Structure and Properties of Organogels Prepared from Rapeseed Oil with Stigmasterol
Abstract
:1. Introduction
2. Materials and Methods
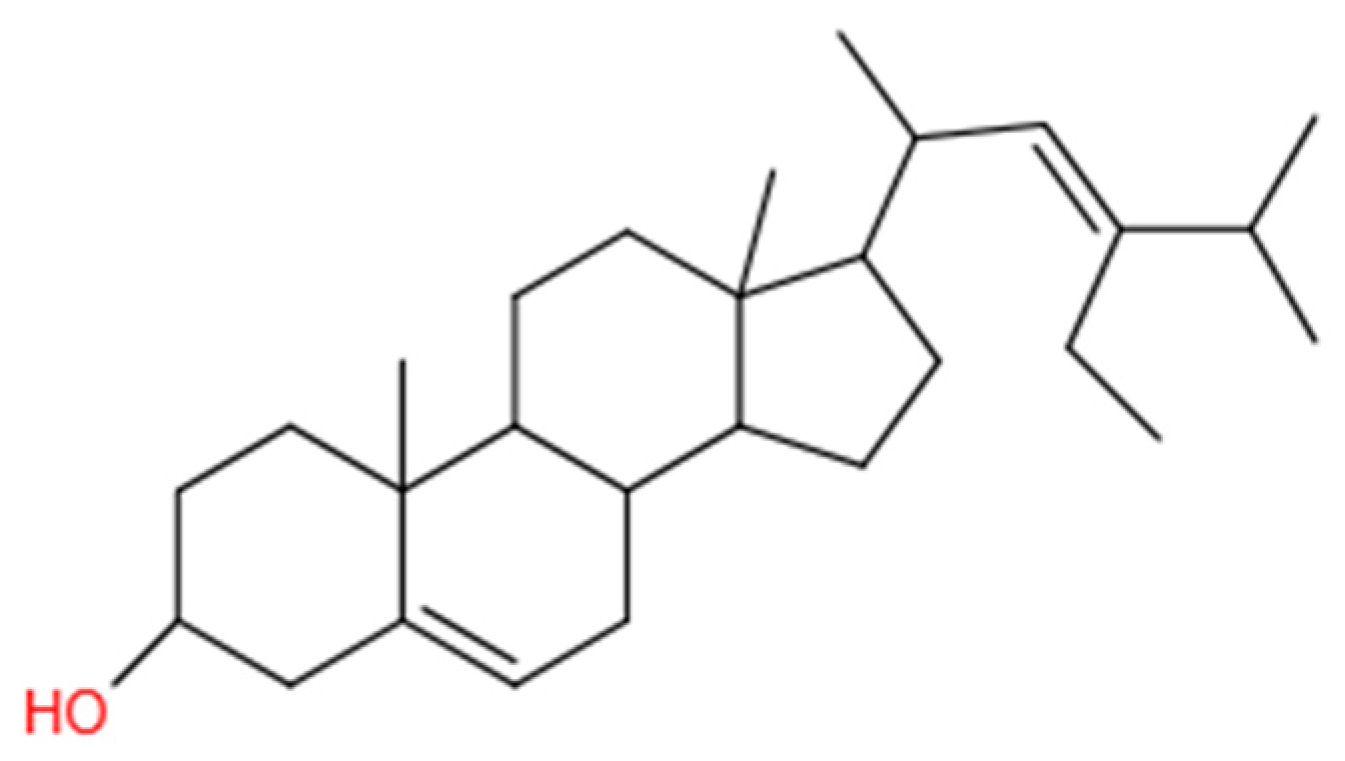
2.1. Materials
2.2. Methods
2.2.1. Organogel Preparation
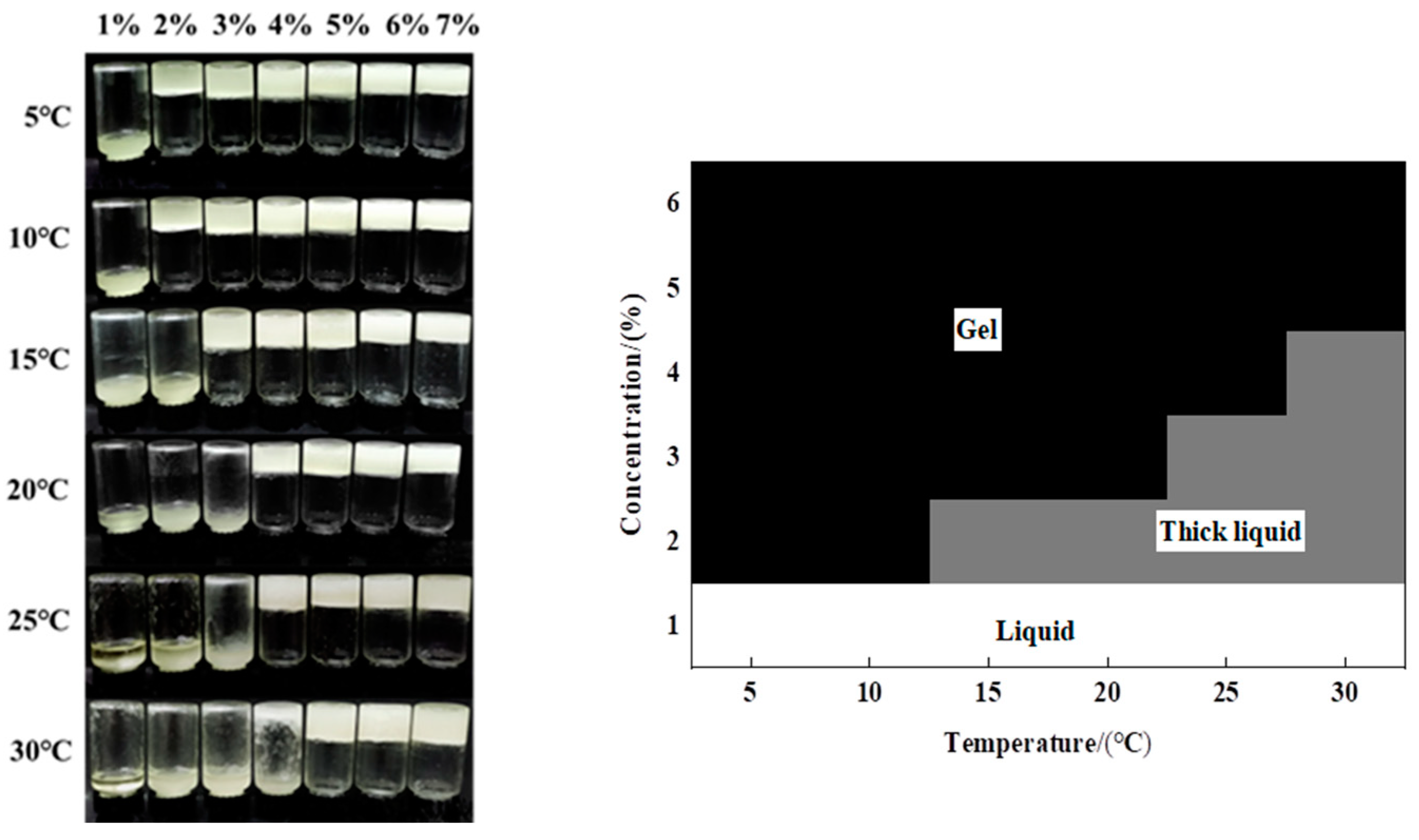
2.2.2. Gelation Temperature Phase Diagram
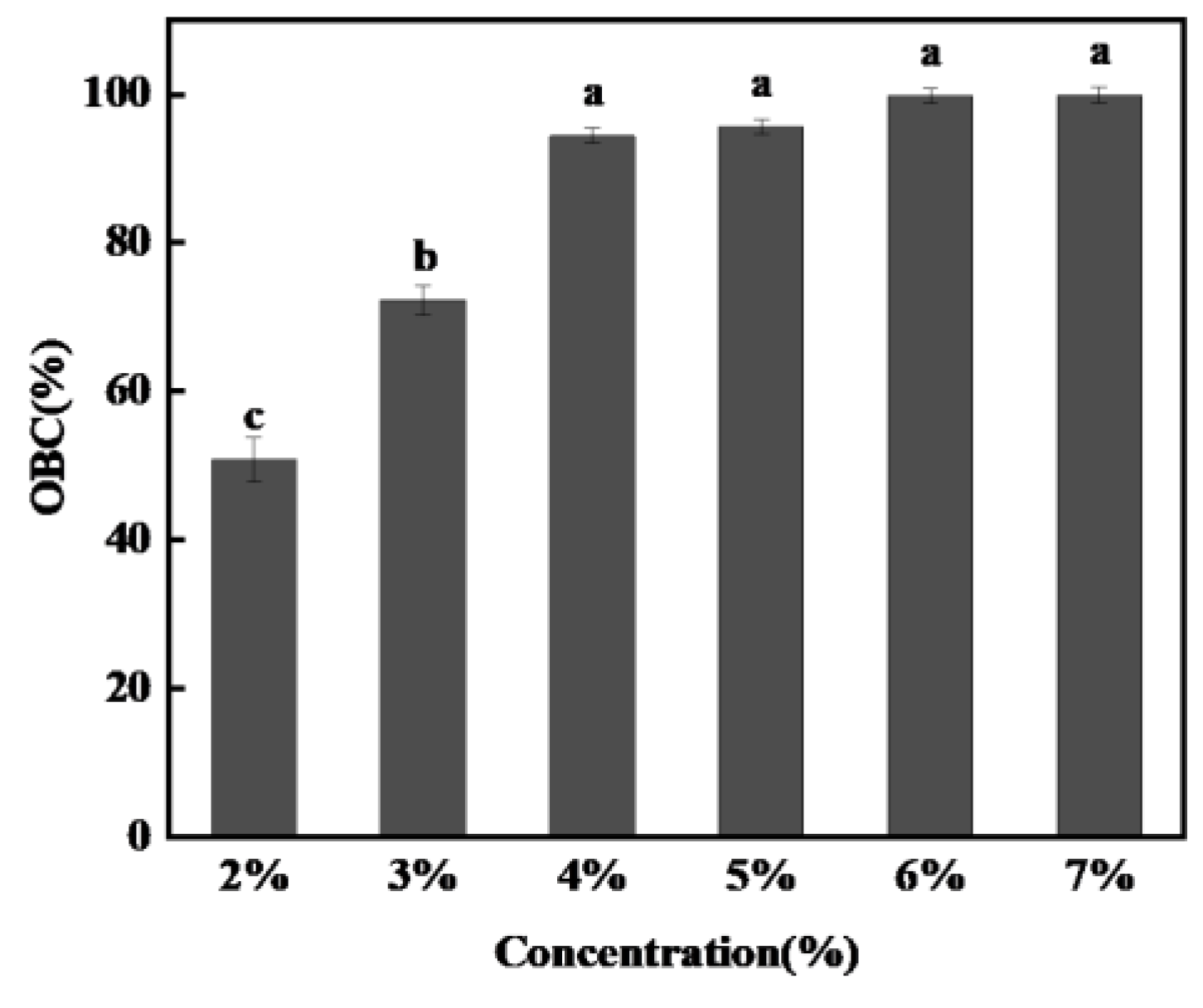
2.2.3. Oil Binding Capacity
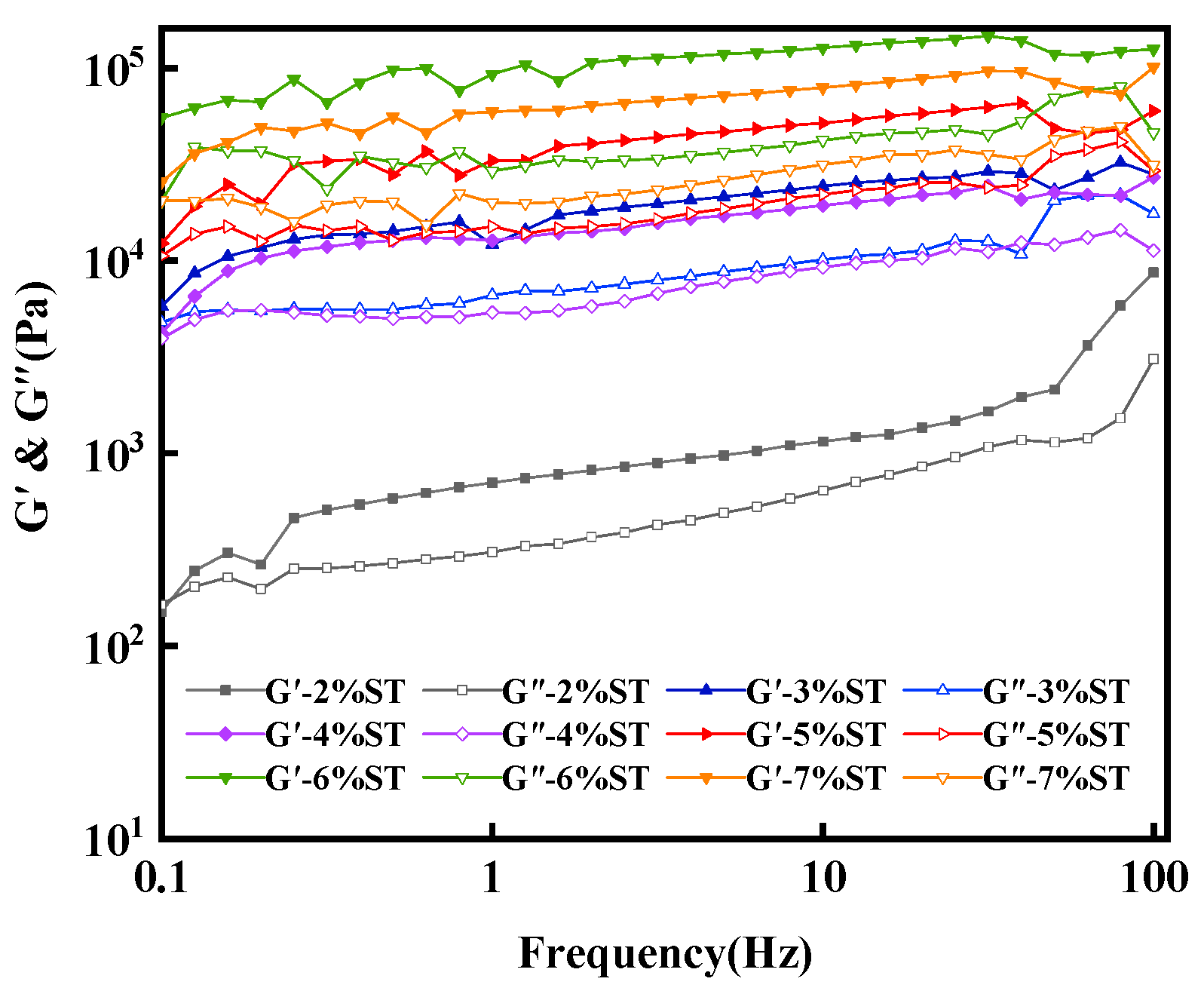
2.2.4. Rheological Characterization
2.2.5. Polarized Light Microscopy
2.2.6. X-ray Diffraction Analysis
2.2.7. Fourier Transform Infrared Spectroscopy
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Gelation Phase Diagram
3.2. Physicochemical Properties of Stigmasterol Organogels
3.2.1. Oil Binding Capacity
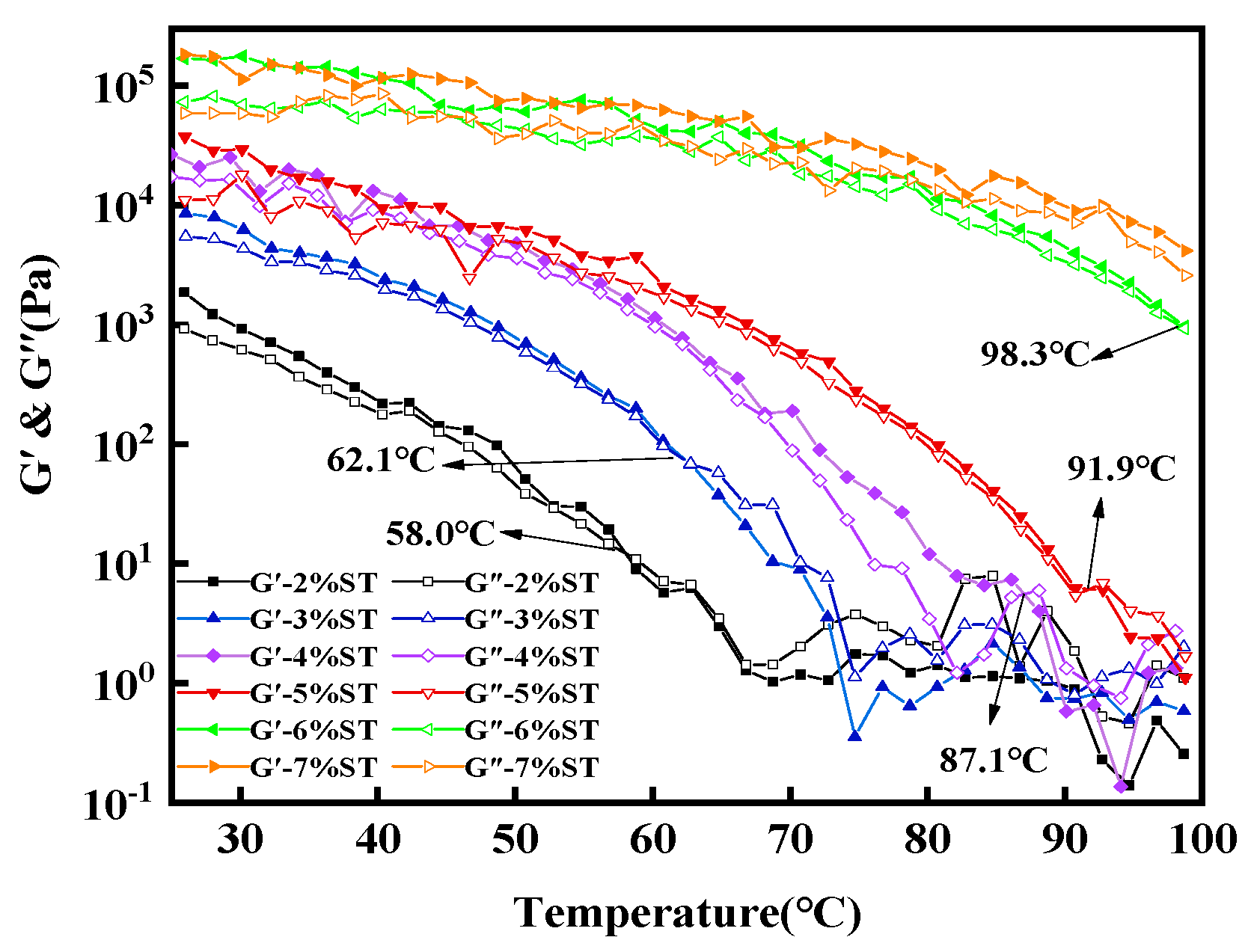
3.2.2. Rheological Properties
3.3. Microstructure Properties of Stigmasterol Organogels
3.3.1. Polarized Light Microscopy
3.3.2. X-ray Diffraction
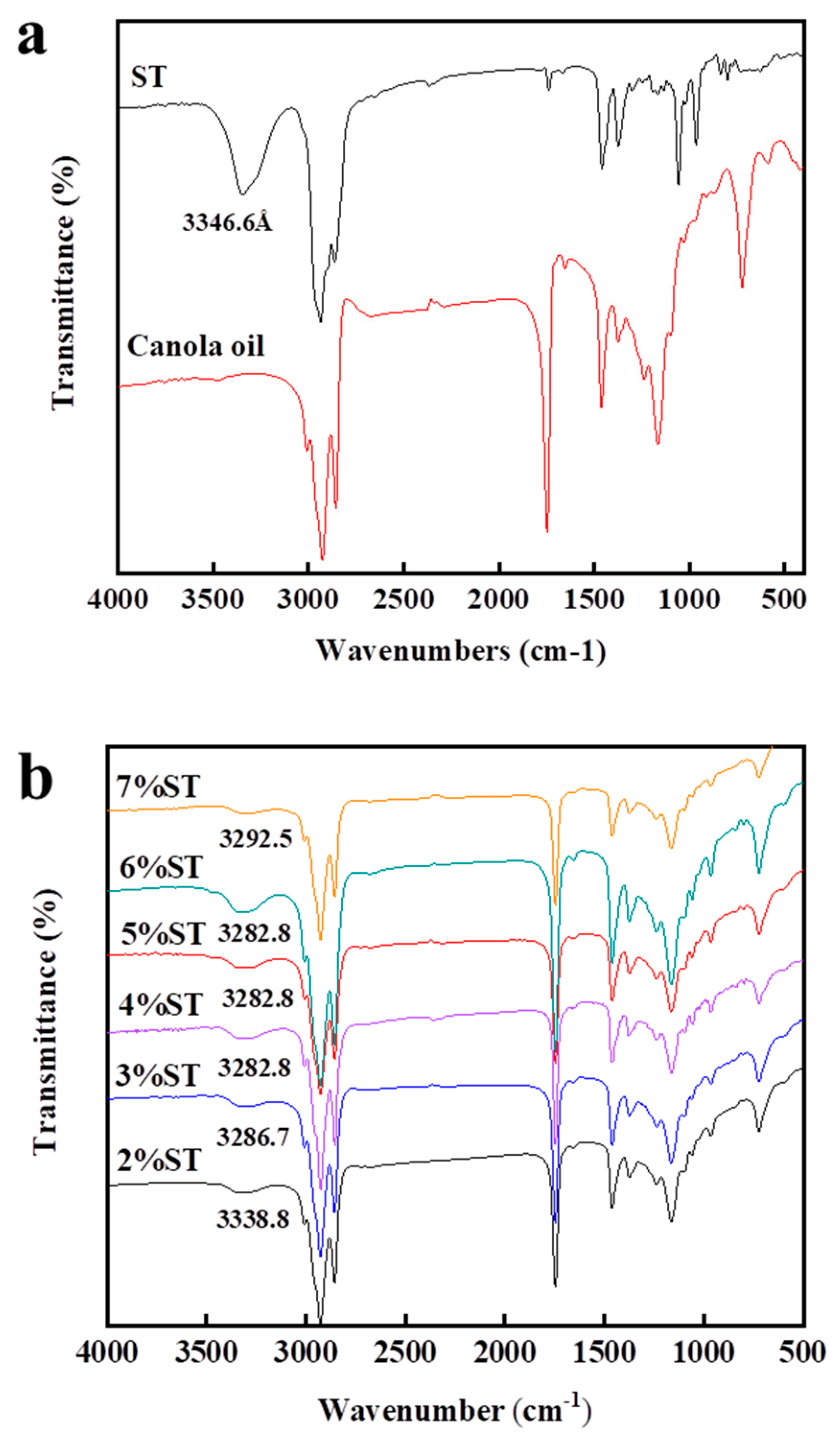
3.3.3. Fourier Transform Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, B.; Wang, Q.; Liu, W.; Xu, T.C.; Liu, L.N.; Zong, A.Z.; Jia, M.; Li, J.; Du, F.L. Biological effects of trans fatty acids and their possible roles in the lipid rafts in apoptosis regulation. Cell Biol. Int. 2018, 42, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Te, M.L.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef] [Green Version]
