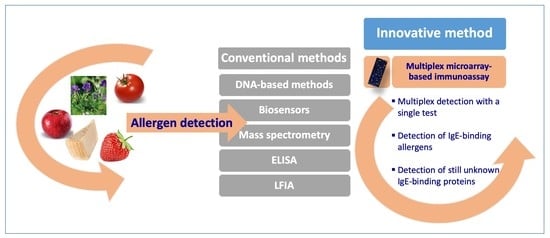
Detection of Allergenic Proteins in Foodstuffs: Advantages of the Innovative Multiplex Allergen Microarray-Based Immunoassay Compared to Conventional Methods
Abstract
:1. Introduction
Food Extract Composition
2. Classical Analytical Methods Generally Used for Allergen Detection in Foods
2.1. DNA Detection
2.2. Mass-Spectrometry-Based Technology
2.3. Biosensor Technology
2.4. ELISA Assay
2.5. LFIA
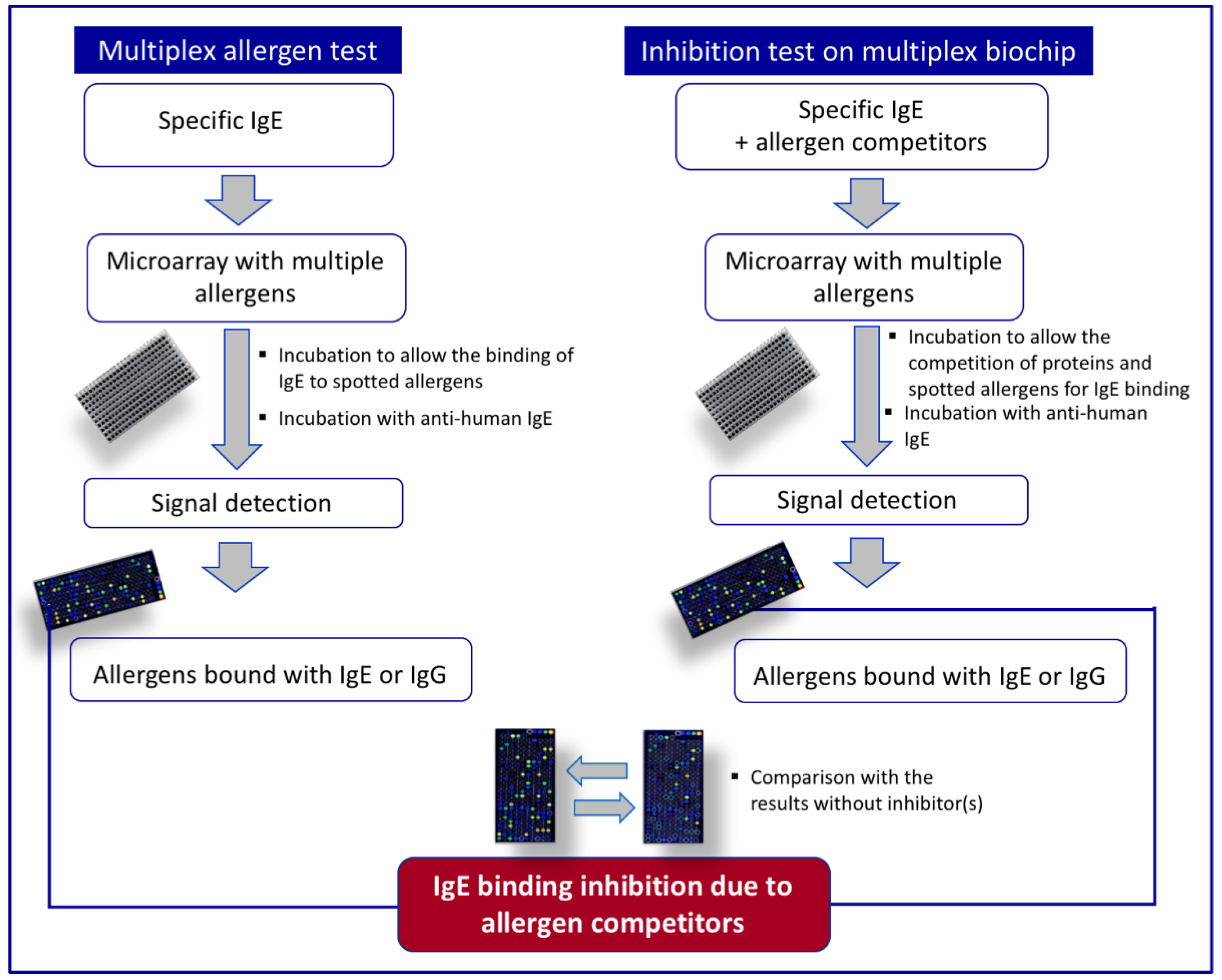
3. Multiplex Allergen Microarray-Based Immunoassay for the Detection and the Identification of IgE Binding Proteins
Combining Multiplex Allergen Immunoassay with Mass-Spectrometry-Based Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pepper, A.N.; Assa’Ad, A.; Blaiss, M.; Brown, E.; Chinthrajah, S.; Ciaccio, C.; Fasano, M.B.; Gupta, R.; Hong, N.; Lang, D.; et al. Consensus report from the Food Allergy Research & Education (FARE) 2019 Oral Immunotherapy for Food Allergy Summit. J. Allergy Clin. Immunol. 2020, 146, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Bilaver, L.A.; Chadha, A.S.; Doshi, P.; O’Dwyer, L.; Gupta, R.S. Economic burden of food allergy: A systematic review. Ann. Allergy Asthma Immunol. 2019, 122, 373–380.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoeckx, K.; Bøgh, K.L.; Constable, A.; Epstein, M.; Sommergruber, K.H.; Holzhauser, T.; Houben, G.; Kuehn, A.; Roggen, E.; O’Mahony, L.; et al. COST Action ‘ImpARAS’: What have we learnt to improve food allergy risk assessment. A summary of a 4 year networking consortium. Clin. Transl. Allergy 2020, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Ferrara, R.; Bernardi, M.L.; Zennaro, D.; Tuppo, L.; Giangrieco, I.; Ricciardi, T.; Tamburrini, M.; Ciardiello, M.A.; Mari, A. Molecular approach to a patient’s tailored diagnosis of the oral allergy syndrome. Clin. Transl. Allergy 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, M.A.; Tamburrini, M.; Liso, M.; Crescenzo, R.; Rafaiani, C.; Mari, A. Food allergen profiling: A big challenge. Food Res. Int. 2013, 54, 1033–1041. [Google Scholar] [CrossRef]
- Kurze, E.; Scalzo, R.L.; Campanelli, G.; Schwab, W. Effect of tomato variety, cultivation, climate and processing on Sola l 4, an allergen from Solanum lycopersicum. PLoS ONE 2018, 13, e0197971. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, M.S.; Palazzo, P.; Tuppo, L.; Liso, M.; Petriccione, M.; Rega, P.; Tartaglia, A.; Tamburrini, M.; Alessandri, C.; Ciardiello, M.A.; et al. Analysis of the potential allergenicity of traditional apple cultivars by Multiplex Biochip-Based Immunoassay. Food Chem. 2012, 135, 219–227. [Google Scholar] [CrossRef]
- Bousfiha, A.; Lotfi, A. Effect of heat and enzymatic treatments on human IgE and rabbit IgG sensitivity to white bean allergens. Iran. J. Allergy Asthma Immunol. 2013, 12, 304–311. [Google Scholar]
- Thomas, K.; Herouet-Guicheney, C.; Ladics, G.; Bannon, G.; Cockburn, A.; Crevel, R.; Fitzpatrick, J.; Mills, C.; Privalle, L.; Vieths, S. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: International workshop report. Food Chem. Toxicol. 2007, 45, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Okon, K.; Yoshida, T.; Hattori, M.; Matsuda, H.; Osada, M. Preparation of hypoallergenic ovalbumin by high-temperature water treatment. Biosci. Biotechnol. Biochem. 2021, 85, 2442–2449. [Google Scholar] [CrossRef]
- Pi, X.; Yang, Y.; Sun, Y.; Cui, Q.; Wan, Y.; Fu, G.; Chen, H.; Cheng, J. Recent advances in alleviating food allergenicity through fermentation. Crit. Rev. Food Sci. Nutr. 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Jiang, X.; Rao, Q. Effect of Processing on Fish Protein Antigenicity and Allergenicity. Foods 2021, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.A.; Nesbit, J.B.; Hurlburt, B.K.; Cheng, H.; Maleki, S.J. Processing Can Alter the Properties of Peanut Extract Preparations. J. Agric. Food Chem. 2009, 58, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Chung, S.-Y.; Champagne, E.T.; Raufman, J.-P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, A.C.Y.; Mueller, G.A. Abundance and Stability as Common Properties of Allergens. Front. Allergy 2021, 2, 769728. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Sun, D. An overview of tropomyosin as an important seafood allergen: Structure, cross-reactivity, epitopes, allergenicity, and processing modifications. Compr. Rev. Food Sci. Food Saf. 2021, 21, 127–147. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible insects: Cross-recognition of IgE from crustacean- and house dust mite allergic patients, and reduction of allergenicity by food processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [Green Version]
