Impact of an Agriphotovoltaic System on Metabolites and the Sensorial Quality of Cabbage (Brassica oleracea var. capitata) and Its High-Temperature-Extracted Juice
Abstract
:1. Introduction
2. Materials and Methods
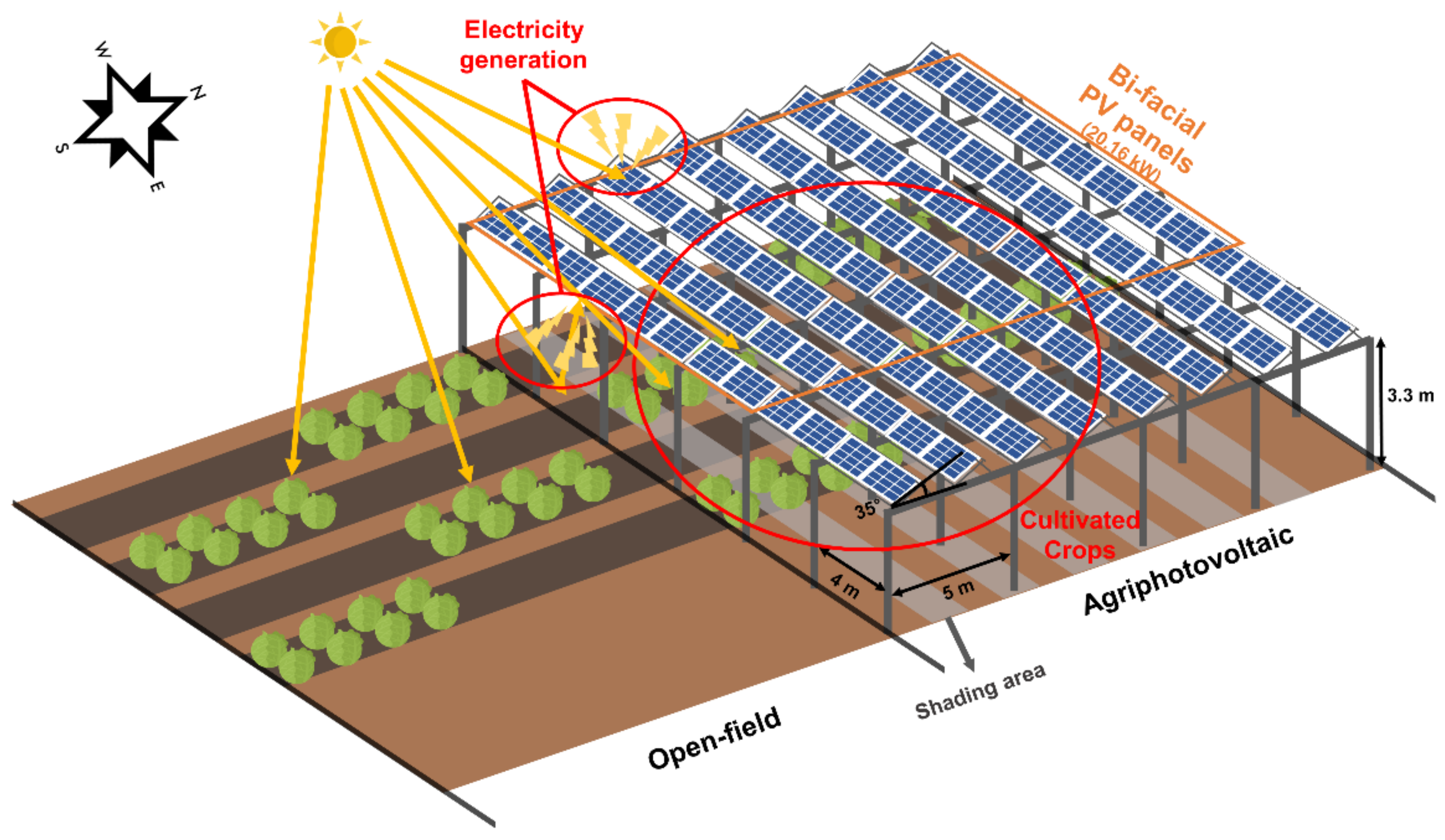
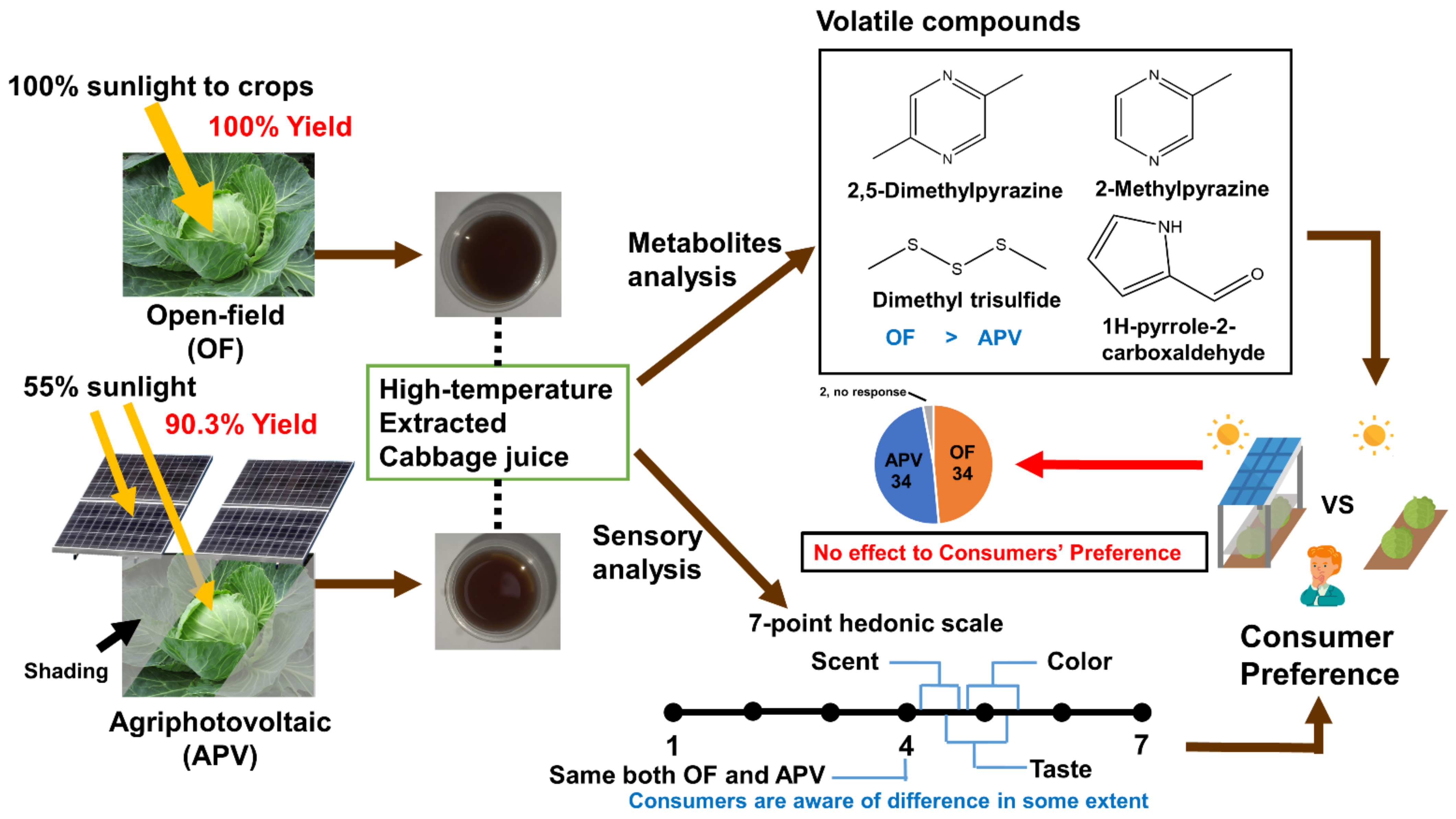
2.1. APV System
2.2. Collection of Microclimate Data
2.3. Cultivation of Cabbage
2.4. Preparation of High-Temperature-Extracted Cabbage Juice
2.5. Quantification of Glucosinolates
2.6. Determination of Glucosinolate Hydrolysis Products and Volatile Compounds
2.7. Sensorial Evaluation
2.8. Profiling of Primary Metabolites
2.9. Determination of Color Difference of Cabbage Juice
2.10. Data Processing and Statistical Analysis
3. Results and Discussions
3.1. Differences in Microclimate and Growth of Cabbage Grown under Open-Field Cultivation and Agriphotovoltaic System
3.1.1. Changed Microclimatic Conditions under the Agriphotovoltaic System
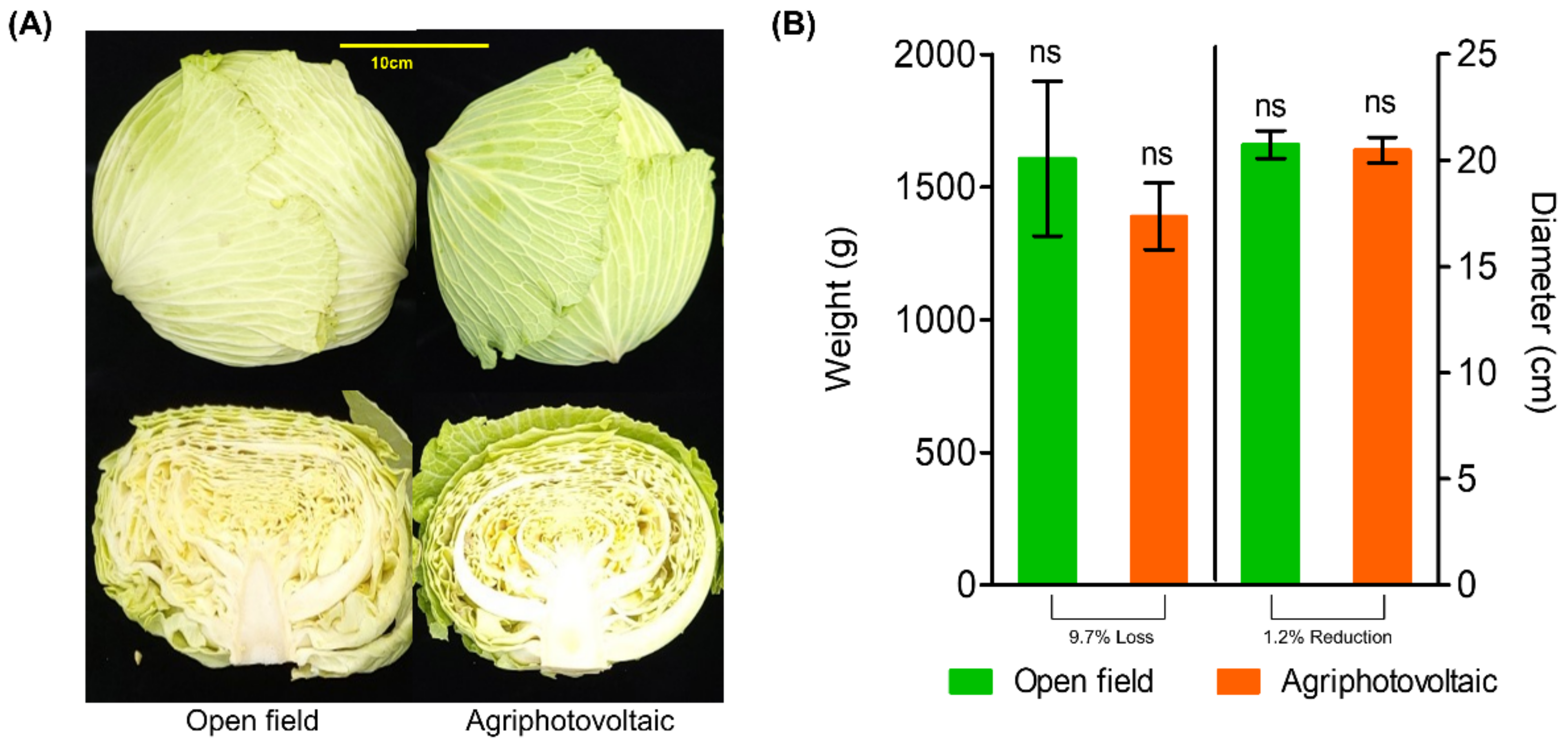
3.1.2. Cabbage Yield under Agriphotovoltaic System
3.2. Comparison of Metabolites between Grown under Open-Field and Agriphotovoltaic Systems
3.2.1. Glucosinolates and their Hydrolysis Products
3.2.2. Difference in Primary Metabolites
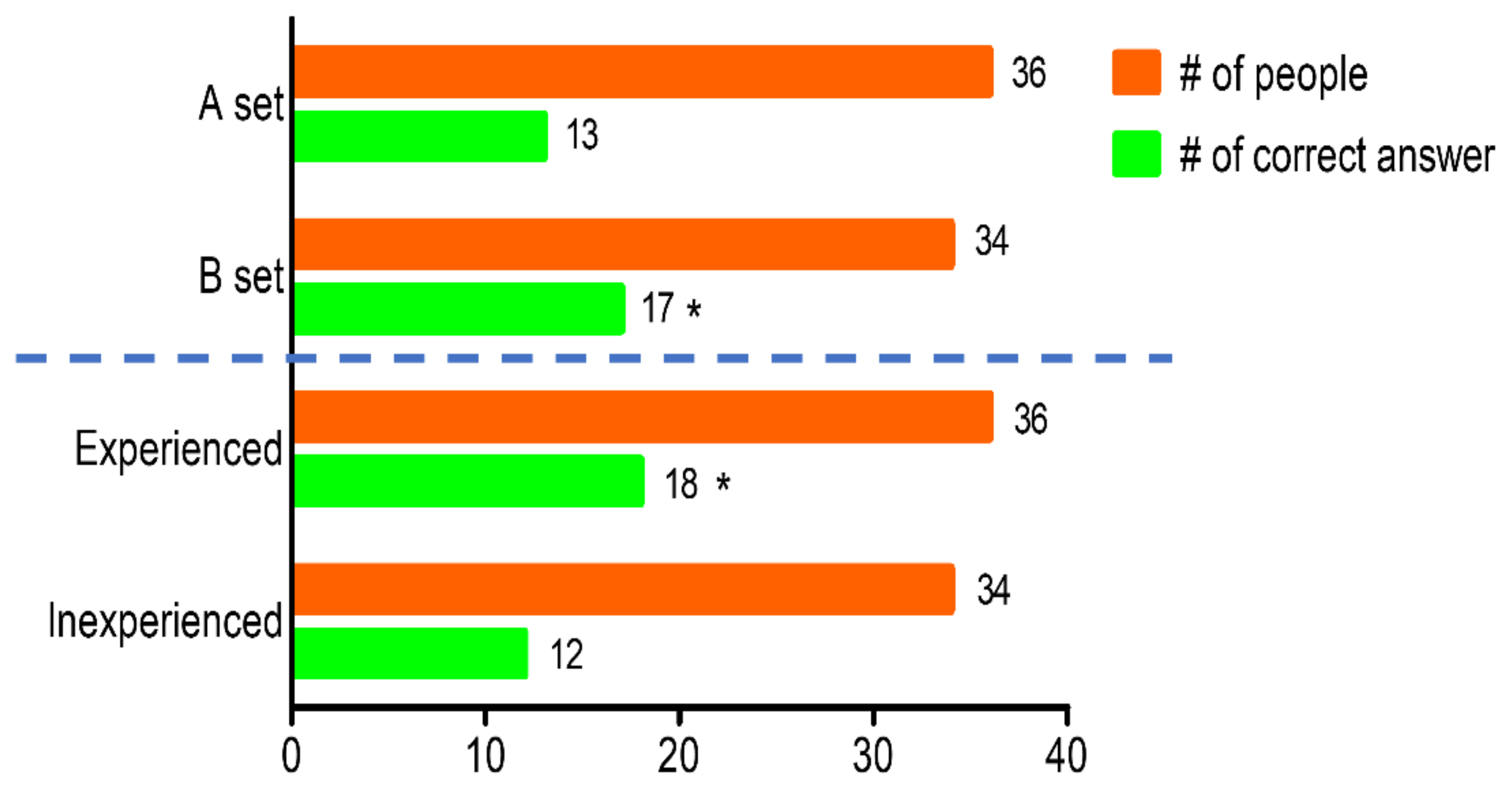
3.3. Sensory Evaluation of Cabbage Juice
3.4. Parameters Affecting the Sensory Properties of Cabbage Juice
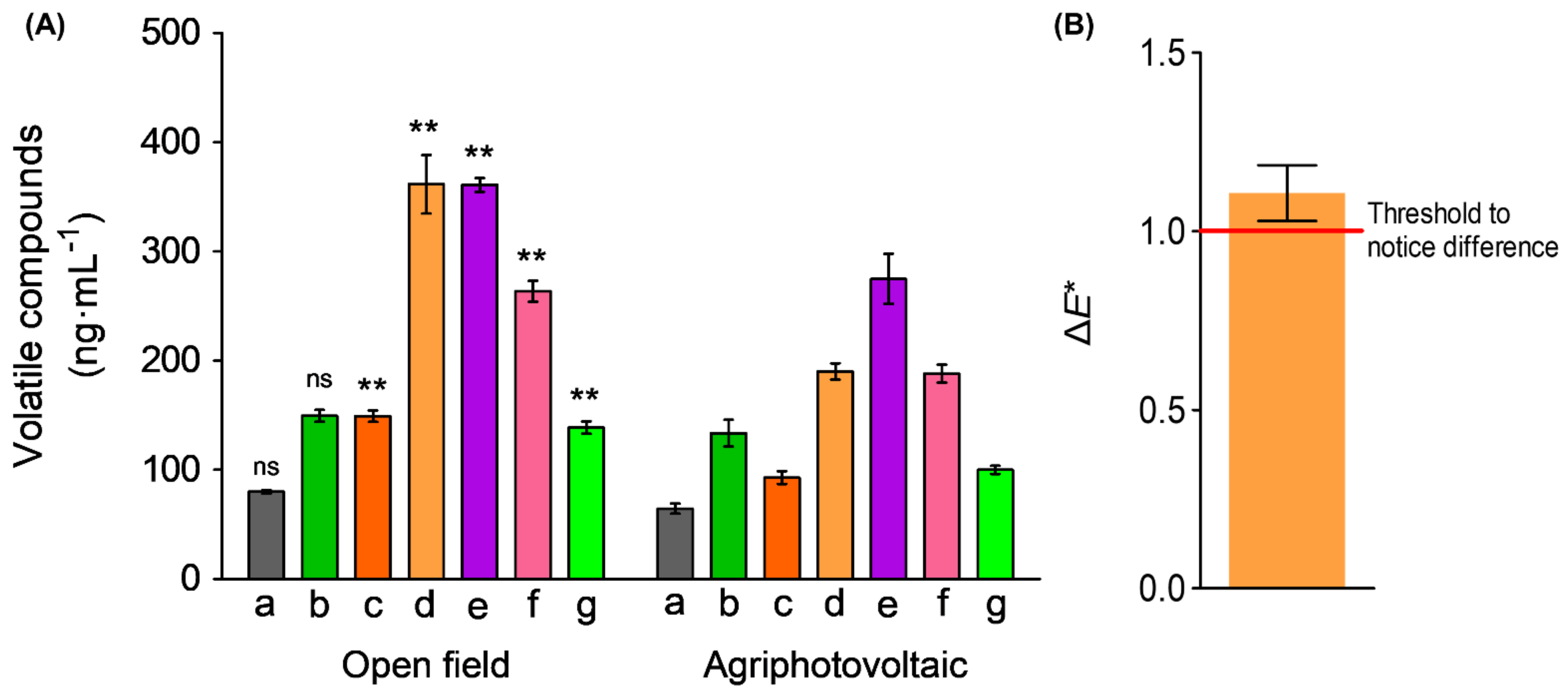
3.4.1. Volatile Organic Compounds
3.4.2. Differences in Colorimetric Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solangi, K.; Islam, M.; Saidur, R.; Rahim, N.; Fayaz, H. A review on global solar energy policy. Renew. Sustain. Energy Rev. 2011, 15, 2149–2163. [Google Scholar] [CrossRef]