- Crupkin, M.; Zambelli, A. Detrimental impact of trans fats on human health: Stearic acid-rich fats as possible substitutes. Compr. Rev. Food Sci. Food Saf. 2018, 7, 271–279. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Pereira, M.A. Trans fatty acids, insulin resistance, and type 2 diabetes. Nutr. Rev. 2006, 64, 364–372. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Azadbakht, L. Consumption of hydrogenated versus nonhydrogenated vegetable oils and risk of insulin resistance and the metabolic syndrome among Iranian adult women. Diabetes Care 2008, 31, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, K.F.; Barrera-Arellano, D.; Ribeiro, A.P.B. Potential application of lipid organogels for food industry. Food Res. Int. 2018, 105, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gao, W.; Du, X.; Zhang, F.; Bai, X. Development of Low-calorie Organogel fromsn-2 Position-modified Coconut Oil Rich in Polyunsaturated Fatty Acids. J. Oleo Sci. 2019, 68, 399–408. [Google Scholar]
- Mert, B.; Demirkesen, I. Reducing saturated fat with oleogel/shortening blends in a baked product. Food Chem. 2016, 199, 809–816. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Edible oil structuring: An overview and recent updates. Food Funct. 2016, 7, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef]
- Stortz, T.A.; Zetzl, A.K.; Barbut, S.; Cattaruzza, A.; Marangoni, A.G. Edible oleogels in food products to help maximize health benefits and improve nutritional profiles. Lipid Technol. 2012, 24, 151–154. [Google Scholar] [CrossRef]
- Singh, V.K.; Pal, K.; Pradhan, D.K.; Pramanik, K. Castor oil and sorbitan monopalmitate based organogel as a probable matrix for controlled drug delivery. J. Appl. Polym. Sci. 2013, 130, 1503–1515. