Ready-to-Use Nutraceutical Formulations from Edible and Waste Organs of Algerian Artichokes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection, NaDES Preparation, and Extraction
2.2. Cell Culture and MTT Test
2.3. In Vitro Digestion Protocol
2.4. UHPLC-HRMS Analyses of Parental Extracts and Digestate Therefrom
2.5. Antiradical Assays: DPPH and ABTS Tests
3. Results and Discussion
3.1. MTT Test on Caco-2 Cell Line
3.2. UHPLC-HRMS Profiling of Artichoke-Based Food Supplements
3.2.1. Simple Phenols
3.2.2. Polyphenols
3.2.3. Triterpenes
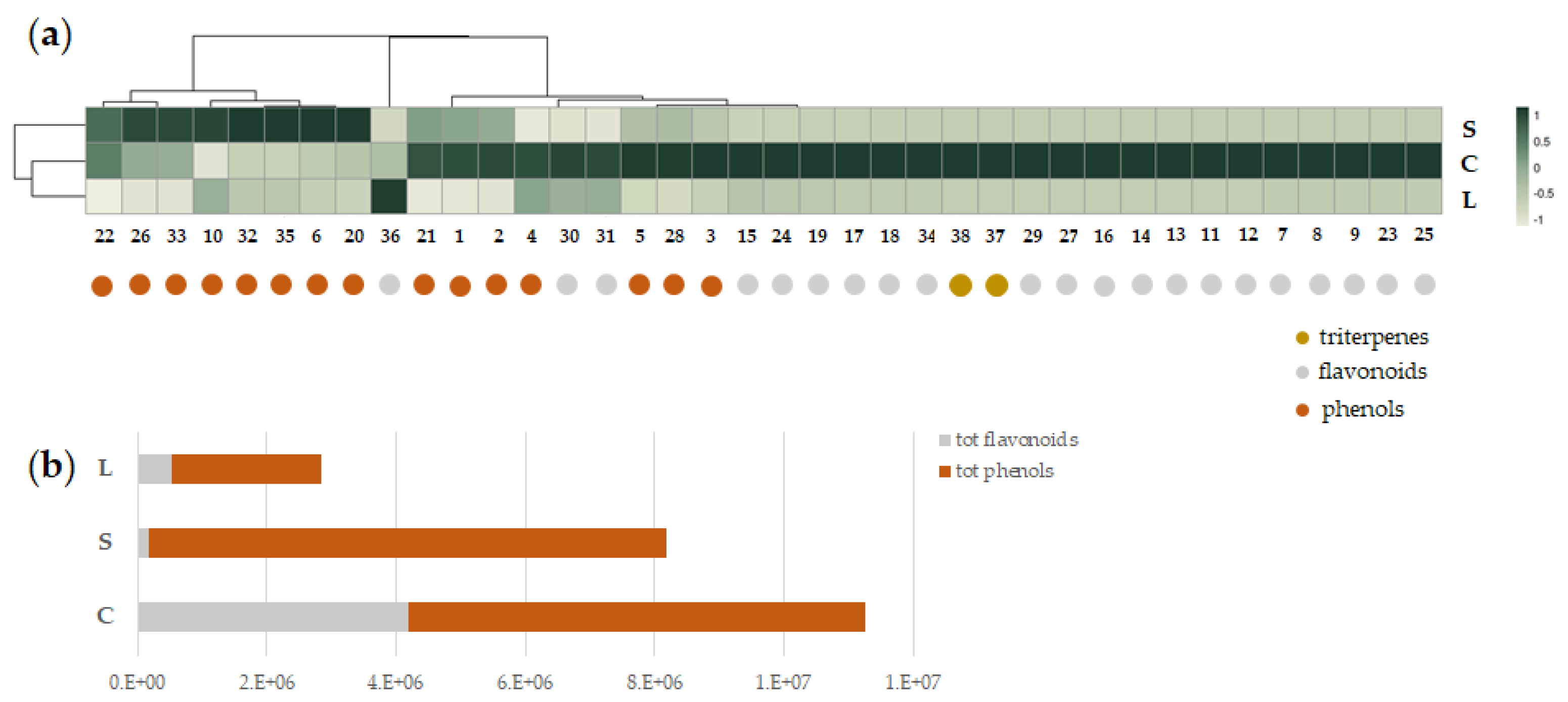
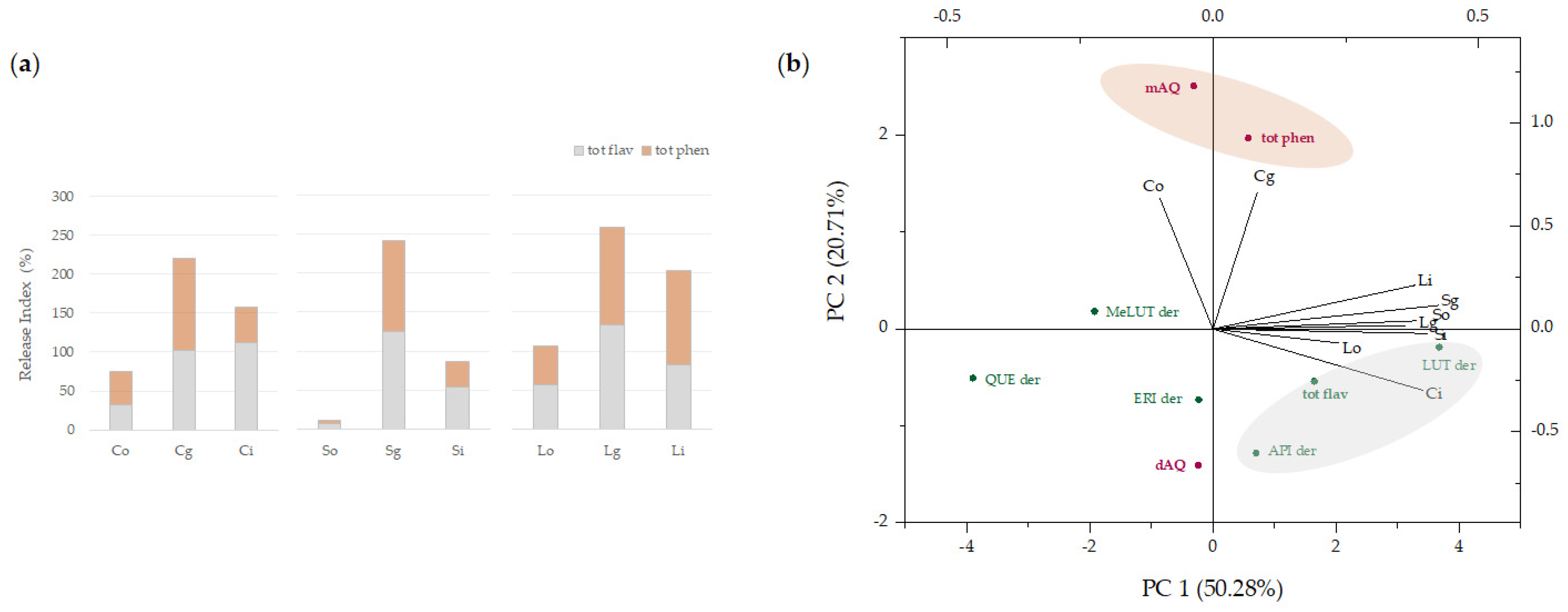
3.2.4. Occurrence and Relative Content of Detected Bioactive Metabolites