- Mari, A.; Ciardiello, M.A.; Tamburrini, M.; Rasi, C.; Palazzo, P. Proteomic analysis in the identification of allergenic molecules. Expert Rev. Proteom. 2010, 7, 723–734. [Google Scholar] [CrossRef]
- de la Cruz, S.; López-Calleja, I.; Martín, R.; González, I.; Alcocer, M.; García, T. Recent Advances in the Detection of Allergens in Foods. In Food Allergens: Methods and Protocols, Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1592, pp. 263–295. [Google Scholar] [CrossRef]
- Shin, J.H.; Reddy, Y.V.M.; Park, T.J.; Park, J.P. Recent advances in analytical strategies and microsystems for food allergen detection. Food Chem. 2021, 371, 131120. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, Y.; Ji, J.; Sun, J.; Sun, X. Advances on the rapid and multiplex detection methods of food allergens. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Yakhlef, M.; Giangrieco, I.; Ciardiello, M.A.; Fiume, I.; Mari, A.; Souiki, L.; Pocsfalvi, G. Potential allergenicity of Medicago sativa investigated by a combined IgE -binding inhibition, proteomics and in silico approach. J. Sci. Food Agric. 2020, 101, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Fiocchi, A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Costa, J.; Gondar, C.; Oliveira, M.B.P.P.; Mafra, I. Effect of food matrix and thermal processing on the performance of a normalised quantitative real-time PCR approach for lupine (Lupinus albus) detection as a potential allergenic food. Food Chem. 2018, 262, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, M.A.; Giangrieco, I.; Tuppo, L.; Tamburrini, M.; Buccheri, M.; Palazzo, P.; Bernardi, M.L.; Ferrara, R.; Mari, A. Influence of the Natural Ripening Stage, Cold Storage, and Ethylene Treatment on the Protein and IgE-Binding Profiles of Green and Gold Kiwi Fruit Extracts. J. Agric. Food Chem. 2009, 57, 1565–1571. [Google Scholar] [CrossRef]
- Nugraha, R.; Ruethers, T.; Johnston, E.; Rolland, J.; O’Hehir, R.; Kamath, S.; Lopata, A. Effects of Extraction Buffer on the Solubility and Immunoreactivity of the Pacific Oyster Allergens. Foods 2021, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, M.; Cerasuolo, I.; Carratore, V.; Stanziola, A.A.; Zofra, S.; Romano, L.; Camardella, L.; Ciardiello, M.A. Kiwellin, a Novel Protein from Kiwi Fruit. Purification, Biochemical Characterization and Identification as an Allergen*. J. Protein Chem. 2005, 24, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, M.A.; D’Avino, R.; Amoresano, A.; Tuppo, L.; Carpentieri, A.; Carratore, V.; Tamburrini, M.; Giovane, A.; Pucci, P.; Camardella, L. The peculiar structural features of kiwi fruit pectin methylesterase: Amino acid sequence, oligosaccharides structure, and modeling of the interaction with its natural proteinaceous inhibitor. Proteins Struct. Funct. Bioinform. 2008, 71, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Weber, P.; Steinhart, H.; Paschke, A. Investigation of the Allergenic Potential of Wines Fined with Various Proteinogenic Fining Agents by ELISA. J. Agric. Food Chem. 2007, 55, 3127–3133. [Google Scholar] [CrossRef]
- Rolland, J.M.; Apostolou, E.; de Leon, M.P.; Stockley, C.S.; O’Hehir, R.E. Specific and Sensitive Enzyme-Linked Immunosorbent Assays for Analysis of Residual Allergenic Food Proteins in Commercial Bottled Wine Fined with Egg White, Milk, and Nongrape-Derived Tannins. J. Agric. Food Chem. 2007, 56, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, B.; Jiang, X.; Hsieh, Y.-H.P.; Rao, Q. Matrix effect on food allergen detection—A case study of fish parvalbumin. Food Chem. 2019, 274, 526–534. [Google Scholar] [CrossRef]