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery—A review. J. Control. Release 2007, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, C.; Kumar, N.; Sagiri, S.S.; Pal, K.; Ray, S.S. Development of span 80-tween 80 based fluid-filled organogels as a matrix for drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Sagiri, S.S.; Singh, V.K.; Pal, K.; Banerjee, I.; Basak, P. Stearic acid based oleogels: A study on the molecular, thermal and mechanical properties. Mater. Sci. Eng. C 2015, 48, 688–699. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical properties of rice bran wax in bulk and organogels. J. Am. Oil Chem. Soc. 2009, 86, 1163. [Google Scholar] [CrossRef]
- Jaiyanth, D.; Ram, R. Organogelation of plant oils and hydrocarbons by long-chain saturated FA, fatty alcohols, wax esters, and dicarboxylic acid. J. Am. Oil Chem. Soc. 2003, 80, 417–421. [Google Scholar] [CrossRef]
- Sawalha, H.; Venema, P.; Bot, A.; Flöter, E.; Van der Linden, E. The influence of concentration and temperature on the formation of γ-oryzanol+β-sitosterol tubules in edible oil organogels. Food Biophys. 2016, 16, 20–25. [Google Scholar]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A comprehensive review. Int. J. Pharm. Sci. Res. 2011, 2, 2259. [Google Scholar]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2008, 80, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Heras, B.; Villar, A. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens Clem. Biol. Pharm. Bull. 2001, 24, 470–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Maloney, D.J.; Dedkova, L.M.; Hecht, S.M. Inhibitors of DNA polymerase β: Activity and mechanism. Bioorg. Med. Chem. 2008, 6, 4331–4340. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.K.; Zhao, P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef] [Green Version]