3.3. In Vitro Digestion of Artichoke-Based Food Supplements
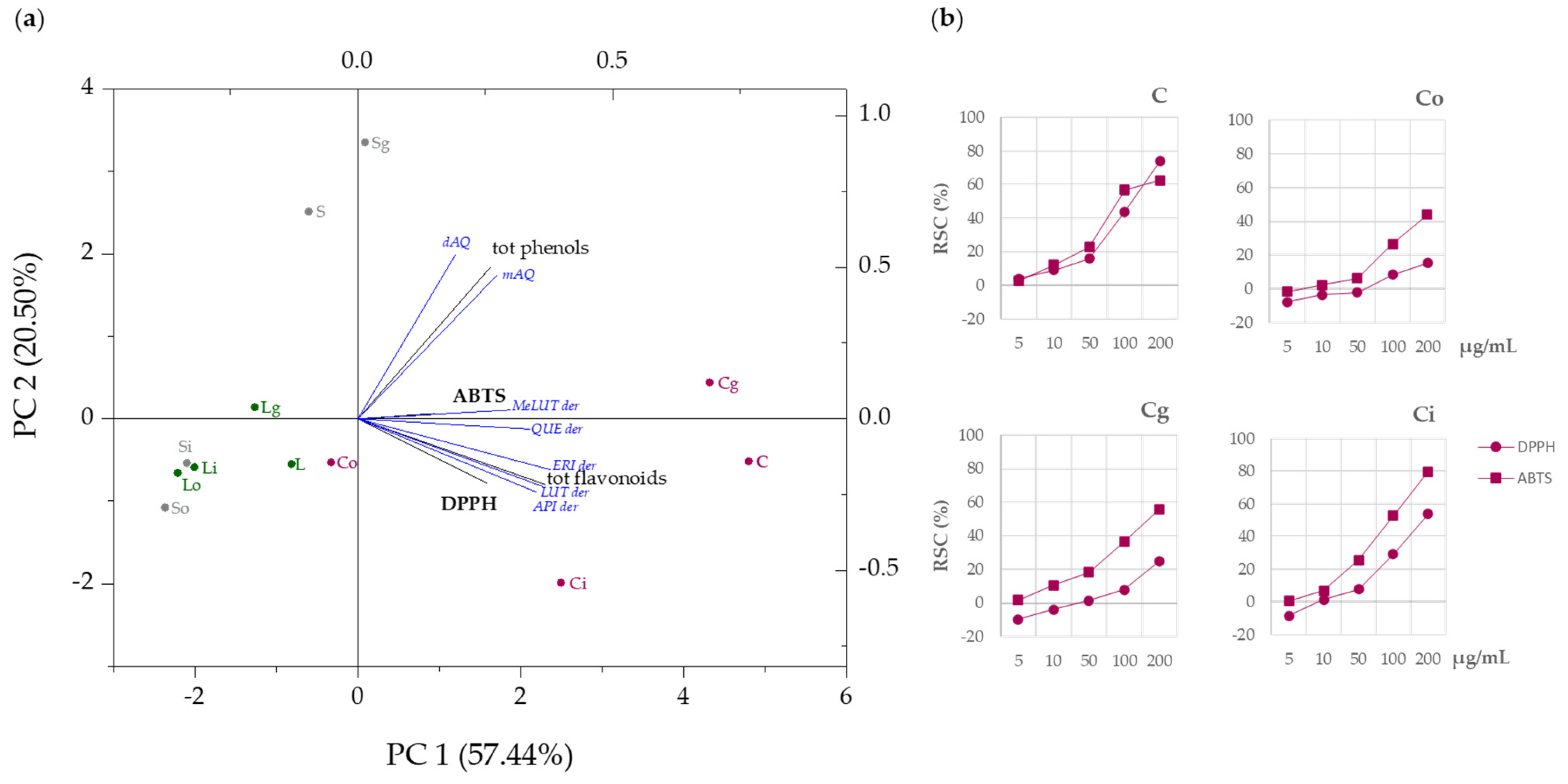
3.4. DPPH and ABTS+ Radical Scavenging Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piccolella, S.; Pacifico, S. Plant-Derived Polyphenols: A Chemopreventive and Chemoprotectant Worth-Exploring Resource in Toxicology. Adv. Mol. Toxicol. 2015, 9, 161–214. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite Profiling of “Green” Extracts of Cynara cardunculus subsp. scolymus, Cultivar “Carciofo di Paestum” PGI by 1H NMR and HRMS-Based Metabolomics. Molecules 2022, 27, 3328. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I. Nutritional Value and Bioactive Compounds Characterization of Plant Parts from Cynara cardunculus L. (Asteraceae) Cultivated in Central Greece. Front. Plant Sci. 2018, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- López-Salas, L.; BorrásLinares, I.; Quintin, D.; GarcíaGomez, P.; Giménez-Martínez, R.; Segura-Carretero, A.; LozanoSánchez, J. Artichoke By-Products as Natural Source of Phenolic Food Ingredient. Appl. Sci. 2021, 11, 3788. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA/HMPC/194014/2017. European Union Herbal Monograph on Cynara cardunculus L. (syn. Cynara scolymus L.), Folium. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-cynara-cardunculus-l-syn-cynara-scolymus-l-folium_en.pdf (accessed on 16 October 2022).