- Waiblinger, H.-U.; Boernsen, B.; Geppert, C.; Demmel, A.; Peterseil, V.; Koeppel, R. Ring trial validation of single and multiplex real-time PCR methods for the detection and quantification of the allergenic food ingredients mustard, celery, soy, wheat and rye. J. Consum. Prot. Food Saf. 2016, 12, 55–72. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Jansen, A.P.H.; Roovers, M.H.W.M.; Ruinemans-Koerts, J.; Wichers, H.J.; Savelkoul, H.F.J. Maillard-type neoallergens present in processed soy extract may cause an allergic reaction in soy allergic patients. Clin. Transl. Allergy 2015, 5, P21. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Chua, J.V.; Le, Q.; Trujillo, F.; Oh, M.-H.; Campbell, D.; Mehr, S.; Lee, N. A Response Surface Methodology (RSM) Approach for Optimizing the Attenuation of Human IgE-Reactivity to β-Lactoglobulin (β-Lg) by Hydrostatic High Pressure Processing. Foods 2021, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Peng, P.; Song, H.; Zhang, T.; Addy, M.; Zhang, Y.; Cheng, Y.; Hatzenbeller, R.; Zhu, X.; Liu, S.; Liu, Y.; et al. Concentrated high intensity electric field (CHIEF) system for non-thermal pasteurization of liquid foods: Modeling and simulation of fluid mechanics, electric analysis, and heat transfer. Comput. Chem. Eng. 2017, 97, 183–193. [Google Scholar] [CrossRef]
- Roberts, P.B. Food irradiation is safe: Half a century of studies. Radiat. Phys. Chem. 2014, 105, 78–82. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Demand, V.; Kern, K.; Strube, A.; Szardenings, M.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Enzymatic Hydrolysis and Fermentation of Pea Protein Isolate and Its Effects on Antigenic Proteins, Functional Properties, and Sensory Profile. Foods 2022, 11, 118. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Coimbra, J.S.D.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Garcia-Rojas, E.E. Food Protein-polysaccharide Conjugates Obtained via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2013, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Teodorowicz, M.; Van Neerven, J.; Savelkoul, H. Food Processing: The Influence of the Maillard Reaction on Immunogenicity and Allergenicity of Food Proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of Processing Technologies on the Allergenicity of Food Products. Crit. Rev. Food Sci. Nutr. 2013, 55, 1902–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuppo, L.; Alessandri, C.; Giangrieco, I.; Tamburrini, M.; Arriaza, R.H.; Chruszcz, M.; Mari, A.; Ciardiello, M.A. When the Frequencies of Sensitization and Elicitation of Allergic Reaction Do Not Correlate—The Case of Apple Gibberellin-Regulated Protein Tested in an Italian Population. Front. Allergy 2021, 2, 745825. [Google Scholar] [CrossRef]
- Alessandri, C.; Giangrieco, I.; Tuppo, L.; Ferrara, R.; Zennaro, D.; Bernardi, M.L.; Ciancamerla, M.; Rafaiani, C.; Rafaiani, C.; Tamburrini, M.; et al. Are peas a safe food for lipid transfer protein allergic patients? Allergy 2021, 76, 2587–2589. [Google Scholar] [CrossRef]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.; Delerue-Matos, C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit. Rev. Food Sci. Nutr. 2015, 56, 2304–2319. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; Ortea, I.; Vial, S.; Rivas, J.; Calo-Mata, P.; Barros-Velázquez, J. Advanced DNA- and Protein-based Methods for the Detection and Investigation of Food Allergens. Crit. Rev. Food Sci. Nutr. 2015, 56, 2511–2542. [Google Scholar] [CrossRef] [PubMed]
- Madrid, R.; García-García, A.; Cabrera, P.; González, I.; Martín, R.; García, T. Survey of Commercial Food Products for Detection of Walnut (Juglans regia) by Two ELISA Methods and Real Time PCR. Foods 2021, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Platteau, C.; De Loose, M.; De Meulenaer, B.; Taverniers, I. Quantitative Detection of Hazelnut (Corylus avellana) in Cookies: ELISA versus Real-Time PCR. J. Agric. Food Chem. 2011, 59, 11395–11402. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Nishitsuji, Y.; Kikuchi, Y.; Fukudome, S.-I.; Hayashida, T.; Kawakami, H.; Kurimoto, Y.; Noguchi, A.; Kondo, K.; Teshima, R.; et al. Quantification of DNA fragmentation in processed foods using real-time PCR. Food Chem. 2017, 226, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Iniesto, E.; Jiménez, A.; Prieto, N.; Cabanillas, B.; Burbano, C.; Pedrosa, M.M.; Rodríguez, J.; Muzquiz, M.; Crespo, J.F.; Cuadrado, C.; et al. Real Time PCR to detect hazelnut allergen coding sequences in processed foods. Food Chem. 2013, 138, 1976–1981. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, S.; Garber, E.A. Effects of processing on detection and quantification of the parvalbumin gene in Atlantic salmon (Salmo salar). Food Chem. 2010, 119, 75–80. [Google Scholar] [CrossRef]