- Batta, A.K.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 2006, 55, 292–299. [Google Scholar] [CrossRef]
- Bag, B.G.; Barai, A.C. Self-assembly of naturally occurring stigmasterol in liquids yielding a fibrillar network and gel. RSC Adv. 2020, 10, 4755–4762. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Teh, S.S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M.A. Novel propolis wax-based organogel: Effect of oil type on its formation, crystal structure and thermal properties. J. Am. Oil Chem. Soc. 2017, 94, 47–55. [Google Scholar] [CrossRef]
- Patel, A.R.; Schatteman, D.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Preparation and rheological characterization of shellac oleogels and oleogel-based emulsions. J. Colloid Interface Sci. 2013, 411, 114–121. [Google Scholar] [CrossRef]
- Rocha, J.C.B.; Lopes, J.D.; Mascarenhas, M.C.N.; Arellano, D.B.; Guerreiro, L.M.R.; Da Cunha, R.L. Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Res. Int. 2013, 50, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Wu, S.; Liu, S.; Li, B.; Li, Y. Structure and Rheological Properties of Glycerol Monolaurate-Induced Organogels: Influence of Hydrocolloids with Different Surface Charge. Molecules 2020, 25, 5117. [Google Scholar] [CrossRef]
- Wright, A.J.; Marangoni, A.G. Formation, structure, and rheological properties of ricinelaidic acid-vegetable oil organogels. J. Am. Oil Chem. Soc. 2016, 83, 497–503. [Google Scholar] [CrossRef]
