Minutina | Swiss Chard | Okahijiki | Kale | Swiss Chard | |
| pH | ns | * | *** | *** | *** | * |
| Additional C | *** | *** | ns | *** | * | *** |
| pH × Additional C | *** | ns | *** | ns | ns | ns |
| Antioxidant capacity (ABTS) | ||||||
| ANOVA | First Harvest | Second Harvest | ||||
| Red orache | Minutina | Swiss chard | Okahijiki | Kale | Swiss chard | |
| pH | *** | * | ** | *** | ns | ns |
| Additional C | *** | *** | ns | ns | ns | *** |
| pH × Additional C | *** | ns | *** | ns | ** | ns |
| Antioxidant capacity (DPPH) | ||||||
| ANOVA | First Harvest | Second Harvest | ||||
| Red orache | Minutina | Swiss chard | Okahijiki | Kale | Swiss chard | |
| pH | ns | ns | *** | *** | *** | ns |
| Additional C | *** | *** | ns | *** | ns | *** |
| pH × Additional C | ns | ns | *** | * | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.-T.; Bao, Y.; Huang, J.-Y.; Kim, H.-J.; Brown, P.B. Supplemental C Addressed the pH Conundrum in Sustainable Marine Aquaponic Food Production Systems. Foods 2023, 12, 69. https://doi.org/10.3390/foods12010069
Chu Y-T, Bao Y, Huang J-Y, Kim H-J, Brown PB. Supplemental C Addressed the pH Conundrum in Sustainable Marine Aquaponic Food Production Systems. Foods. 2023; 12(1):69. https://doi.org/10.3390/foods12010069
Chicago/Turabian StyleChu, Yu-Ting, Yiwen Bao, Jen-Yi Huang, Hye-Ji Kim, and Paul B. Brown. 2023. "Supplemental C Addressed the pH Conundrum in Sustainable Marine Aquaponic Food Production Systems" Foods 12, no. 1: 69. https://doi.org/10.3390/foods12010069
APA StyleChu, Y.-T., Bao, Y., Huang, J.-Y., Kim, H.-J., & Brown, P. B. (2023). Supplemental C Addressed the pH Conundrum in Sustainable Marine Aquaponic Food Production Systems. Foods, 12(1), 69. https://doi.org/10.3390/foods12010069