- Domínguez-Fernández, M.; Irigoyen, Á.; de los Angeles Vargas-Alvarez, M.; Ludwig, I.A.; De Peña, M.P.; Cid, C. Influence of culinary process on free and bound (poly) phenolic compounds and antioxidant capacity of artichokes. Int. J. Gastron. Food Sci. 2021, 25, 100389. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-HerreraB, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; Gutiérrez Fernández, M.Y.; Escuadra Burrieza, J.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining green extracts rich in phenolic compounds from underexploited food by-products using natural deep eutectic solvents. Opportunities and challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- De Faria, E.L.P.; Do Carmo, R.S.; Cláudio, A.F.M.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Deep Eutectic Solvents as Efficient Media for the Extraction and Recovery of Cynaropicrin from Cynara cardunculus L. Leaves. Int. J. Mol. Sci. 2017, 18, 2276. [Google Scholar] [CrossRef]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Nocera, P.; Pacifico, F.; Manti, L.; Pacifico, S. Ultrasound-assisted aqueous extraction, LC-MS/MS analysis and radiomodulating capability of autochthonous Italian sweet cherry fruits. Food Funct. 2018, 9, 1840. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Marciano, S.; Galasso, S.; Nocera, P.; Piscopo, V.; Fiorentino, A.; Monaco, P. LC-MS/MS Profiling of a Mastic Leaf Phenol Enriched Extract and Its Effects on H2O2 and Aβ(25–35) Oxidative Injury in SK-B-NE(C)-2 Cells. J. Agric. Food. Chem. 2014, 62, 11957–11966. [Google Scholar] [CrossRef] [PubMed]
- Morgana, N.M.; Magdalena, E.; de los Angeles Fernandez, M.; Fernanda, S.M. NADES for food industry innovation: Novel bioadditives based on olive oil byproducts. Food Bioprod. Process. 2022, 134, 193–201. [Google Scholar] [CrossRef]
- Brahmi-Chendouh, N.; Piccolella, S.; Nigro, E.; Hamri-Zeghichi, S.; Madani, K.; Daniele, A.; Pacifico, S. Urtica dioica L. leaf chemical composition: A never-ending disclosure by means of HR-MS/MS techniques. J. Pharm. Biomed. Anal. 2021, 195, 113892. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yao, L.; Gong, K.; Li, K.; Sun, L.; Cai, W. Identification and Quantification of Chlorogenic Acids from the Root Bark of Acanthopanax gracilistylus by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. ACS Omega 2022, 7, 25675–25685. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- March, R.E.; Lewars, E.G.; Stadey, C.J.; Miao, X.S.; Zhao, X.; Metcalfe, C.D. A comparison of flavonoid glycosides by electrospray tandem mass spectrometry. Int. J. Mass Spectrom. 2006, 248, 61–85. [Google Scholar] [CrossRef]
- Ramos, P.A.; Santos, S.A.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crops Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Marengo, A.; Maxia, A.; Sanna, C.; Bertea, C.M.; Bicchi, C.; Ballero, M.; Cagliero, C.; Rubiolo, P. Characterization of four wild edible Carduus species from the Mediterranean region via phytochemical and biomolecular analyses. Food Res. Int. 2017, 100, 822–831. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Claeys, M. Determination of the glycosylation site in flavonoid mono-O-glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. J. Mass Spectrom. 2005, 40, 364–372. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Volpe, M.G.; Paolucci, M.; Pacifico, S. UHPLC-HR-MS/MS-Guided Recovery of Bioactive Flavonol Compounds from Greco di Tufo Vine Leaves. Molecules 2019, 24, 3630. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Gravina, C.; Piccolella, S.; Pecoraro, M.T.; Formato, M.; Stinca, A.; Pacifico, S.; Esposito, A. Calendula arvensis (Vaill.) L.: A Systematic Plant Analysis of the Polar Extracts from Its Organs by UHPLC-HRMS. Foods 2022, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Desseva, I.; Stoyanova, M.; Petkova, N.; Terzyiska, M.; Lante, A. Impact of In Vitro Gastrointestinal Digestion on the Bioaccessibility of Phytochemical Compounds from Eight Fruit Juices. Molecules 2021, 26, 1187. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef]
- Swallah, M.S.; Fu, H.; Sun, H.; Affoh, R.; Yu, H. The impact of polyphenol on general nutrient metabolism in the monogastric gastrointestinal tract. J. Food Qual. 2020, 2020, 5952834. [Google Scholar] [CrossRef]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl Flavonol Glycosides and More in Marketed Green Teas: An Intrinsic Value beyond Much-Lauded Catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef]