- Mustafa, O.; Nazan, A.; Emin, Y. Preparation and Characterization of Virgin Olive Oil-Beeswax Oleogel Emulsion Products. J. Am. Oil Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Huang, X.; Terech, P.; Raghavan, S.R.; Weiss, R.G. Kinetics of 5α-cholestan-3β-yl N-(2-naphthyl) carbamate/n-alkane organogel formation and its influence on the fibrillar networks. J. Am. Chem. Soc. 2005, 127, 4336–4344. [Google Scholar] [PubMed]
- Li, J.L.; Liu, X.Y.; Wang, R.Y.; Xiong, J.Y. Architecture of a biocompatible supramolecular material by supersaturation-driven fabrication of its fiber network. J. Phys. Chem. B 2005, 109, 24231–24235. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.G. Organogels: An alternative edible oil-structuring method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Bascuas, S.; Hernando, I.; Moraga, G.; Quiles, A. Structure and stability of edible oleogels prepared with different unsaturated oils and hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1458–1467. [Google Scholar] [CrossRef]
- Dai, M.; Bai, L.; Zhang, H.; Ma, Q.; Luo, R.; Lei, F.; Fei, Q.; He, N. A novel flunarizine hydrochloride-loaded organogel for intraocular drug delivery in situ: Design, physicochemical characteristics and inspection. Int. J. Pharm. 2020, 576, 119027. [Google Scholar] [CrossRef]
- Chauvelon, G.; Doublier, J.L.; Buléon, A.; Thibault, J.F.; Saulnier, L. Rheological properties of sulfoacetate derivatives of cellulose. Carbohydr. Res. 2003, 338, 751–759. [Google Scholar] [CrossRef]