- Herrero, B.; Vieites, J.M.; Espiñeira, M. Fast Real-Time PCR for the Detection of Crustacean Allergen in Foods. J. Agric. Food Chem. 2012, 60, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Eischeid, A.C.; Kim, B.-H.; Kasko, S.M. Two Quantitative Real-Time PCR Assays for the Detection of Penaeid Shrimp and Blue Crab, Crustacean Shellfish Allergens. J. Agric. Food Chem. 2012, 61, 5669–5674. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campuzano, S.; Montiel, V.R.-V.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Cutting-Edge Advances in Electrochemical Affinity Biosensing at Different Molecular Level of Emerging Food Allergens and Adulterants. Biosensors 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, M.; Neves, M.; Nouws, H.; Delerue-Matos, C. Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices. Foods 2021, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, X.; Teng, S.; Xu, X.; Zhou, G. Development and Validation of a Surface Plasmon Resonance Biosensor for Specific Detection of Porcine Serum Albumin in Food. J. AOAC Int. 2018, 101, 1868–1872. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, Q.; Zhang, C.; Sun, Z.; Weng, X. Microfluidic origami nano-aptasensor for peanut allergen Ara h1 detection. Food Chem. 2021, 365, 130511. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Oh, J.-H.; Park, M.-K.; Lee, J. Detection of Peanut Allergen Ara h 6 in Commercially Processed Foods using a Single-Walled Carbon Nanotube–Based Biosensor. J. AOAC Int. 2018, 101, 1558–1565. [Google Scholar] [CrossRef]
- Gamella, M.; Bueno-Díaz, C.; Montiel, V.R.-V.; Povedano, E.; Reviejo, A.; Villalba, M.; Campuzano, S.; Pingarrón, J. First electrochemical immunosensor for the rapid detection of mustard seeds in plant food extracts. Talanta 2020, 219, 121247. [Google Scholar] [CrossRef]
- Sun, X.; Li, C.; Zhu, Q.; Huang, H.; Jing, W.; Chen, Z.; Kong, L.; Han, L.; Wang, J.; Li, Y. A label-free photoelectrochemical immunosensor for detection of the milk allergen β-lactoglobulin based on Ag2S -sensitized spindle-shaped BiVO4/BiOBr heterojunction by an in situ growth method. Anal. Chim. Acta 2020, 1140, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Costa, J.; Sagastizábal, I.; Brandão, A.T.; Moreira, P.; Mafra, I.; Silva, A.F.; Pereira, C.M. Electrochemical and optical biosensing platforms for the immunorecognition of hazelnut Cor a 14 allergen. Food Chem. 2021, 361, 130122. [Google Scholar] [CrossRef] [PubMed]
- González-Buitrago, J.M.; Ferreira, L.; Isidoro-García, M.; Sanz, C.; Lorente, F.; Dávila, I. Proteomic approaches for identifying new allergens and diagnosing allergic diseases. Clin. Chim. Acta 2007, 385, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Aslanian, A.; Yates, J.R. Mass spectrometry for proteomics. Curr. Opin. Chem. Biol. 2008, 12, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmelik, J.; Zidkova, J.; Rehulka, P.; Petry-Podgorska, I.; Bobalova, J. Influence of different proteomic protocols on degree of high-coverage identification of nonspecific lipid transfer protein 1 modified during malting. Electrophoresis 2009, 30, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Kim, H.; Antonucci, G.; Fiume, I.; Guescini, M.; Kim, K.P.; Ciardiello, M.A.; Giangrieco, I.; Mari, A.; Pocsfalvi, G. Crosstalk Between the Immune System and Plant-Derived Nanovesicles: A Study of Allergen Transporting. Front. Bioeng. Biotechnol. 2021, 9, 760730. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, S.; Fourdrilis, S.; Dobson, R.; Scippo, M.-L.; Maghuin-Rogister, G.; De Pauw, E. Quantitative methods for food allergens: A review. Anal. Bioanal. Chem. 2009, 395, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vlierberghe, K.; Gavage, M.; Dieu, M.; Renard, P.; Arnould, T.; Gillard, N.; Coudijzer, K.; De Loose, M.; Gevaert, K.; Van Poucke, C. Selecting processing robust markers using high resolution mass spectrometry for the detection of milk in food products. J. AOAC Int. 2021, 105, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.P.; Heupel, L.; Zimmermann, L.; Peters, Y.; Vongehr, K.U.; El-Bedewy, H.; Siebeneicher, S.; Weiß, T.; Hektor, T.; Lindemann, B.; et al. Investigation of Reduced ELISA Recovery of Almond and Hazelnut Traces from Roasted Nut Samples by SDS-PAGE and Mass Spectrometry. J. AOAC Int. 2019, 102, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Röder, M.; Wiacek, C.; Lankamp, F.; Kreyer, J.; Weber, W.; Ueberham, E. Improved Sensitivity of Allergen Detection by Immunoaffinity LC-MS/MS Using Ovalbumin as a Case Study. Foods 2021, 10, 2932. [Google Scholar] [CrossRef] [PubMed]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 1972, 109, 129–135. [Google Scholar] [PubMed]