- Fritsche, J.; Beindor, C.M.; Dachtler, M.; Zhang, H.; Lammers, J.G. Isolation, characterization and determination of minor artichoke (Cynara scolymus L.) leaf extract compounds. Eur. Food Res. Technol. 2002, 215, 149–157. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Mejri, F.; Baati, T.; Martins, A.; Selmi, S.; Luisa Serralheiro, M.; Falé, P.L.; Rauter, A.; Casabianca, H.; Hosni, K. Phytochemical analysis and in vitro and in vivo evaluation of biological activities of artichoke (Cynara scolymus L.) floral stems: Towards the valorization of food by-products. Food Chem. 2020, 333, 127506. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, M.; Zhang, T.; Wu, J.; Wang, J.; Liu, K.; Zhan, N. Antioxidant and Anti-inflammatory Activities of Cynaroside from Elsholtiza bodinieri. Nat. Prod. Commun. 2018, 13, 1501–1504. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Hu, X.; Feng, J.; Wang, H.; Xiong, F. Protective effects of cynaroside on oxidative stress in retinal pigment epithelial cells. J. Biochem. Mol. Toxicol. 2019, 33, e22352. [Google Scholar] [CrossRef]

| Peak n. | Rt (min) | Tentative Assignment | Formula | [M-H]− Calc. (m/z) | [M-H]− Found (m/z) | Error (ppm) | RDB | MS/MS Fragment Ions (m/z) | C | S | L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.606 | 1-O-caffeoylquinic acid | C16H18O9 | 353.0878 | 353.0881 | 0.8 | 8 | 353.0863; 191.0563 | × | × | × |
| 2 | 5.182 | 5-O-caffeoylquinic acid | C16H18O9 | 353.0878 | 353.0879 | 0.3 | 8 | 353.0868; 191.0559 | × | × | × |
| 3 | 5.830 | p-coumaroylquinic acid 1 | C16H18O8 | 337.0929 | 337.0929 | 0 | 8 | 337.0921; 191.0560;173.0441; 119.0508; 93.0343 | × | × | × |
| 4 | 6.174 | p-coumaroylquinic acid 2 | C16H18O8 | 337.0929 | 337.0929 | 0 | 8 | 337.0932; 191.0560; 119.0517 | × | × | |
| 5 | 6.227 | 1,3-dicaffeoylquinic acid (cynarin) | C25H24O12 | 515.1195 | 515.1211 | 3.1 | 14 | 515.1209; 353.0878; 335.0796; 191.0553; 179.0338; 135.0442; 93.0356 | × | × | × |
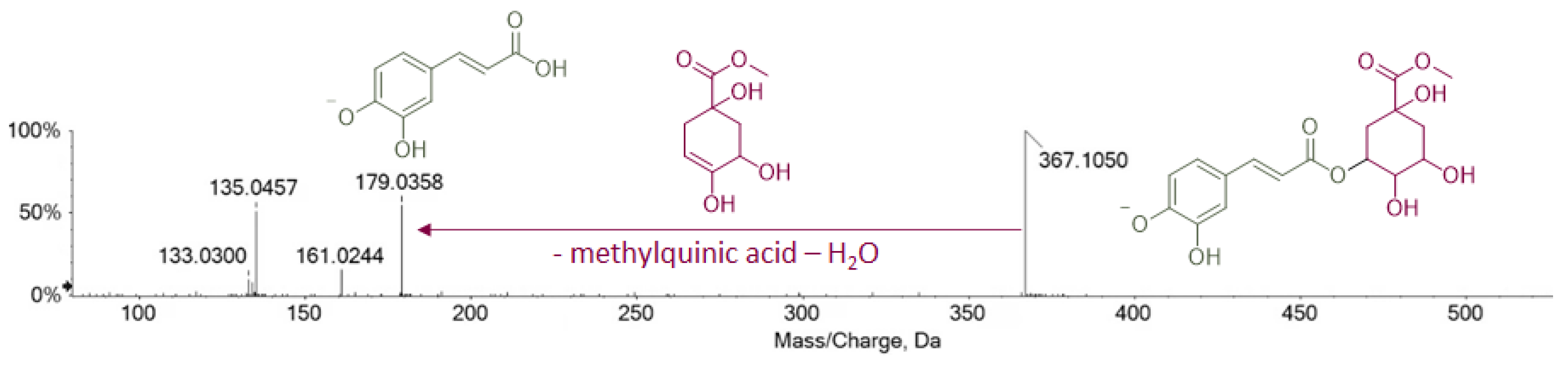