- Herazo, M.Á.; Ciro-Velásquez, H.J.; Márquez, C.J. Rheological and thermal study of structured oils: Avocado (Persea americana) and sacha inchi (Plukenetia volubilis L.) systems. J. Food Sci. Technol. 2019, 56, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Rojas, J.; Cabrera, S.; Ciro, G.; Naranjo, A. Lipidic matrixes containing lemon essential oil increases storage stability: Rheological, thermal, and microstructural studies. Appl. Sci. 2020, 10, 3909. [Google Scholar] [CrossRef]
- Zuo, F.; Li, X.; Yang, S.; Wang, P.; Yang, Q.; Wang, N.; Xiao, Z. Formation, rheological behavior and morphological structure of cinnamic acid based rice bran oil organogel. Food Sci. 2018, 39, 16–21. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.Y.; Xiong, J.; Li, J. Real-time observation of fiber network formation in molecular organogel: Supersaturation-dependent microstructure and its related rheological property. J. Phys. Chem. B 2006, 110, 7275–7280. [Google Scholar] [CrossRef]
- Meng, Z.; Guo, Y.; Wang, Y.; Liu, Y. Organogels based on the polyglyceryl fatty acid ester and sunflower oil: Macroscopic property, microstructure, interaction force, and application. LWT 2019, 116, 108590. [Google Scholar] [CrossRef]
- Ojijo, N.K.; Neeman, I.; Eger, S.; Shimoni, E. Effects of monoglyceride content, cooling rate and shear on the rheological properties of olive oil/monoglyceride gel networks. J. Sci. Food Agric. 2004, 84, 1585–1593. [Google Scholar] [CrossRef]
- Lan, Y.; Corradini, M.G.; Weiss, A.G.; Raghavan, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef]
- Mallia, V.A.; Terech, P.; Weiss, R.G. Correlations of properties and structures at different length scales of hydro-and organo-gels based on N-alkyl-(R)-12-hydroxyoctadecylammonium chlorides. J. Phys. Chem. B 2011, 115, 12401–12414. [Google Scholar] [CrossRef]
