Investigation of Bioaccumulation and Human Health Risk Assessment of Heavy Metals in Crayfish (Procambarus clarkii) Farming with a Rice-Crayfish-Based Coculture Breeding Modes
Abstract
:1. Introduction
2. Materials and Methods
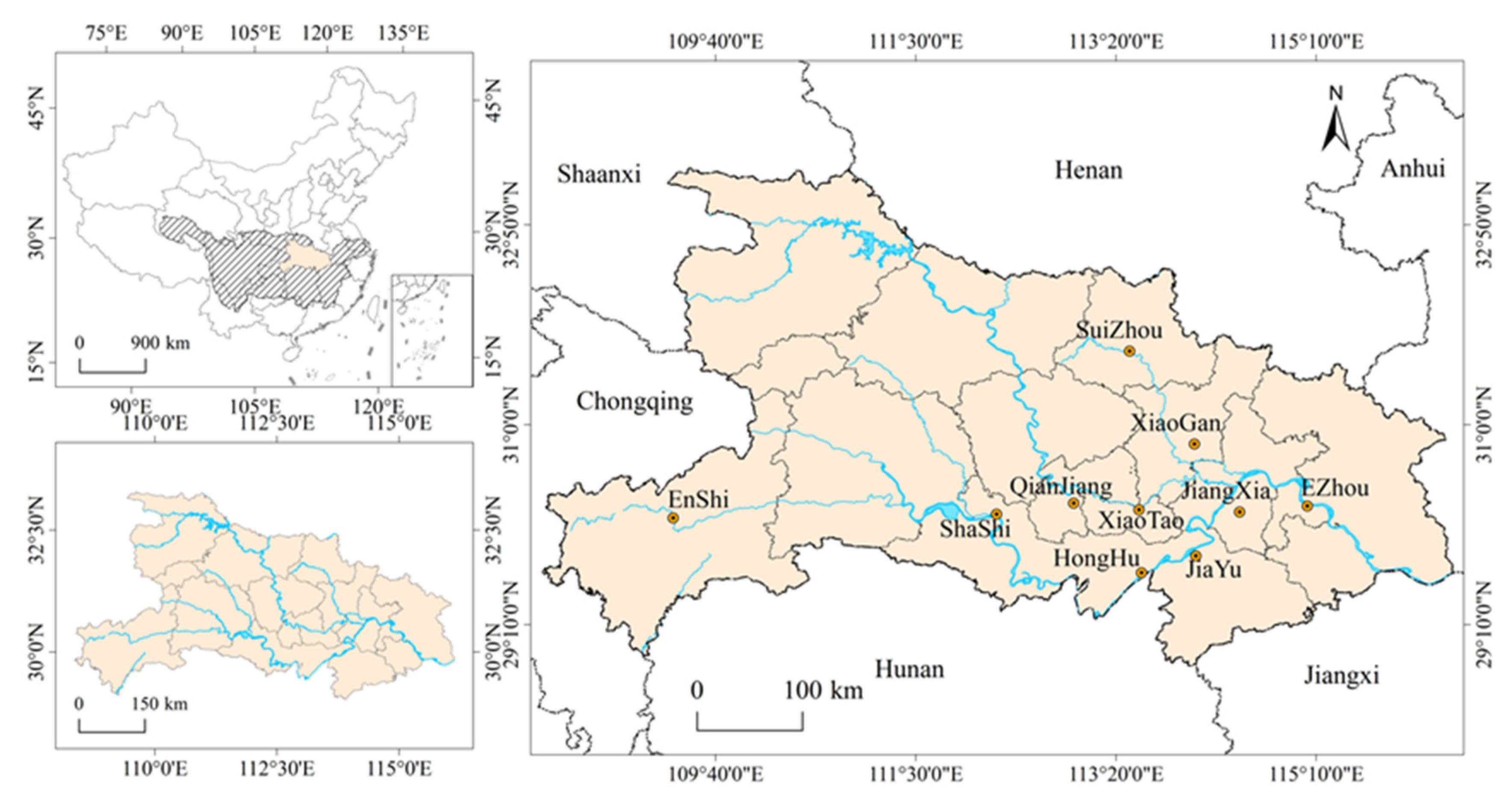
2.1. Sample Collection
2.2. Chemicals and Reagents
2.3. Sample Digestion and Analysis
2.4. Quality Assurance and Quality Control
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Health Risk Assessment
2.6.1. Detection of Estimated Daily Intake (EDI) of Metal
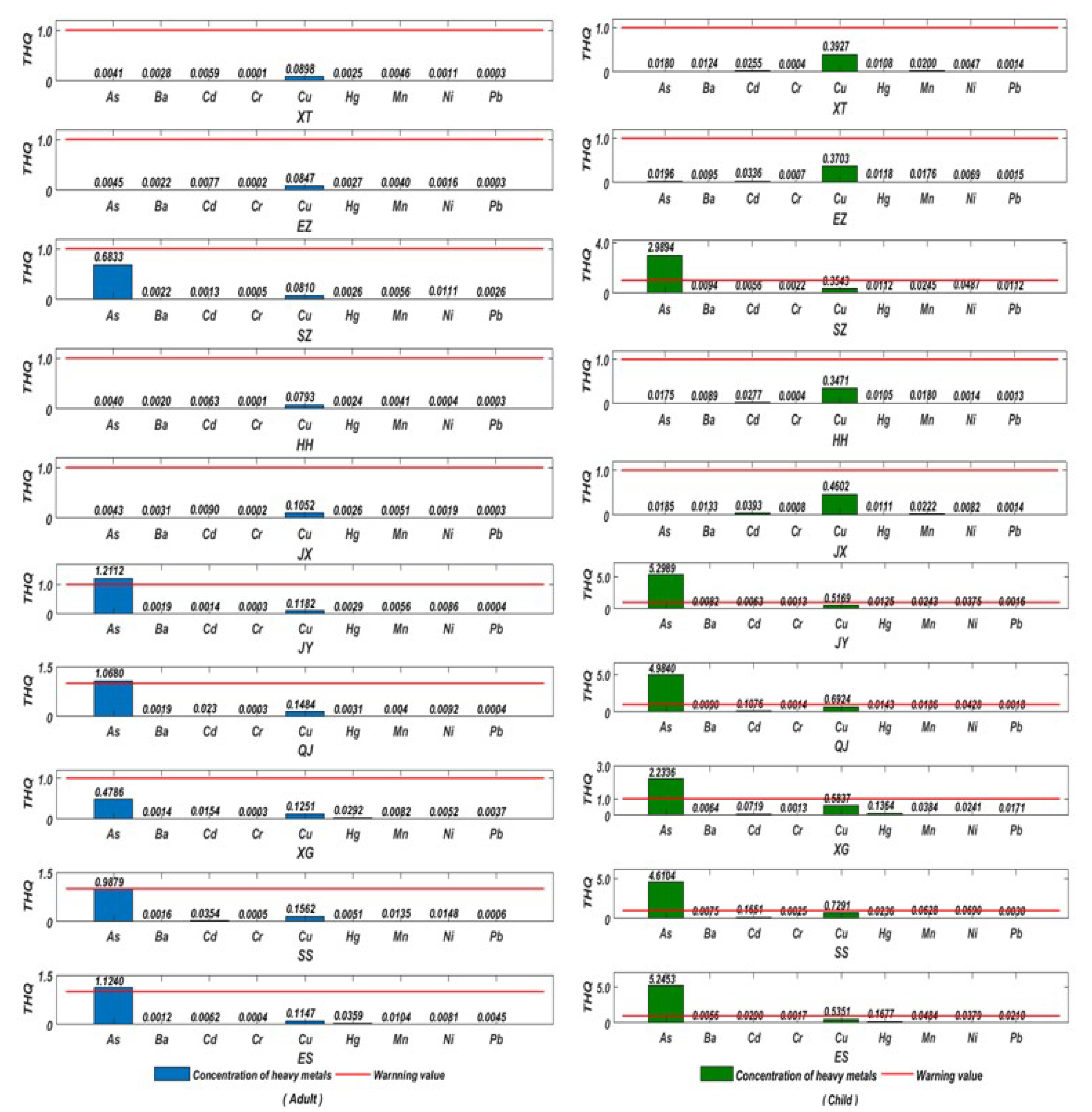
2.6.2. Detection of Target Hazard Quotient (THQ)
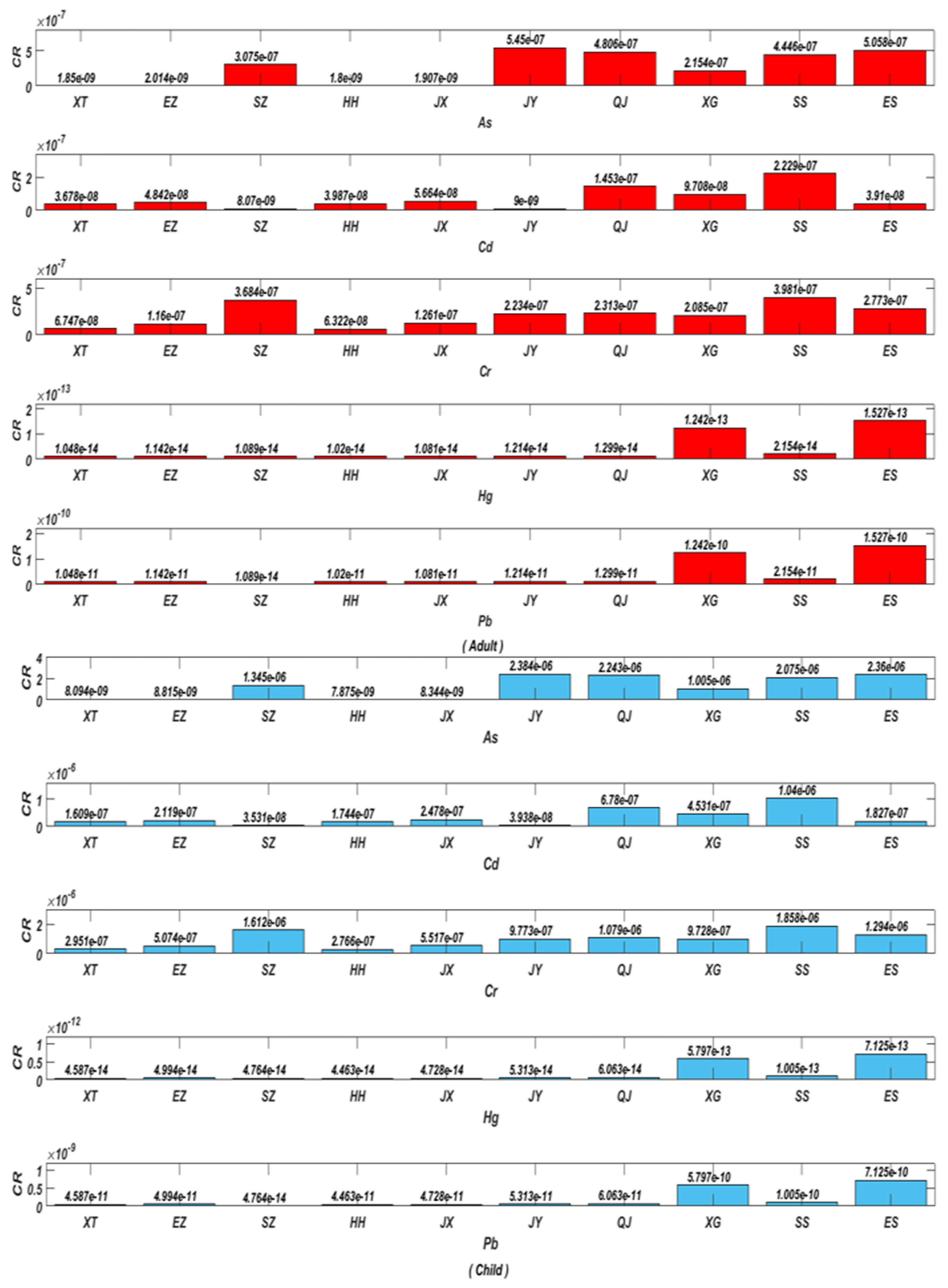
2.6.3. Evaluating the Cancer Risk (CR)
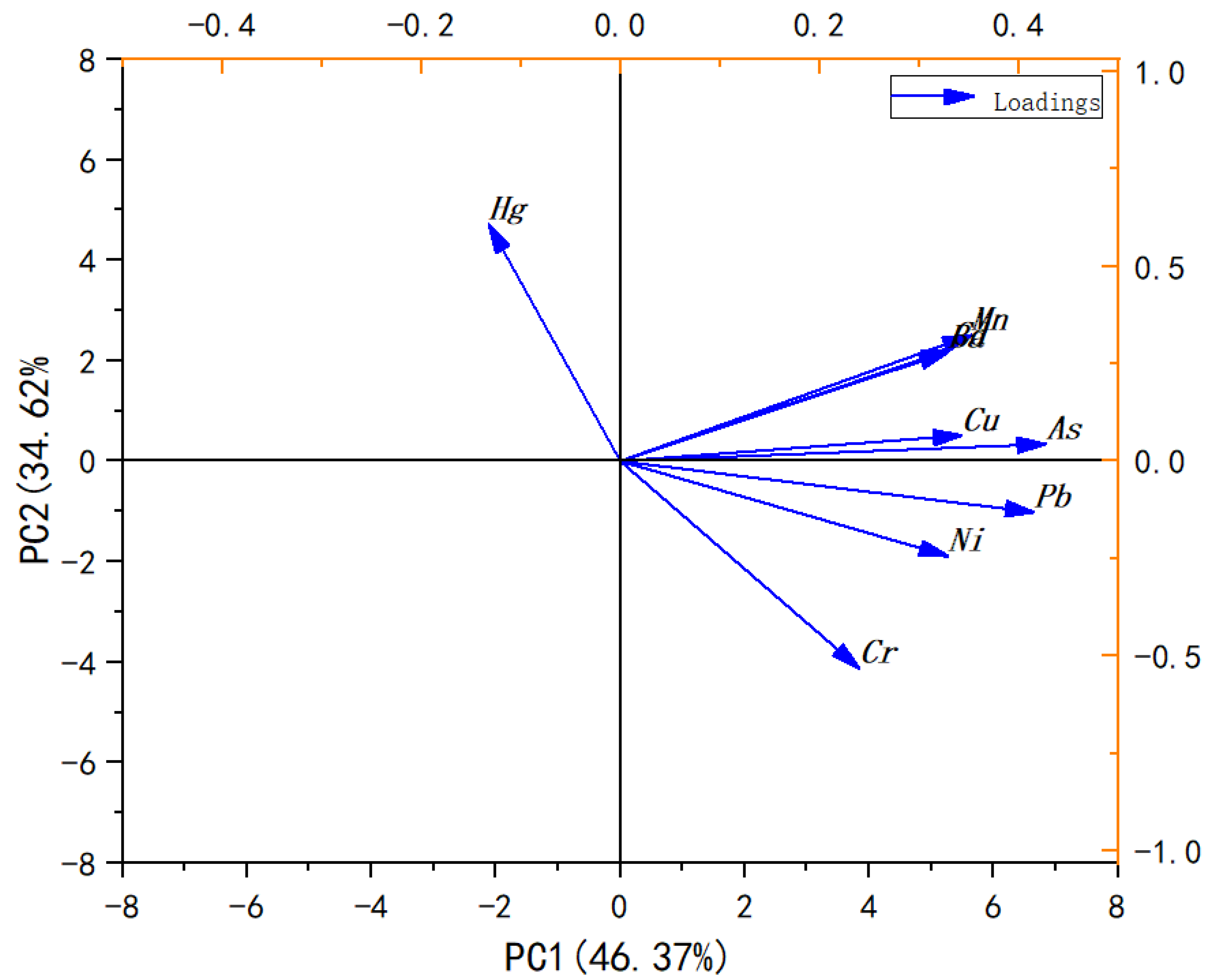
2.7. Principal Component Analysis (PCA)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Mean Concentrations of Heavy Metals in Crayfish and Abdominal Muscles of Crayfish
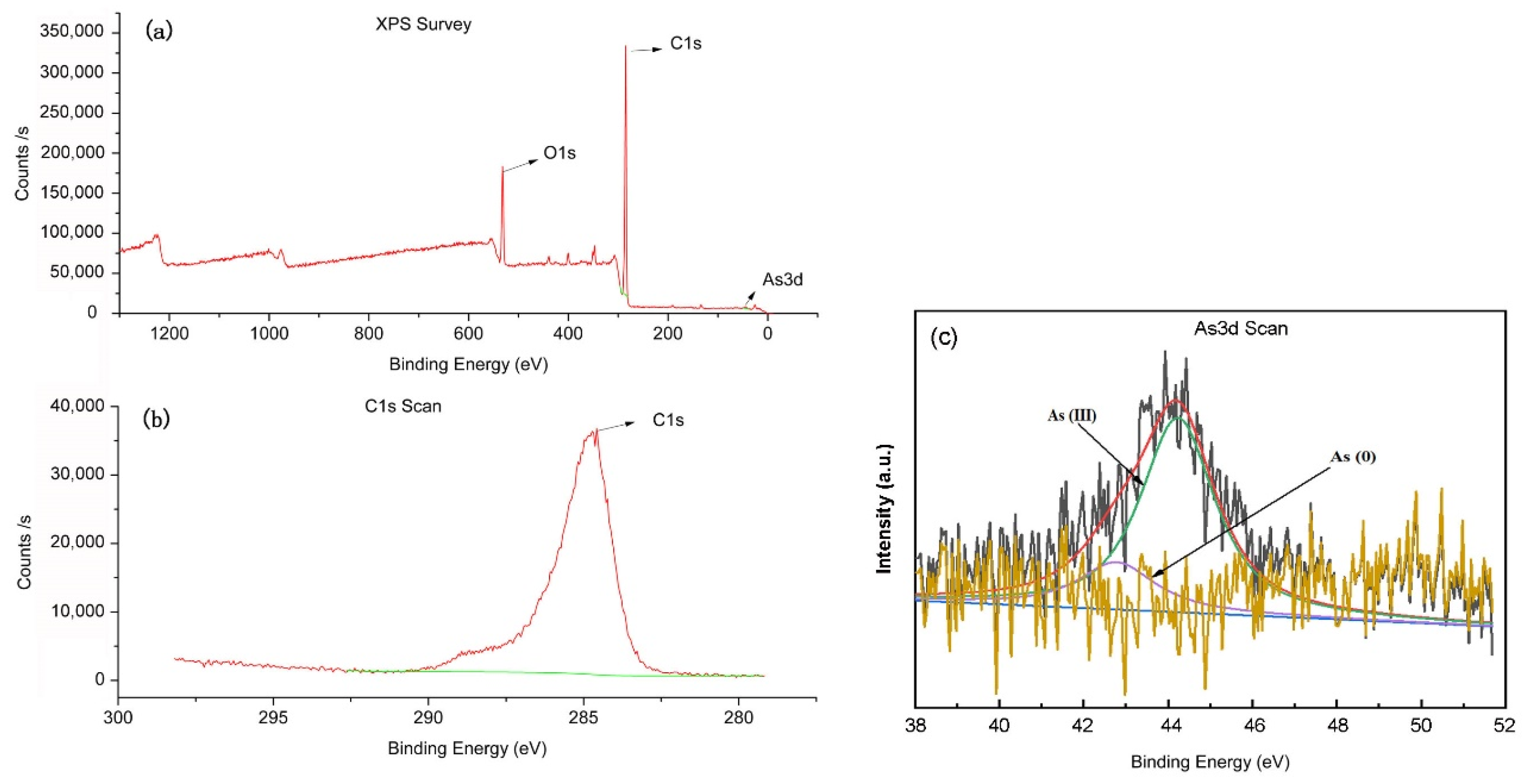
3.2. XPS Measurement
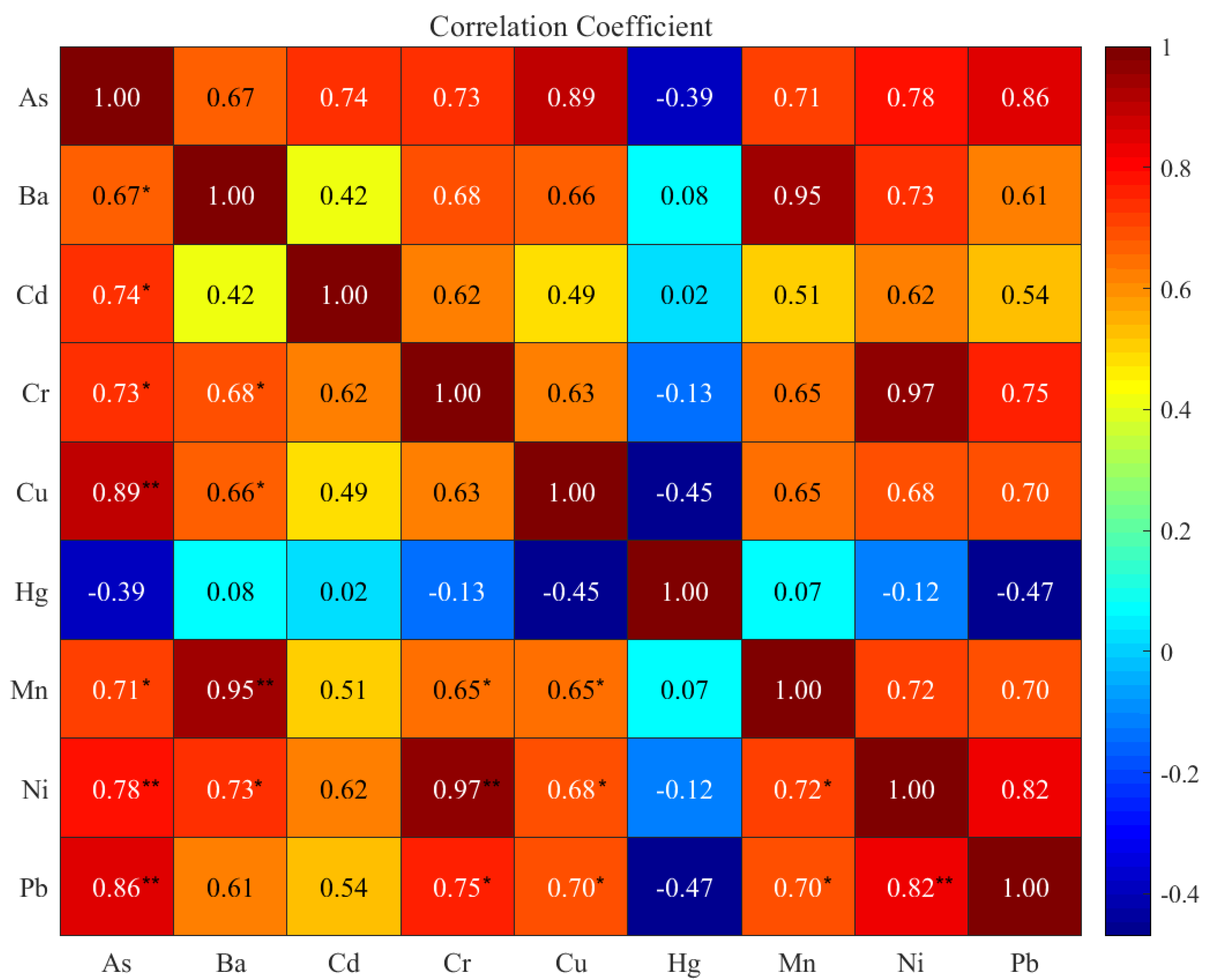
3.3. Correlation Matrix Analysis (CMA)
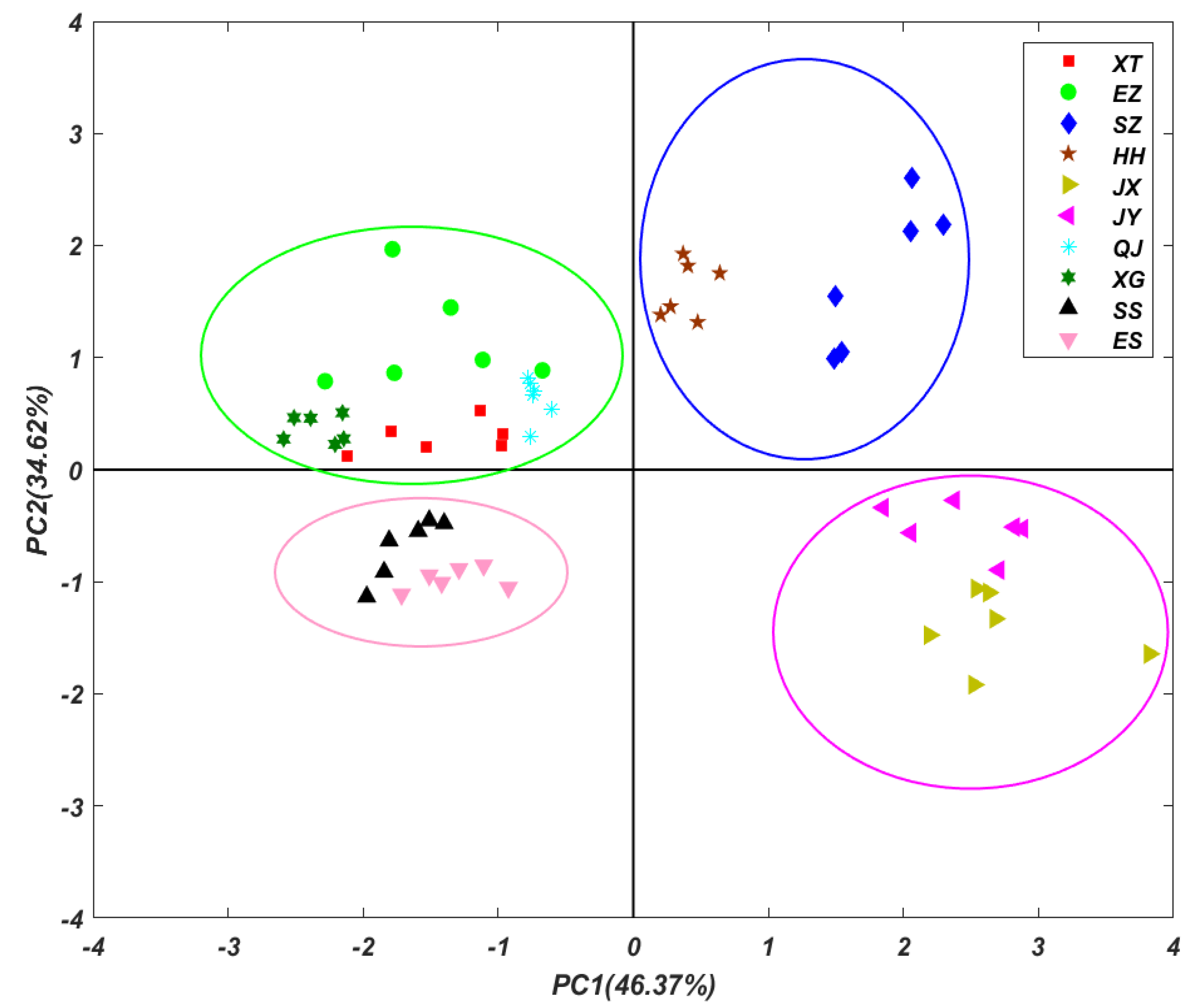
3.4. Principal Component Analysis (PCA)
3.5. Human Health Risk Assessment
3.5.1. Evaluation of the Estimated Daily Intake
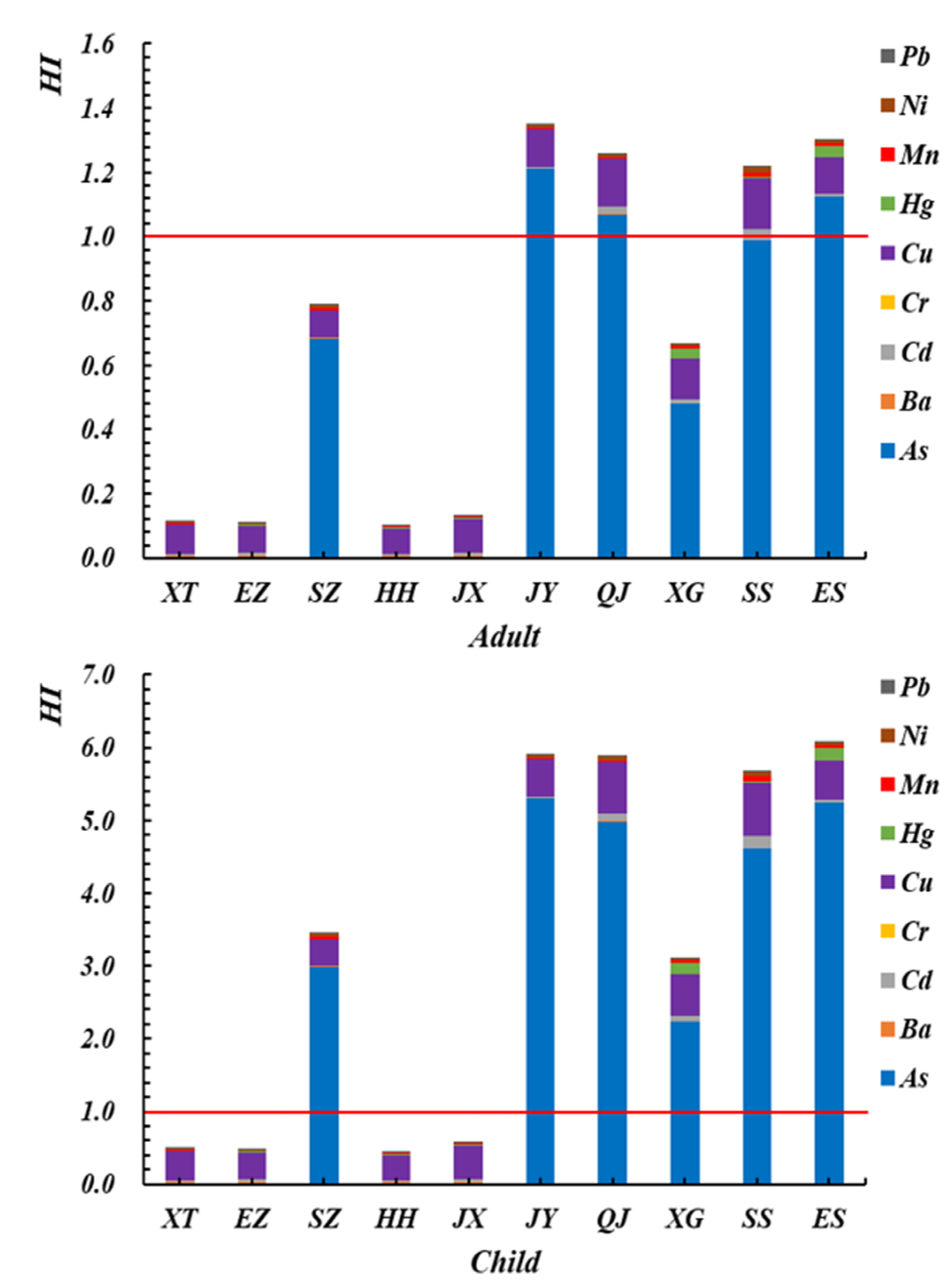
3.5.2. Target Hazard Quotient (THQ)