- Baumert, J.L. Detecting and Measuring Allergens in Food. Risk Manag. Food Allergy 2014, 2014, 215–226. [Google Scholar] [CrossRef]
- Immer, U.; Lacorn, M. Enzyme-linked immunosorbent assays (ELISAs) for detecting allergens in food. In Handbook of Food Allergen Detection and Control; Woodhead Publishing: Cambridge, UK, 2015; pp. 199–217. [Google Scholar] [CrossRef]
- Kadooka, Y.; Idota, T.; Gunji, H.; Shimatani, M.; Kawakami, H.; Dosako, S.-I.; Samori, T. A method for measuring specific IgE in sera by direct ELISA without interference by IgG competition or IgG autoantibodies to IgE. Int. Arch. Allergy Immunol. 2000, 122, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Orcajo, J.; Lavilla, M.; Martínez-De-Marañón, I. Specific and sensitive ELISA for measurement of IgE-binding variations of milk allergen β-lactoglobulin in processed foods. Anal. Chim. Acta 2018, 1052, 163–169. [Google Scholar] [CrossRef]
- Enck, K.M.; Lee, K.W.; McKinney, B.H.; Blankenship, K.D.; Montesano, C. Detection and inhibition of IgE antibodies reactive with cross-reactive carbohydrate determinants in an ELISA for allergen-specific IgE in horses. Vet. -Dermatol. 2021, 32, 685. [Google Scholar] [CrossRef] [PubMed]
- Fall, B.I.; Nießner, R. Detection of Known Allergen-Specific IgE Antibodies by Immunological Methods. Methods Mol. Biol. 2009, 509, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.D.; Mazzella, M.J.; Nixon, R.A.; Mathews, P.M. Aβ Measurement by Enzyme-Linked Immunosorbent Assay. Methods Mol. Biol. 2012, 849, 507–527. [Google Scholar] [CrossRef] [PubMed]
- Lacorn, M.; Dubois, T.; Gößwein, C.; Kredel, R.; Ferkinghoff, B.; Brunelle, S.; Théolier, J.; Dominguez, S.; Weiss, T. Validation of the RIDASCREEN® Peanut for Determination of Peanut Protein in Cookies, Milk Chocolate, Ice Cream, Trail Mix, Puffed Rice Cereals, and Granola Bar: AOAC Performance Tested MethodSM 112102. J. AOAC Int. 2021, 2021, qsab168. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Lardizabal, A.L.; Niemann, L.; Baumert, J.L.; Taylor, S.L. Development of a Sandwich Enzyme-Linked Immunosorbent Assay for Detection and Quantification of Clam Residues in Food Products. BioMed Res. Int. 2021, 2021, 6685575. [Google Scholar] [CrossRef] [PubMed]
- Pomés, A.; Vinton, R.; Chapman, M. Peanut Allergen (Ara h 1) Detection in Foods Containing Chocolate. J. Food Prot. 2004, 67, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.S.; Cassola, A. Novel sensitive monoclonal antibody based competitive enzyme-linked immunosorbent assay for the detection of raw and processed bovine beta-casein. PLoS ONE 2017, 12, e0182447. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.T.; Fæste, C.K.; Egaas, E. Quantitative Sandwich ELISA for the Determination of Tropomyosin from Crustaceans in Foods. J. Agric. Food Chem. 2007, 55, 8025–8032. [Google Scholar] [CrossRef] [PubMed]
- Garber, E.A.E.; Cho, C.Y.; Rallabhandi, P.; Nowatzke, W.L.; Oliver, K.G.; Venkateswaran, K.V.; Venkateswaran, N. Multi-laboratory validation of the xMAP—Food Allergen Detection Assay: A multiplex, antibody-based assay for the simultaneous detection of food allergens. PLoS ONE 2020, 15, e0234899. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, R.M.; Jackson, E.S.; Lin, A.V.; Haff, R.P.; McGarvey, J.A. A rapid and sensitive lateral flow immunoassay (LFIA) for the detection of gluten in foods. Food Chem. 2021, 355, 129514. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Russo, A.; Cavalera, S.; Giovannoli, C.; Spano, G.; Baumgartner, S.; Lauter, K.; Baggiani, C. Silver and gold nanoparticles as multi-chromatic lateral flow assay probes for the detection of food allergens. Anal. Bioanal. Chem. 2018, 411, 1905–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civera, A.; Galan-Malo, P.; Segura-Gil, I.; Mata, L.; Tobajas, A.P.; Sánchez, L.; Pérez, M.D. Development of sandwich ELISA and lateral flow immunoassay to detect almond in processed food. Food Chem. 2021, 371, 131338. [Google Scholar] [CrossRef] [PubMed]
- Harwanegg, C.; Hiller, R. Protein microarrays for the diagnosis of allergic diseases: State-of-the-art and future development. Clin. Chem. Lab. Med. (CCLM) 2005, 43, 1321–1326. [Google Scholar] [CrossRef]