- Elborg, M. Reducing land competition for agriculture and photovoltaic energy generation–a comparison of two agro-photovoltaic plants in japan. In Proceedings of the International Conference on Sustainable and Renewable Energy Development and Design (SREDD2017), Mohithang Thimphu, Bhutan, 3–5 April 2017. [Google Scholar]
- Marrou, H.; Wéry, J.; Dufour, L.; Dupraz, C. Productivity and radiation use efficiency of lettuces grown in the partial shade of photovoltaic panels. Eur. J. Agron. 2013, 44, 54–66. [Google Scholar] [CrossRef]
- Amaducci, S.; Yin, X.; Colauzzi, M. Agrivoltaic systems to optimise land use for electric energy production. Appl. Energy 2018, 220, 545–561. [Google Scholar] [CrossRef]
- Touil, S.; Richa, A.; Fizir, M.; Bingwa, B. Shading effect of photovoltaic panels on horticulture crops production: A mini review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 281–296. [Google Scholar] [CrossRef]
- Weselek, A.; Bauerle, A.; Zikeli, S.; Lewandowski, I.; Högy, P. Effects on crop development, yields and chemical composition of celeriac (apium graveolens l. Var. Rapaceum) cultivated underneath an agrivoltaic system. Agronomy 2021, 11, 733. [Google Scholar] [CrossRef]
- Hassanien, R.H.E.; Ming, L. Influences of greenhouse-integrated semi-transparent photovoltaics on microclimate and lettuce growth. Int. J. Agricult. Biol. Eng. 2017, 10, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Blando, F.; Gerardi, C.; Renna, M.; Castellano, S.; Serio, F. Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses. J. Berry Res. 2018, 8, 55–69. [Google Scholar] [CrossRef]
- López-Díaz, G.; Carreño-Ortega, A.; Fatnassi, H.; Poncet, C.; Díaz-Pére, M.Z. The effect of different levels of shading in a photovoltaic greenhouse with a north–south orientation. Appl. Sci. 2020, 10, 882. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Ma, X.; Li, M.; Wang, Y. The effect of temperature and light on strawberry production in a solar greenhouse. Solar Energy 2020, 195, 318–328. [Google Scholar] [CrossRef]