3.5.3. Estimation of the Cancer Risk (CR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the yangtze river basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
- McGeer, J.C.; Szebedinszky, C.; McDonald, D.G.; Wood, C.M. Effects of chronic sublethal exposure to waterborne cu, cd or zn in rainbow trout. 1: Iono-regulatory disturbance and metabolic costs. Aquat. Toxicol. 2000, 50, 231–243. [Google Scholar] [CrossRef]
- Almeida, J.A.; Diniz, Y.S.; Marques, S.F.G.; Faine, L.A.; Ribas, B.O.; Burneiko, R.C.; Novelli, E.L.B. The use of the oxidative stress responses as biomarkers in nile tilapia (oreochromis niloticus) exposed to in vivo cadmium contamination. Environ. Int. 2002, 27, 673–679. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Mohammed, M.A. Heavy metal residues in some fishes from manzala lake, egypt, and their health-risk assessment. J. Food. Sci. 2019, 84, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Griboff, J.; Wunderlin, D.A.; Monferran, M.V. Metals, as and se determination by inductively coupled plasma-mass spectrometry (icp-ms) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem. J. 2017, 130, 236–244. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Tuzen, M.; Mendil, D.; Soylak, M. Trace metal content in nine species of fish from the black and aegean seas, turkey. Food Chem. 2007, 104, 835–840. [Google Scholar] [CrossRef]