- Teixeira, A.C.; Garcia, A.R.; Ilharco, L.M.; da Silva, A.M.G.; Fernandes, A.C. Phase behaviour of oleanolic acid, pure and mixed with stearic acid: Interactions and crystallinity. Chem. Phys. Lipids 2010, 163, 655–666. [Google Scholar] [CrossRef]
- Gong, N.; Wang, Y.; Zhang, B.; Yang, D.; Du, G.; Lu, Y. Screening, preparation and characterization of diosgenin versatile solvates. Steroids 2019, 143, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wan, Z.; Xia, H.; Zhao, H.; Guo, S. Structure and properties of organogels developed by diosgenin in canola oil. Food Biophys. 2020, 15, 452–462. [Google Scholar] [CrossRef]
- Rogers, M.A.; Wright, A.J.; Marangoni, A.G. Nanostructuring fiber morphology and solvent inclusions in 12-hydroxystearic acid/canola oil organogels. Curr. Opin. Colloid Interface Sci. 2009, 14, 33–42. [Google Scholar] [CrossRef]
- Xing, P.; Sun, T.; Li, S.; Hao, A.; Su, J.; Hou, Y. An instant-formative heat-set organogel induced by small organic molecules at a high temperature. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 44–50. [Google Scholar] [CrossRef]
- Baran, N.; Singh, V.K.; Pal, K.; Anis, A.; Pradhan, D.K.; Pramanik, K. Development and characterization of soy lecithin and palm oil-based organogels. Polym.-Plast. Technol. Eng. 2014, 53, 865–879. [Google Scholar] [CrossRef]
- Kulkarni, C.; Balasubramanian, S.; George, S.J. What molecular features govern the mechanism of supramolecular polymerization? ChemPhysChem 2013, 14, 661–673. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakajima, Y.; Yumoto, M.; Kimura, M.; Shirai, H.; Hanabusa, K. Effects of hydrogen bonding and van der Waals interactions on organogelation using designed low-molecular-weight gelators and gel formation at room temperature. Langmuir 2003, 19, 8622–8624. [Google Scholar] [CrossRef] [Green Version]