- Hassanpour Adeh, E.; Selker, J.S.; Higgins, C.W. Remarkable agrivoltaic influence on soil moisture, micrometeorology and water-use efficiency. PLoS ONE 2018, 13, e0203256. [Google Scholar] [CrossRef] [Green Version]
- Marrou, H.; Guilioni, L.; Dufour, L.; Dupraz, C.; Wéry, J. Microclimate under agrivoltaic systems: Is crop growth rate affected in the partial shade of solar panels? Agricult. For. Meteorol. 2013, 177, 117–132. [Google Scholar] [CrossRef]
- Deng, B.; Shang, X.; Fang, S.; Li, Q.; Fu, X.; Su, J. Integrated effects of light intensity and fertilization on growth and flavonoid accumulation in cyclocarya paliurus. J. Agricult. Food Chem. 2012, 60, 6286–6292. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, N.; Aguirrezábal, L.; Andrade, F.; Geroudet, C.; Valentinuz, O.; Iraola, M.P. Intercepted solar radiation affects oil fatty acid composition in crop species. Field Crops Res. 2009, 114, 66–74. [Google Scholar] [CrossRef]
- Johnson, I.T. Glucosinolates: Bioavailability and importance to health. Int. J. Vitam. Nutr. Res. 2002, 72, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, D.T.; Verhagen, H.; Goldbohm, R.A.; van den Brandt, P.A.; van Poppel, G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem. Biol. Interact. 1997, 103, 79–129. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef]
- Engel, E.; Baty, C.; le Corre, D.; Souchon, I.; Martin, N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J. Agricult. Food Chem. 2002, 50, 6459–6467. [Google Scholar] [CrossRef]
- Van Doorn, H.E.; van der Kruk, G.C.; van Holst, G.J.; Raaijmakers-Ruijs, N.C.; Postma, E.; Groeneweg, B.; Jongen, W.H. The glucosinolates sinigrin and progoitrin are important determinants for taste preference and bitterness of brussels sprouts. J. Sci. Food Agricult. 1998, 78, 30–38. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Matak, K.; Ku, K.-M. Methyl jasmonate treated broccoli: Impact on the production of glucosinolates and consumer preferences. Food Chem. 2019, 299, 125099. [Google Scholar] [CrossRef]