- Zheng, R.; Peng, X.; Zeng, H.; Zhang, S.; Chen, T.; Wang, H.; Chen, W. Incidence, mortality and survival of childhood cancer in china during 2000–2010 period: A population-based study. Cancer Lett. 2015, 363, 176–180. [Google Scholar] [CrossRef]
- Conti, G.O.; Copat, C.; Ledda, C.; Fiore, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Evaluation of heavy metals and polycyclic aromatic hydrocarbons (pahs) in mullus barbatus from sicily channel and risk-based consumption limits. Bull. Environ. Contam. Toxicol. 2012, 88, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Yu, B.; Dong, K.F.; Ma, D.; Xiao, G.; Zhang, C. Heavy metals in aquatic products and the health risk assessment to population in China. Environ. Sci. Pollut. Res. 2020, 27, 22708–22719. [Google Scholar] [CrossRef]
- Adebiyi, F.M.; Ore, O.T.; Ogunjimi, I.O. Evaluation of human health risk assessment of potential toxic metals in commonly consumed crayfish (palaemon hastatus) in nigeria. Heliyon 2020, 6, e03092. [Google Scholar] [CrossRef] [Green Version]
- Kalantzi, I.; Pergantis, S.A.; Black, K.D.; Shimmield, T.M.; Papageorgiou, N.; Tsapakis, M.; Karakassis, I. Metals in tissues of seabass and seabream reared in sites with oxic and anoxic substrata and risk assessment for consumers. Food Chem. 2016, 194, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, X.; Li, X.; Ru, S.; Wang, Y.; Yin, J.; Liu, D. Oxidative damage induced by copper in testis of the red swamp crayfish procambarus clarkii and its underlying mechanisms. Aquat. Toxicol. 2019, 207, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y. The growth performance and nonspecific immunity of red swamp crayfish procambarus clarkia affected by dietary rhodiola rosea polysaccharide. Fish Shellfish. Immunol. 2019, 93, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liao, Y.; Shou, L. Concentration and potential health risk of heavy metals in seafoods collected from sanmen bay and its adjacent areas, China. Mar. Pollut. Bull. 2018, 131, 356–364. [Google Scholar] [CrossRef]
- Burger, J. Assessment and management of risk to wildlife from cadmium. Sci. Total Environ. 2008, 389, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Gui, D.; Liu, W.; Wu, Y. Risk for indo-pacific humpback dolphins (sousa chinensis) and human health related to the heavy metal levels in fish from the pearl river estuary, China. Chemosphere 2020, 240, 124844. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Yang, J. Oxidative damage induced by copper and beta-cypermethrin in gill of the freshwater crayfish procambarus clarkii. Ecotoxicol. Environ. Saf. 2015, 113, 446–453. [Google Scholar] [CrossRef]
- Rodriguez-Estival, J.; Ortiz-Santaliestra, M.E.; Mateo, R. Assessment of ecotoxicological risks to river otters from ingestion of invasive red swamp crayfish in metal contaminated areas: Use of feces to estimate dietary exposure. Environ. Res. 2020, 181, 108907. [Google Scholar] [CrossRef] [PubMed]
- Goretti, E.; Pallottini, M.; Ricciarini, M.I.; Selvaggi, R.; Cappelletti, D. Heavy metals bioaccumulation in selected tissues of red swamp crayfish: An easy tool for monitoring environmental contamination levels. Sci. Total Environ. 2016, 559, 339–346. [Google Scholar] [CrossRef]
- Peng, Q.; Nunes, L.M.; Greenfield, B.K.; Dang, F.; Zhong, H. Are chinese consumers at risk due to exposure to metals in crayfish? A bioaccessibility-adjusted probabilistic risk assessment. Environ. Int. 2016, 88, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Xiong, B.; Xu, T.; Li, R.; Johnson, D.; Ren, D.; Liu, H.; Xi, Y.; Huang, Y. Heavy metal accumulation and health risk assessment of crayfish collected from cultivated and uncultivated ponds in the middle reach of yangtze river. Sci. Total Environ. 2020, 739, 139963. [Google Scholar] [CrossRef]
- Li, C.-l.; Bai, J.-y.; Shao-hui, Y.; Zhong-guo, Y. Simultaneous determination of zinc, copper, lead, cadmium, manganese, chromium, iron, nickel and arsenic in mineral processing wastewater by ic p-oes. World Nonferrous Met. 2019, 18, 208–212. [Google Scholar]