| 6 | 7.112 | Caffeoyl methylquinate 1 | C17H20O9 | 367.1035 | 367.1042 | 2.0 | 8 | 367.1050; 179.0358; 161.0244; 135.0457; 133.0300 | × | × | × |
| 7 | 7.124 | Apigenin hexosyl-hexuronide | C27H28O16 | 607.1305 | 607.1310 | 0.9 | 14 | 607.1330; 431.1007; 269.0449; 268.0378; 175.0252; 113.0238 | × | × | |
| 8 | 7.177 | Luteolin hexosyl-hexuronide | C27H28O17 | 623.1254 | 623.1269 | 2.4 | 14 | 623.1290; 461.0737; 447.0939; 285.0400; 284.0310; | × | × | × |
| 9 | 7.300 | Eriodictyol hexoside | C21H22O11 | 449.1089 | 449.1087 | −0.5 | 11 | 449.1090; 287.0554; 193.0124; 151.0026; 107.0127 | × | × | × |
| 10 | 7.308 | Caffeoyl methylquinate 2 | C17H20O9 | 367.1035 | 367.1031 | −1.0 | 8 | 367.1032; 179.0350; 161.0245; 135.0450 | × | × | × |
| 11 | 7.322 | Eriodictyol hexuronide | C21H20O12 | 463.0882 | 463.0877 | −1.1 | 12 | 463.0882; 287.0556; 151.0041 | × | ||
| 12 | 7.449 | Eriodictyol rutinoside | C27H32O15 | 595.1668 | 595.1676 | 1.3 | 12 | 595.1669; 287.0551; 151.0033; 135.0440 | × | ||
| 13 | 7.500 | Quercetin hexuronide | C21H18O13 | 477.0675 | 477.0683 | 1.8 | 13 | 477.0678; 301.0341 | × | ||
| 14 | 7.595 | Quercetin hexoside | C17H20O12 | 463.0882 | 463.0901 | 4.1 | 12 | 463.0899; 301.0346; 300.0275 | × | ||
| 15 | 7.921 | Methyl-luteolin derivative | C27H31NO14 | 592.1672 | 592.1685 | 2.2 | 13 | 592.1713; 546.1614; 475.0880; 299.0542; 285.0400; 284.0308 | × | × | |
| 16 | 8.197 | Luteolin pentosyl-hexoside | C26H28O15 | 579.1355 | 579.1366 | 1.8 | 13 | 579.1375; 285.0401 | × | ||
| 17 | 8.300 | Luteolin hexuronide | C21H18O12 | 461.0725 | 461.0733 | 1.6 | 13 | 461.0745; 285.0401 | × | × | × |
| 18 | 8.397 | Luteolin hexoside 1 | C21H20O11 | 447.0933 | 447.0935 | 0.5 | 12 | 447.0945; 285.0396; 284.0322 | × | × | × |
| 19 | 8.493 | Luteolin rutinoside (e.g., scolymoside) | C27H30O15 | 593.1512 | 593.1526 | 2.4 | 13 | 593.1551; 285.0405 | × | × | × |
| 20 | 8.654 | 1,4-dicaffeoylquinic acid | C25H24O12 | 515.1195 | 515.1208 | 2.5 | 14 | 515.1218; 353.0884; 335.0772; 191.0557; 179.0352; 173.0447; 135.0451; 93.0337 | × | × | × |
| 21 | 9.045 | 3,5-dicaffeoylquinic acid | C25H24O12 | 515.1195 | 515.1203 | 1.6 | 14 | 515.1195; 353.0868; 191.0560; 179.0347; 135.0451 | × | × | × |