- Bohinc, T.; Trdan, S. Environ. factors affecting the glucosinolate content in brassicaceae. J. Food Agric. Environ. 2012, 10, 357. [Google Scholar]
- Ciska, E.; Martyniak-Przybyszewska, B.; Kozlowska, H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J. Agricult. Food Chem. 2000, 48, 2862–2867. [Google Scholar] [CrossRef] [PubMed]
- Mølmann, J.A.; Steindal, A.L.; Bengtsson, G.B.; Seljåsen, R.; Lea, P.; Skaret, J.; Johansen, T.J. Effects of temperature and photoperiod on sensory quality and contents of glucosinolates, flavonols and vitamin c in broccoli florets. Food Chem. 2015, 172, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cheney, G. Rapid healing of peptic ulcers in patients receiving fresh cabbage juice. Calif. Med. 1949, 70, 10. [Google Scholar] [PubMed]
- Butz, P.; Garcia, A.F.; Lindauer, R.; Dieterich, S.; Bognar, A.; Tauscher, B. Influence of ultra high pressure processing on fruit and vegetable products. J. Food Eng. 2003, 56, 233–236. [Google Scholar] [CrossRef]
- Han, D.-C.; Kyung, K.-H. Antimicrobial activity of autoclaved cabbage juice. Korean J. Food Sci. Technol. 1995, 27, 74–79. [Google Scholar]
- Artru, S.; Garré, S.; Dupraz, C.; Hiel, M.-P.; Blitz-Frayret, C.; Lassois, L. Impact of spatio-temporal shade dynamics on wheat growth and yield, perspectives for temperate agroforestry. Eur. J. Agron. 2017, 82, 60–70. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Pavao-Zuckerman, M.A.; Minor, R.L.; Sutter, L.F.; Barnett-Moreno, I.; Blackett, D.T.; Thompson, M.; Dimond, K.; Gerlak, A.K.; Nabhan, G.P. Agrivoltaics provide mutual benefits across the food–energy–water nexus in drylands. Nat. Sustain. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Valle, B.; Simonneau, T.; Sourd, F.; Pechier, P.; Hamard, P.; Frisson, T.; Ryckewaert, M.; Christophe, A. Increasing the total productivity of a land by combining mobile photovoltaic panels and food crops. Appl. Energy 2017, 206, 1495–1507. [Google Scholar] [CrossRef]
- Weselek, A.; Ehmann, A.; Zikeli, S.; Lewandowski, I.; Schindele, S.; Högy, P. Agrophotovoltaic systems: Applications, challenges, and opportunities. A review. Agron. Sustain. Develop. 2019, 39, 35. [Google Scholar] [CrossRef]
- Ku, K.-M.; Kim, M.J.; Jeffery, E.H.; Kang, Y.-H.; Juvik, J.A. Profiles of glucosinolates, their hydrolysis products, and quinone reductase inducing activity from 39 arugula (eruca sativa mill.) accessions. J. Agricult. Food Chem. 2016, 64, 6524–6532. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Ku, K.-M. Optimization of allyl isothiocyanate sanitizing concentration for inactivation of salmonella typhimurium on lettuce based on its phenotypic and metabolome changes. Food Chem. 2021, 364, 130438. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Influence of seasonal variation and methyl jasmonate mediated induction of glucosinolate biosynthesis on quinone reductase activity in broccoli florets. J.Agricult. Food Chem. 2013, 61, 9623–9631. [Google Scholar] [CrossRef] [PubMed]
- Ciska, E.; Kozłowska, H. The effect of cooking on the glucosinolates content in white cabbage. Eur. Food Res. Technol. 2001, 212, 582–587. [Google Scholar] [CrossRef]
- Oerlemans, K.; Barrett, D.M.; Suades, C.B.; Verkerk, R.; Dekker, M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006, 95, 19–29. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Patras, A.; Mullen, A.M.; Marcos, B. Assessing the impact of high-pressure processing on selected physical and biochemical attributes of white cabbage (brassica oleracea l. Var. Capitata alba). Food Bioprocess. Technol. 