- Ahmed, A.S.S.; Rahman, M.; Sultana, S.; Babu, S.; Sarker, M.S.I. Bioaccumulation and heavy metal concentration in tissues of some commercial fishes from the meghna river estuary in bangladesh and human health implications. Mar. Pollut. Bull. 2019, 145, 436–447. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Q.; Wu, H.; Liu, J.; Ning, Y.; Xie, S.; Huang, W.; Bi, X. Enrichment of trace elements in red swamp crayfish: Influences of region and production method, and human health risk assessment. Aquaculture 2021, 535, 736366. [Google Scholar] [CrossRef]
- Maurya, P.K.; Malik, D.S.; Yadav, K.K.; Kumar, A.; Kumar, S.; Kamyab, H. Bioaccumulation and potential sources of heavy metal contamination in fish species in river ganga basin: Possible human health risks evaluation. Toxicol. Rep. 2019, 6, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Estival, J.; Morales-Machuca, C.; Pareja-Carrera, J.; Ortiz-Santaliestra, M.E.; Mateo, R. Food safety risk assessment of metal pollution in crayfish from two historical mining areas: Accounting for bioavailability and cooking extractability. Ecotoxicol. Environ. Saf. 2019, 185, 109682. [Google Scholar] [CrossRef] [PubMed]