- Alessandri, C.; Ferrara, R.; Bernardi, M.L.; Zennaro, D.; Tuppo, L.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Diagnosing allergic sensitizations in the third millennium: Why clinicians should know allergen molecule structures. Clin. Transl. Allergy 2017, 7, 21. [Google Scholar] [CrossRef]
- Giangrieco, I.; Ricciardi, T.; Alessandri, C.; Farina, L.; Crescenzo, R.; Tuppo, L.; Ciancamerla, M.; Rafaiani, C.; Bernardi, M.L.; Digilio, A.F.; et al. ENEA, a peach and apricot IgE-binding protein cross-reacting with the latex major allergen Hev b 5. Mol. Immunol. 2019, 112, 347–357. [Google Scholar] [CrossRef]
- Akarsu, A.; Ocak, M.; Sahiner, U.M.; Soyer, O.; Sekerel, B.E. Multiplex component-based allergen macroarray test is useful to predict clinical reactivity to tree nuts in children. Allergol. Int. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Zennaro, D.; Scala, E.; Ferrara, R.; Bernardi, M.L.; Santoro, M.; Palazzo, P.; Mari, A. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen’s egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin. Exp. Allergy 2011, 42, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Schulten, V.; Nagl, B.; Scala, E.; Bernardi, M.L.; Mari, A.; Ciardiello, M.A.; Lauer, I.; Scheurer, S.; Briza, P.; Jürets, A.; et al. Pru p 3, the nonspecific lipid transfer protein from peach, dominates the immune response to its homolog in hazelnut. Allergy 2011, 66, 1005–1013. [Google Scholar] [CrossRef]
- Bernardi, M.L.; Giangrieco, I.; Camardella, L.; Ferrara, R.; Palazzo, P.; Panico, M.R.; Crescenzo, R.; Carratore, V.; Zennaro, D.; Liso, M.; et al. Allergenic Lipid Transfer Proteins from Plant-Derived Foods Do Not Immunologically and Clinically Behave Homogeneously: The Kiwifruit LTP as a Model. PLoS ONE 2011, 6, e27856. [Google Scholar] [CrossRef] [Green Version]
- Tuppo, L.; Alessandri, C.; Giangrieco, I.; Ciancamerla, M.; Rafaiani, C.; Tamburrini, M.; Ciardiello, M.A.; Mari, A. Isolation of cypress gibberellin-regulated protein: Analysis of its structural features and IgE binding competition with homologous allergens. Mol. Immunol. 2019, 114, 189–195. [Google Scholar] [CrossRef] [PubMed]
- D’Avino, R.; Bernardi, M.L.; Wallner, M.; Palazzo, P.; Camardella, L.; Tuppo, L.; Alessandri, C.; Breiteneder, H.; Ferreira, F.; Ciardiello, M.A.; et al. Kiwifruit Act d 11 is the first member of the ripening-related protein family identified as an allergen. Allergy 2011, 66, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Sforza, S.; Palazzo, P.; Lambertini, F.; Paolella, S.; Zennaro, D.; Rafaiani, C.; Ferrara, R.; Bernardi, M.L.; Santoro, M.; et al. Tolerability of a Fully Maturated Cheese in Cow’s Milk Allergic Children: Biochemical, Immunochemical, and Clinical Aspects. PLoS ONE 2012, 7, e40945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberer, W.; Holzweber, F.; Hemmer, W.; Koch, L.; Bokanovic, D.; Fellner, W.; Altmann, F. Inhibition kreuzreaktiver Kohlenhydratdeterminanten (CCDs) erhöht die Treffsicherheit der In-vitro-Allergiediagnostik. Allergologie 2014, 37, 46–54. [Google Scholar] [CrossRef]
- Roccotiello, E.; Nicosia, E.; Pierdonà, L.; Marescotti, P.; Ciardiello, M.A.; Giangrieco, I.; Mari, A.; Zennaro, D.; Dozza, D.; Brancucci, M.; et al. Tomato (Solanum Lycopersicum L.) Response to Nickel Stress: Bioavailability, Accumulation and Allergenicity. Res. Sq. 2021, in press. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczynska, J.; Fotopoulos, V. Influence of Heavy Metals (Ni, Cu, and Zn) on Nitro-Oxidative Stress Responses, Proteome Regulation and Allergen Production in Basil (Ocimum basilicum L.) Plants. Front. Plant Sci. 2018, 9, 862. [Google Scholar] [CrossRef] [Green Version]
- Tuppo, L.; Spadaccini, R.; Alessandri, C.; Wienk, H.; Boelens, R.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Picone, D.; Ciardiello, M.A. Structure, stability, and IgE binding of the peach allergen Peamaclein (Pru p 7). Biopolymers 2014, 102, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Tuppo, L.; Alessandri, C.; Pasquariello, M.S.; Petriccione, M.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Pomegranate Cultivars: Identification of the New IgE-Binding Protein Pommaclein and Analysis of Antioxidant Variability. J. Agric. Food Chem. 2017, 65, 2702–2710. [Google Scholar] [CrossRef]
- Hazebrouck, S.; Canon, N.; Dreskin, S.C. The Effector Function of Allergens. Front. Allergy 2022, 3. [Google Scholar] [CrossRef]