2014, 7, 682–692. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef] [Green Version]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic variation of glucosinolates and their breakdown products in leaves of brassica rapa. J. Agricult. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef]
- O’Mahony, M.; Odbert, N. A comparison of sensory difference testing procedures: Sequential sensitivity analysis and aspects of taste adaptation. J. Food Sci. 1985, 50, 1055–1058. [Google Scholar] [CrossRef]
- Agresti, P.D.M.; Franca, A.S.; Oliveira, L.S.; Augusti, R. Discrimination between defective and non-defective brazilian coffee beans by their volatile profile. Food Chem. 2008, 106, 787–796. [Google Scholar] [CrossRef]
- Maruyama, F. Identification of dimethyl trisulfide as a major aroma component of cooked brassicaceous vegetables. J. Food Sci. 1970, 35, 540–543. [Google Scholar] [CrossRef]
- Chin, H.W.; Lindsay, R. Volatile sulfur compounds formed in disrupted tissues of different cabbage cultivars. J. Food Sci. 1993, 58, 835–839. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour difference∆ e-a survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Kim, M.J.; Chiu, Y.-C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.-M. Cultivar-specific changes in primary and secondary metabolites in pak choi (Brassica rapa, Chinensis group) by methyl jasmonate. Int. J. Mol. Sci. 2017, 18, 1004. [Google Scholar]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. Influence of cabbage (Brassica oleracea) accession and growing conditions on myrosinase activity, glucosinolates and their hydrolysis products. Foods 2021, 10, 2903. [Google Scholar]