- Boutrif, E. Institutions involved in food safety: Food and agriculture organization of the united nations (fao). In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 354–358. [Google Scholar]
- Varol, M.; Kaya, G.K.; Alp, A. Heavy metal and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the firat (euphrates) river: Risk-based consumption advisories. Sci. Total Environ. 2017, 599–600, 1288–1296. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, J.; Wang, H.; Han, X.; Ma, J.; Ma, Y.; Luan, H. Distribution and health risk assessment of potentially toxic elements in soils around coal industrial areas: A global meta-analysis. Sci. Total Environ. 2020, 713, 135292. [Google Scholar] [CrossRef]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and health risk assessment of heavy metals in china’s lead–zinc mine tailings: A meta–analysis. Chemosphere 2020, 267, 128909. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wu, W.-L.; Zhang, Y.-H.; Zhou, S.-J.; Long, C.-Y.; Wen, J.; Wang, B.-Y.; Liu, Z.-T.; Zhang, C.-Z.; Huang, P.-P.; et al. Levels, temporal trend and health risk assessment of five heavy metals in fresh vegetables marketed in guangdong province of china during 2014–2017. Food Control 2018, 92, 107–120. [Google Scholar] [CrossRef]
- Gb 2762-2017 national food safety standard limits of pollutants in food. Chin. J. Food Hyg. 2018, 30, 329–340.