- Cerecedo, I.; Zamora, J.; Fox, M.; Voordouw, J.; Plana, N.; Rokicka, E.; Fernandez-Rivas, M.; Cortés, S.V.; Reche, M.; Fiandor, A.; et al. The impact of double-blind placebo- controlled food challenge (DBPCFC) on the socioeconomic cost of food allergy in Europe. J. Investig. Allergy Clin. Immunol. 2014, 24, 418–424. [Google Scholar]

| Methodology | References | Comments | Best Suited for… |
|---|---|---|---|
| DNA-based methods | [48,49,50,51,52,53,54,55] |
|
|
| Biosensors | [56,57,58,59,60,61,62,63,64] |
|
|
| Mass-spectrometry-based methods | [23,65,66,67,68,69,70,71,72] |
|
|
| ELISA | [73,74,75,76,77,78,79,80,81,82,83,84,85,86] |
|
|
| LFIA | [87,88,89] |
|
|
| Multiplex allergen technology | [90,91,92,93,94,95,96,97,98,99,100,101,102] |
|
|
| Allergen Source | FABER Extracts * | FABER Allergens * | ISAC Allergens * |
|---|---|---|---|
| Gold kiwifruit | Act c (fruit) | Act c 11, Act c chitinase IV | – |
| Green kiwifruit | Act d (fruit) | Act d 1, Act d 2, Act d 5, Act d 10 | Act d 1, Act d 2, Act d 5, Act d 8 |
| Mosquito | Aed c (saliva) | – | – |
| Onion | All c (bulb) | – | – |
| Leek | All p (bulb) | – | – |
| Garlic | All s (bulb) | – | – |
| Alder | – | – | Aln g 1 |
| Alternaria | – | Alt a 1, Alt a 6.0101 | Alt a 1, Alt a 6 |
| Amaranth | Ama cr (seed) | – | – |
| Ragweed | Amb a (pollen) | Amb a 1 | Amb a 1 |
| Pineapple | – | Ana c 2 | – |
| Cashew | Ana o (seed) | Ana o 3 | Ana o 2 |
| Duck | Ana p (egg yolk), Ana p (egg white) | _ | _ |
| Anisakis parasite | Ani pe (larva) | Ani s 1, Ani s 3 | Ani s 1, Ani s 3 |
| Celery | Api g (stalk) | Api g 1.0101 | Api g 1 |
| Honey bee | Api m (venom) | Api m 1, Api m 4 | Api m 1, Api m 4 |
| Peanut | Ara h (seed) | Ara h 1, Ara h 2, Ara h 3, Ara h 6, Ara h 8.0101, Ara h 9, Ara h agglutinin | Ara h 1, Ara h 2, Ara h 3, Ara h 6, Ara h 8 |
| Horseradish | – | Arm r horseradish peroxidase | – |
| Mugwort | Art v (pollen) | Art v 1 | Art v 1, Art v 3 |
| Aspergillus | Asp f (whole body) | Asp r 1 | Asp f 1, Asp f 3, Asp f 6 |
| Asparagus | Aspa o (stem) | – | – |
| Brazil nut | Ber e (seed) | – | Ber e 1 |
| Birch | Bet v (pollen) | Bet v 1.0101, Bet v 2.0101 | Bet v 1, Bet v 2, Bet v 4 |
| Common beet | Beta v (leaf) | – | – |
| German cockroach | Bla g (whole body) | Bla g 1, Bla g 2, Bla g 4, Bla g 5, | Bla g 1, Bla g 2, Bla g 5, Bla g 7 |
| Blomia | Blo t (whole body) | – | Blo t 5 |
| Cow | Bos d (milk), Bos d (muscle) | Bos d 4, Bos d 5, Bos d 6, Bos d 8, Bos d carbonic anhydrase, Bos d gelatin, Bos d lactoferrin | Bos d 4, Bos d 5, Bos d 6, Bos d 8, Bos d lactoferrin |
| Buffalo | Bub b (milk) | – | – |
| Camel | Cam d (milk) | – | – |
| Dog | Can f (epithelium) | Can f 1, Can f 2, Can f 3, Can f 5 | Can f 1, Can f 2, Can f 3, Can f 5 |
| Candida | Cand a (whole body) | – | – |
| Goat | Cap h (milk) | – | – |
| Chestnut | Cas s (seed) | – | – |
| Guinea pig | Cav p (epithelium) | – | – |
| Carob | Cer si (seed) | – | – |
| Goosefoot | – | – | Che a 1 |
| Quinoa | Que qu (seed) | – | – |
| Chickpea | Cic a (seed) | – | – |
| Tangerine | Cit r (fruit) | – | – |
| Cladosporium | Cla h (whole body) | – | Cla h 8 |
| Hazelnut | Cor a (seed) | Cor a 1.0103, Cor a 14, Cor a 8, Cor a 9 | Cor a 1.0101, Cor a 1.0401, Cor a 8, Cor a 9 |
| Common quail | Cot c (egg yolk), Cot c (egg white) | – | – |
| Hamster | Cri c (epithelium) | – | – |
| Japanese cedar | Cry j (pollen) | – | Cry j 1 |
| Cantaloupe melon | Cuc m (fruit) | – | – |
| Cucumber | Cuc s (fruit) | – | – |
| Cypress | – | Cup a 1 | – |
| Bermuda grass | – | – | Cyn d 1 |
| Carrot | Dau c (root) | – | – |
| Mites | Der p (whole body) | Der f 1, Der f 2, Der p 1, Der p 2, Der p 10, Der p 23.0101, Der p 7, Der p 9 | Der f 1, Der f 2, Der p 1, Der p 2, Der p 10, Lep d 2 |
| European anchovy | Eng e (muscle) | – | – |
| Donkey | Equ as (milk) | – | – |
| Horse | Equ c (epithelium), Equ c (milk) | Equ c 3, Equ c myoglobin | Equ c 1, Equ c 3 |
| House dust mite | – | Eur m 2 | - |
| Buckwheat | Fag e (seed) | - | Fag e 2 |
| Cat | Fel d (epithelium) | Fel d 1, Fel d 2 | Fel d 1, Fel d 2, Fel d 4 |
| Fennel | Foe v (bulb) | – | – |
| Strawberry | Fra a (fruit) | – | – |
| Atlantic cod | Gad m (muscle) | – | Gad c 1 |
| Chicken | Gal d (egg yolk), Gal d (egg white), Gal d (muscle) | Gal d 1, Gal d 2, Gal d 3, Gal d 4, Gal d 5 | Gal d 1, Gal d 2, Gal d 3, Gal d 5 |
| Soybean | Gly m (seed) | Gly m 1, Gly m agglutinin, Gly m trypsin inhibitor | Gly m 4, Gly m 5, Gly m 6 |
| Snail | Hel as (muscle) | Hel as 1 | – |