| Area | Mean Air Temperature (°C) | Max Air Temperature a (°C) | Min Air Temperature a (°C) | Mean Soil Temperature (°C) | CGDD a,b (°C) | Mean Humidity (%) | Mean PPFD per Day a (µmol·m−2·s−¹) |
|---|---|---|---|---|---|---|---|
| OF | 9.2 | 32.9 | −17.3 | 12.2 | 1670.9 | 54.4 | 545.1 |
| APV | 9.0 | 32.7 | −17.1 | 11.7 | 1651.0 | 55.5 | 301.3 |
| Freeze-dried (μmol·g−1 DW) | Juice (μmol·mL−1) | |||
|---|---|---|---|---|
| Glucosinolates | OF | APV | OF | APV |
| Glucoiberin | 2.39 ± 0.37 a | 2.19 ± 0.38 | n.d. | n.d. |
| Progoitrin | 3.15 ± 0.57 | 2.89 ± 0.68 | n.d. | n.d. |
| Glucoraphanin | 2.70 ± 0.40 | 2.53 ± 0.47 | n.d. | n.d. |
| Sinigrin | 2.91 ± 0.40 | 3.03 ± 0.60 | n.d. | n.d. |
| Gluconapin | 0.65 ± 0.07 | 0.62 ± 0.09 | n.d. | n.d. |
| Glucobrassicin | 3.54 ± 0.31 | 3.52 ± 0.56 | n.d. | n.d. |
| 4-Methoxyglucobrassicin | 0.80 ± 0.05 | 0.77 ± 0.03 | n.d. | n.d. |
| 4-Hydroxyglucobrassicin | 0.44 ± 0.05 | 0.40 ± 0.05 | n.d. | n.d. |
| Neoglucobrassicin | 0.03 ± 0.01 | 0.02 ± 0.01 | n.d. | n.d. |
| Gluconasturtiin | 0.21 ± 0.03 | 0.23 ± 0.03 | n.d. | n.d. |
| Total aliphatic GS | 11.82 ± 1.75 | 11.26 ± 2.12 | - | - |
| Total indolyl GS | 4.84 ± 0.37 | 4.71 ± 0.58 | - | - |
| Total GS | 16.86 ± 1.79 | 16.21 ± 2.47 | - | - |
| Freeze-dried (μg·g−1 DW) | Juice (μg·mL−1) | |||
| Glucosinolate hydrolysis | OF | APV | OF | APV |
| 1-cyano-2,3-epithiopropane | 55.81 ± 12.11 | 52.42 ± 10.47 | n.d. | n.d. |
| 1-cyano-3,4-epithiobutane | 12.84 ± 0.91 | 10.44 ± 3.46 | n.d. | n.d. |
| 3-phenylpropanenitrile | 7.05 ± 1.48 | 6.80 ± 0.74 | 0.49 ± 0.02 | 0.55 ± 0.03 *b |
| Erucin nitrile | 2.34 ± 1.00 | 2.32 ± 1.19 | 0.73 ± 0.02 * | 0.66 ± 0.03 |
| Iberverin nitrile | 1.27 ± 0.36 | 1.17 ± 0.59 | 0.44 ± 0.01 | 0.44 ± 0.02 |
| Indole-3-acetonitrile | 13.03 ± 2.22 | 14.30 ± 1.77 | 0.87 ± 0.08 | 1.00 ± 0.07 * |
| Sulforaphane nitrile | 23.30 ± 4.04 | 21.03 ± 3.49 | 0.49 ± 0.06 | 0.51 ± 0.03 |