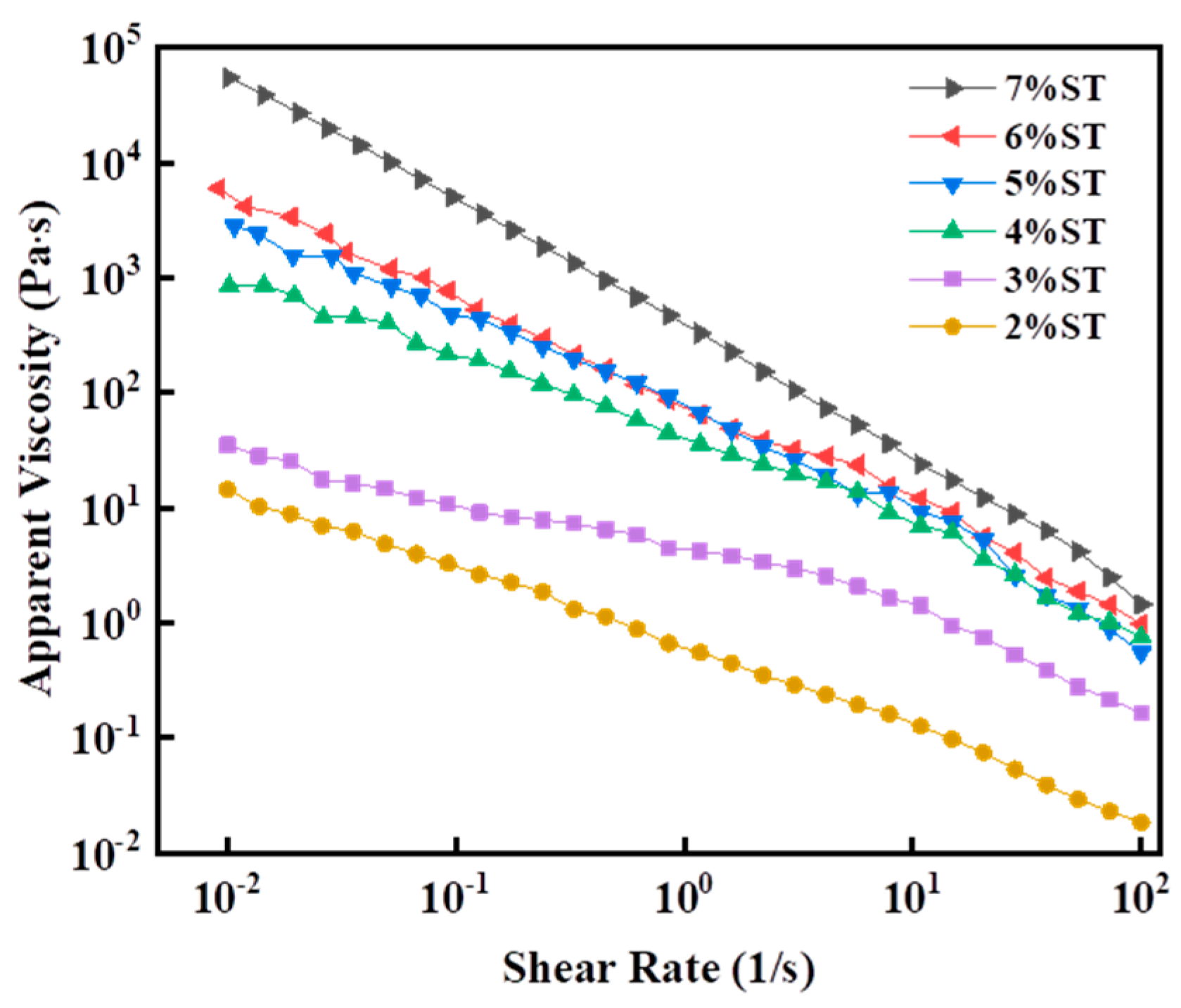


| ST Concentration | K/Pa·s | n | R2 |
|---|---|---|---|
| 2% | 0.42 ± 0.027 | 0.47 ± 0.087 | 0.997 |
| 3% | 12.71 ± 0.088 | 0.42 ± 0.068 | 0.991 |
| 4% | 39.49 ± 0.069 | 0.25 ± 0.025 | 0.991 |
| 5% | 63.66 ± 0.032 | 0.14 ± 0.017 | 0.998 |
| 6% | 81.59 ± 0.019 | 0.08 ± 0.022 | 0.997 |
| 7% | 253.60 ± 0.048 | 0.03 ± 0.046 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Wan, Z.; Chen, Y.; Tang, Y.; Fan, W.; Cao, Y.; Song, M.; Qin, J.; Xiao, H.; Guo, S.; et al. Structure and Properties of Organogels Prepared from Rapeseed Oil with Stigmasterol. Foods 2022, 11, 939. https://doi.org/10.3390/foods11070939
Tang C, Wan Z, Chen Y, Tang Y, Fan W, Cao Y, Song M, Qin J, Xiao H, Guo S, et al. Structure and Properties of Organogels Prepared from Rapeseed Oil with Stigmasterol. Foods. 2022; 11(7):939. https://doi.org/10.3390/foods11070939
Chicago/Turabian StyleTang, Caili, Zheng Wan, Yilu Chen, Yiyun Tang, Wei Fan, Yong Cao, Mingyue Song, Jingping Qin, Hang Xiao, Shiyin Guo, and et al. 2022. "Structure and Properties of Organogels Prepared from Rapeseed Oil with Stigmasterol" Foods 11, no. 7: 939. https://doi.org/10.3390/foods11070939
APA StyleTang, C., Wan, Z., Chen, Y., Tang, Y., Fan, W., Cao, Y., Song, M., Qin, J., Xiao, H., Guo, S., & Tang, Z. (2022). Structure and Properties of Organogels Prepared from Rapeseed Oil with Stigmasterol. Foods, 11(7), 939. https://doi.org/10.3390/foods11070939