- Alcorlo, P.; Otero, M.; Crehuet, M.; Baltanas, A.; Montes, C. The use of the red swamp crayfish (procambarus clarkii, girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the river guadiamar (sw, Spain). Sci. Total Environ. 2006, 366, 380–390. [Google Scholar] [CrossRef]
- Yue, C.-F.; Wang, T.-T.; Wang, Y.-F.; Peng, Y. Effect of combined photoperiod, water calcium concentration and ph on survival, growth, and moulting of juvenile crayfish (procambarus clarkii) cultured under laboratory conditions. Aquac. Res. 2009, 40, 1243–1250. [Google Scholar] [CrossRef]
- Cheung, K.C.; Leung, H.M.; Wong, M.H. Metal concentrations of common freshwater and marine fish from the pearl river delta, south china. Arch. Environ. Contam. Toxicol. 2007, 54, 705–715. [Google Scholar] [CrossRef]
- Oliveira, C.; Chaves, C.R.; Bargiela, P.; da Rocha, M.d.G.C.; da Silva, A.F.; Chubaci, J.F.D.; Boström, M.; Persson, C.; Malta, M. Surface studies of the chemical environment in gold nanorods supported by X-ray photoelectron spectroscopy (xps) and ab initio calculations. J. Mater. Res. Technol. 2021, 15, 768–776. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, M.; Zheng, L.; Wang, B.; Wang, Y. Uranium speciation in coal bottom ash investigated via x-ray absorption fine structure and x-ray photoelectron spectra. J. Environ. Sci. (China) 2018, 74, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Kunene, S.C.; Lin, K.S.; Mdlovu, N.V.; Shih, W.C. Bioaccumulation of trace metals and speciation of copper and zinc in pacific oysters (crassostrea gigas) using xanes/exafs spectroscopies. Chemosphere 2021, 265, 129067. [Google Scholar] [CrossRef]
- Lavorgna, M.; Attianese, I.; Buonocore, G.G.; Conte, A.; Del Nobile, M.A.; Tescione, F.; Amendola, E. Mmt-supported ag nanoparticles for chitosan nanocomposites: Structural properties and antibacterial activity. Carbohydr. Polym. 2014, 102, 385–392. [Google Scholar] [CrossRef]
- Du, H.; Fu, J.; Wang, S.; Liu, H.; Zeng, Y.; Yang, J.; Xiong, S. 1h-nmr metabolomics analysis of nutritional components from two kinds of freshwater fish brain extracts. RSC Adv. 2018, 8, 19470–19478. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, P.; Reddy, A.G.; Reddy, Y.R.; Shivakumar, P. Evaluation of protective effect of n-acetyl cysteine on arsenic-induced hepatotoxicity. J. Nat. Sci. Biol. Med. 2013, 4, 393–395. [Google Scholar]
- Suárez-Serrano, A.; Alcaraz, C.; Ibáñez, C.; Trobajo, R.; Barata, C. Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower ebro river and delta. Ecotoxicol. Environ. Saf. 2010, 73, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, R.; Husain, T.; Bose, N.; Veitch, B. Distribution of heavy metals in sediment pore water due to offshore discharges: An ecological risk assessment. Environ. Model. Softw. 2003, 18, 451–461. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Shen, Z.; Niu, J.; Tang, Z. Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the yangtze river catchment of Wuhan, China. J. Hazard. Mater. 2009, 166, 1186–1194. [Google Scholar] [CrossRef]
- Suseno, H.; Hudiyono, S.; Budiawan, B.; Wisnubroto, D. Effects of concentration, body size and food type on the bioaccumulation of hg in farmed tilapia oreochromis mossambicus. Aust. J. Basic Appl. Sci. 2010, 4, 792–799. [Google Scholar]
- Baki, M.A.; Hossain, M.M.; Akter, J.; Quraishi, S.B.; Haque Shojib, M.F.; Atique Ullah, A.K.M.; Khan, M.F. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in saint martin island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef]
- Anandkumar, A.; Li, J.; Prabakaran, K.; Xi Jia, Z.; Leng, Z.; Nagarajan, R.; Du, D. Accumulation of toxic elements in an invasive crayfish species (procambarus clarkii) and its health risk assessment to humans. J. Food Compos. Anal. 2020, 88, 103449. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Yuan, Z.; Ge, M.; Wang, S.; Liu, Y.; Da, C. Occurrence, potential health risk of heavy metals in aquatic organisms from laizhou bay, china. Mar. Pollut. Bull. 2019, 140, 388–394. [Google Scholar] [CrossRef]
- Arisekar, U.; Shakila, R.J.; Shalini, R.; Jeyasekaran, G. Human health risk assessment of heavy metals in aquatic sediments and freshwater fish caught from thamirabarani river, the western ghats of south tamil nadu. Mar. Pollut. Bull. 2020, 159, 111496. [Google Scholar] [CrossRef] [PubMed]
- Ezemonye, L.I.; Adebayo, P.O.; Enuneku, A.A.; Tongo, I.; Ogbomida, E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from benin river, nigeria. Toxicol. Rep. 2019, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the meiliang bay, taihu lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, T.; Zhang, Y.; Hou, Y.; Chang, Q. Health risk assessment of heavy metals in agricultural soils and identification of main influencing factors in a typical industrial park in northwest China. Chemosphere 2020, 252, 126591. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. International agency for research on cancer. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 1067–1069. [Google Scholar]
- Bonsignore, M.; Salvagio Manta, D.; Mirto, S.; Quinci, E.M.; Ape, F.; Montalto, V.; Gristina, M.; Traina, A.; Sprovieri, M. Bioaccumulation of heavy metals in fish, crustaceans, molluscs and echinoderms from the tuscany coast. Ecotoxicol. Environ. Saf. 2018, 162, 554–562. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Li, J.; Gong, Z.; Yue, B.; Wang, X.; Manyande, A.; Du, H. Investigation of Bioaccumulation and Human Health Risk Assessment of Heavy Metals in Crayfish (Procambarus clarkii) Farming with a Rice-Crayfish-Based Coculture Breeding Modes. Foods 2022, 11, 261. https://doi.org/10.3390/foods11030261
Peng F, Li J, Gong Z, Yue B, Wang X, Manyande A, Du H. Investigation of Bioaccumulation and Human Health Risk Assessment of Heavy Metals in Crayfish (Procambarus clarkii) Farming with a Rice-Crayfish-Based Coculture Breeding Modes. Foods. 2022; 11(3):261. https://doi.org/10.3390/foods11030261
Chicago/Turabian StylePeng, Fangjun, Jiawen Li, Zhiyong Gong, Bing Yue, Xueli Wang, Anne Manyande, and Hongying Du. 2022. "Investigation of Bioaccumulation and Human Health Risk Assessment of Heavy Metals in Crayfish (Procambarus clarkii) Farming with a Rice-Crayfish-Based Coculture Breeding Modes" Foods 11, no. 3: 261. https://doi.org/10.3390/foods11030261
APA StylePeng, F., Li, J., Gong, Z., Yue, B., Wang, X., Manyande, A., & Du, H. (2022). Investigation of Bioaccumulation and Human Health Risk Assessment of Heavy Metals in Crayfish (Procambarus clarkii) Farming with a Rice-Crayfish-Based Coculture Breeding Modes. Foods, 11(3), 261. https://doi.org/10.3390/foods11030261