| Iberin nitrile | n.d. | n.d. | 0.34 ± 0.02 | 0.33 ± 0.01 |
| Crembene | n.d. | n.d. | 0.45 ± 0.04 | 0.46 ± 0.04 |
| Goitrin | n.d. | n.d. | 0.13 ± 0.02 | 0.12 ± 0.01 |
| 4-methoxyindole-3-acetonitrile | n.d. | n.d. | 0.15 ± 0.02 | 0.16 ± 0.01 |
| Total GS hydrolysis | 115.63 ± 19.78 | 108.49 ± 15.30 | 4.09 ± 0.22 | 4.23 ± 0.18 |
| Total | Experienced d | Inexperienced | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Color | Scent | Taste | Color | Scent | Taste | Color | Scent | Taste | |
| Mean b | 5.2 ± 1.2 a | 4.5 ± 1.2 | 4.9 ± 1.4 | 5.2 ± 1.2 | 4.7 ± 1.1 | 5.1 ± 1.4 | 5.2 ± 1.2 | 4.3 ± 1.4 | 4.5 ± 1.3 |
| CI c | 4.92–5.48 | 4.19–4.78 | 4.52–5.20 | 4.78–5.56 | 4.29–5.05 | 4.60–5.56 | 4.79–5.64 | 3.82–4.78 | 4.07–5.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.-W.; Ku, K.-M. Impact of an Agriphotovoltaic System on Metabolites and the Sensorial Quality of Cabbage (Brassica oleracea var. capitata) and Its High-Temperature-Extracted Juice. Foods 2022, 11, 498. https://doi.org/10.3390/foods11040498
Moon H-W, Ku K-M. Impact of an Agriphotovoltaic System on Metabolites and the Sensorial Quality of Cabbage (Brassica oleracea var. capitata) and Its High-Temperature-Extracted Juice. Foods. 2022; 11(4):498. https://doi.org/10.3390/foods11040498
Chicago/Turabian StyleMoon, Hyeon-Woo, and Kang-Mo Ku. 2022. "Impact of an Agriphotovoltaic System on Metabolites and the Sensorial Quality of Cabbage (Brassica oleracea var. capitata) and Its High-Temperature-Extracted Juice" Foods 11, no. 4: 498. https://doi.org/10.3390/foods11040498
APA StyleMoon, H.-W., & Ku, K.-M. (2022). Impact of an Agriphotovoltaic System on Metabolites and the Sensorial Quality of Cabbage (Brassica oleracea var. capitata) and Its High-Temperature-Extracted Juice. Foods, 11(4), 498. https://doi.org/10.3390/foods11040498