| Rubber tree | Hev b (latex) | Hev b 1, Hev b 10, Hev b 11, Hev b 3.0101, Hev b 5.0101, Hev b 6.02, Hev b 7.02, Hev b 8 | Hev b 1, Hev b 3, Hev b 5, Hev b 6.01, Hev b 8 |
| American lobster | Hom a (muscle) | – | – |
| Human | – | Hom s serum albumin, Hom s lactoferrin | – |
| Barley | Hor v (seed) | – | – |
| Walnut | Jug r (seed) | Jug r 2, Jug r 3 | Jug r 1, Jug r 2, Jug r 3 |
| Lettuce | Lac s (leaf) | – | – |
| Lentil | Len c (seed) | – | – |
| Linseed | Lin us (seed) | – | – |
| Shrimp | Lit v (whole body) | Lit v 1 | Pen m 1, Pen m 2, Pen m 4 |
| Rye grass | Lol p (pollen) | Lol p 1 | – |
| Lupine | Lup a (seed) | – | – |
| Apple | Mal d (fruit) | Mal d 1.0108 | Mal d 1 |
| Common turkey | Mel g (egg yolk), Mel g (egg white), Mel g (muscle) | – | – |
| Annual Mercury | – | Mer a 1 | Mer a 1 |
| European Hake | – | Mer mr 1 | – |
| Mouse | Mus m (epithelium) | Mus m 1, Mus m 4 | Mus m 1 |
| Mussel | Myt g (muscle) | – | – |
| Olive tree | Ole e (pollen) | Ole e 1, Ole e 2 | Ole e 1, Ole e 7, Ole e 9 |
| Rabbit | Ory c (epithelium), Ory c (muscle) | Ory c 6 | – |
| Rice | Ory s (seed) | – | – |
| Sheep | Ovi a (milk), Ovi a (muscle) | Ovi a 6 | – |
| Pellitory | Par j (pollen) | Par j 2 | Par j 2 |
| Penicillium | Pen ch (whole body) | – | – |
| American cockroach | Per a (whole body) | Per a 7 | – |
| Avocado | Pers a (fruit) | – | – |
| Bean | Pha v (seed) | – | – |
| Timothy grass | Phl p (pollen) | Phl p 1.0102, Phl p 2.0101, Phl p 5.0101, Phl p 6.0101, Phl p 7.0101 | Phl p 1, Phl p 2, Phl p 4, Phl p 5b, Phl p 6, Phl p 11, Phl p 12 |
| Pine nut | Pin p (seed) | – | – |
| Peas | – | Pis s 3 | – |
| Pistachio | Pis v (seed) | – | – |
| American sycamore | Pla a (pollen) | Pla a 1 | Pla a 1, Pla a2, Pla a 3 |
| Ribwort | – | – | Pla l 1 |
| Mushroom | Ple o (whole body) | – | – |
| Paper wasp | Pol spp (venom) | – | Pol d 5 |
| Apricot | Pru ar (fruit) | – | – |
| Almond | Pru du (seed) | – | – |
| Peach | Pru p (pulp), Pru p (peel) | Pru p 3, Pru p 7 | Pru p 1, Pru p 3 |
| Pomegranate | Pun g (fruit) | Pun g 1, Pun g 14, Pun g 5, Pun g 7 | – |
| Oak | Que a (pollen) | – | – |
| Rat | Rat n (epithelium) | Rat n 1, Rat n 4 | – |
| Saccharomyces | Sac c (whole body) | – | – |
| Salsola | – | – | Sal k 1 |
| Salmon | Sal s (muscle) | – | – |
| Sesame | Ses i (seed) | – | Ses i 1 |
| White mustard | Sin a (seed) | – | – |
| Common sole | Sol so (muscle) | – | – |
| Tomato | Sola l (fruit), Sola l (seed) | Sola l 6 | – |
| Eggplant | Sola m (fruit) | – | – |
| Potato | Sola t (tuber) | Sola t 1 | – |
| Spinach | Spi o (leaf) | – | – |
| Domestic pig | Sus s (muscle) | Sus s 1 | – |
| Tuna | Thu a (muscle) | – | – |
| Wheat | Tri a (seed) | Tri a 7k-LTP, Tri a 18, Tri a 28, Tri a gliadin | Tri a 14, Tri a 19.0101, Tri a_trypsin inhibitor |
| Trichophyton | Tri me (whole body) | – | – |
| Kamut | Tri tp (seed) | – | – |
| Squid | Uro du (muscle) | Uro du 1 | – |
| Clam | Ven ga (muscle) | Ven ga 1 | – |
| Wasp | Ves spp (venom) | – | Ves v 5 |
| Grape | Vit v (fruit) | – | – |
| Corn | Zea m (seed) | Zea m 14 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuppo, L.; Giangrieco, I.; Tamburrini, M.; Alessandri, C.; Mari, A.; Ciardiello, M.A. Detection of Allergenic Proteins in Foodstuffs: Advantages of the Innovative Multiplex Allergen Microarray-Based Immunoassay Compared to Conventional Methods. Foods 2022, 11, 878. https://doi.org/10.3390/foods11060878
Tuppo L, Giangrieco I, Tamburrini M, Alessandri C, Mari A, Ciardiello MA. Detection of Allergenic Proteins in Foodstuffs: Advantages of the Innovative Multiplex Allergen Microarray-Based Immunoassay Compared to Conventional Methods. Foods. 2022; 11(6):878. https://doi.org/10.3390/foods11060878
Chicago/Turabian StyleTuppo, Lisa, Ivana Giangrieco, Maurizio Tamburrini, Claudia Alessandri, Adriano Mari, and Maria Antonietta Ciardiello. 2022. "Detection of Allergenic Proteins in Foodstuffs: Advantages of the Innovative Multiplex Allergen Microarray-Based Immunoassay Compared to Conventional Methods" Foods 11, no. 6: 878. https://doi.org/10.3390/foods11060878
APA StyleTuppo, L., Giangrieco, I., Tamburrini, M., Alessandri, C., Mari, A., & Ciardiello, M. A. (2022). Detection of Allergenic Proteins in Foodstuffs: Advantages of the Innovative Multiplex Allergen Microarray-Based Immunoassay Compared to Conventional Methods. Foods, 11(6), 878. https://doi.org/10.3390/foods11060878