| 22 | 9.270 | 1,5-dicaffeoylquinic acid | C25H24O12 | 515.1195 | 515.1217 | 4.3 | 14 | 515.1228; 353.0885; 191.0561 | × | × | × |
| 23 | 9.867 | Apigenin hexoside | C21H20O10 | 431.0984 | 431.0997 | 3.1 | 12 | 431.0983; 269.0438; 268.0361 | × | × | × |
| 24 | 9.869 | Apigenin hexuronide | C21H18O11 | 445.0776 | 445.0793 | 3.7 | 13 | 445.0787; 269.0441; 113.0236 | × | × | × |
| 25 | 9.876 | Apigenin rutinoside | C27H30O14 | 577.1563 | 577.1583 | 3.5 | 13 | 577.1589; 269.0450 | × | × | × |
| 26 | 9.942 | Dicaffeoylquinic acid isomer | C25H24O12 | 515.1195 | 515.1200 | 1.0 | 14 | 515.1213; 353.0877; 191.0560; 179.0343 | × | × | × |
| 27 | 10.104 | Luteolin hexoside 2 | C21H20O11 | 447.0933 | 447.0941 | 1.8 | 12 | 447.0937; 285.0393 | × | × | |
| 28 | 10.710 | 3,4-dicaffeoylquinic acid | C25H24O12 | 515.1195 | 515.1217 | 4.3 | 14 | 515.1220; 353.0875; 191.0557; 179.0343;173.0450; 135.0443 | × | × | × |
| 29 | 11.410 | Luteolin hexoside 3 (e.g., cynaroside) | C21H20O11 | 447.0933 | 447.0949 | 3.6 | 12 | 447.0941; 285.0396 | × | ||
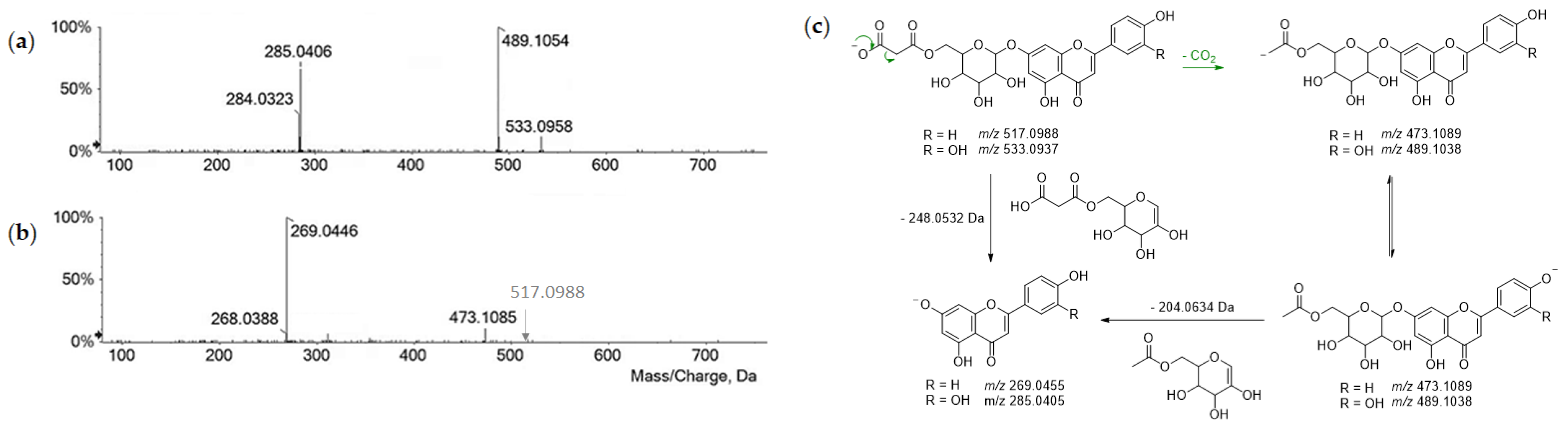
| 30 | 11.584 | Luteolin malonylhexoside | C24H22O14 | 533.0937 | 533.0947 | 1.9 | 14 | 533.0958; 489.1054; 285.0406; 284.0323 | × | × | |
| 31 | 13.115 | Methyl-luteolin hexuronide | C22H20O12 | 475.0882 | 475.0903 | 4.4 | 13 | 475.0888; 299.0553; 285.0398; 284.0308 | × | × | × |
| 32 | 13.262 | Dicaffeoyl methylquinate 1 | C26H26O12 | 529.1352 | 529.1361 | 1.8 | 14 | 529.1371; 367.1030; 349.0922; 179.0350; 161.0246; 135.0452; 133.0298 | × | × | × |
| 33 | 13.277 | 4,5-dicaffeoylquinic acid | C25H24O12 | 515.1195 | 515.1214 | 3.7 | 14 | 515.1230; 353.0889; 191.0560; 179.0355; 173.0445; 135.0466 | × | × | × |
| 34 | 13.340 | Apigenin malonylhexoside | C24H22O13 | 517.0988 | 517.1008 | 3.9 | 14 | 473.1085; 269.0446; 268.0388 | × | × | × |
| 35 | 13.601 | Dicaffeoyl methylquinate 2 | C26H26O12 | 529.1352 | 529.1354 | 0.5 | 14 | 529.1380; 367.1031; 179.0353; 161.0244; 135.0454 | × | × | × |
| 36 | 13.618 | Apigenin methylhexuronide | C22H20O11 | 459.0933 | 459.0943 | 2.2 | 13 | 459.0935; 269.0454; 268.0374 | × | × | × |
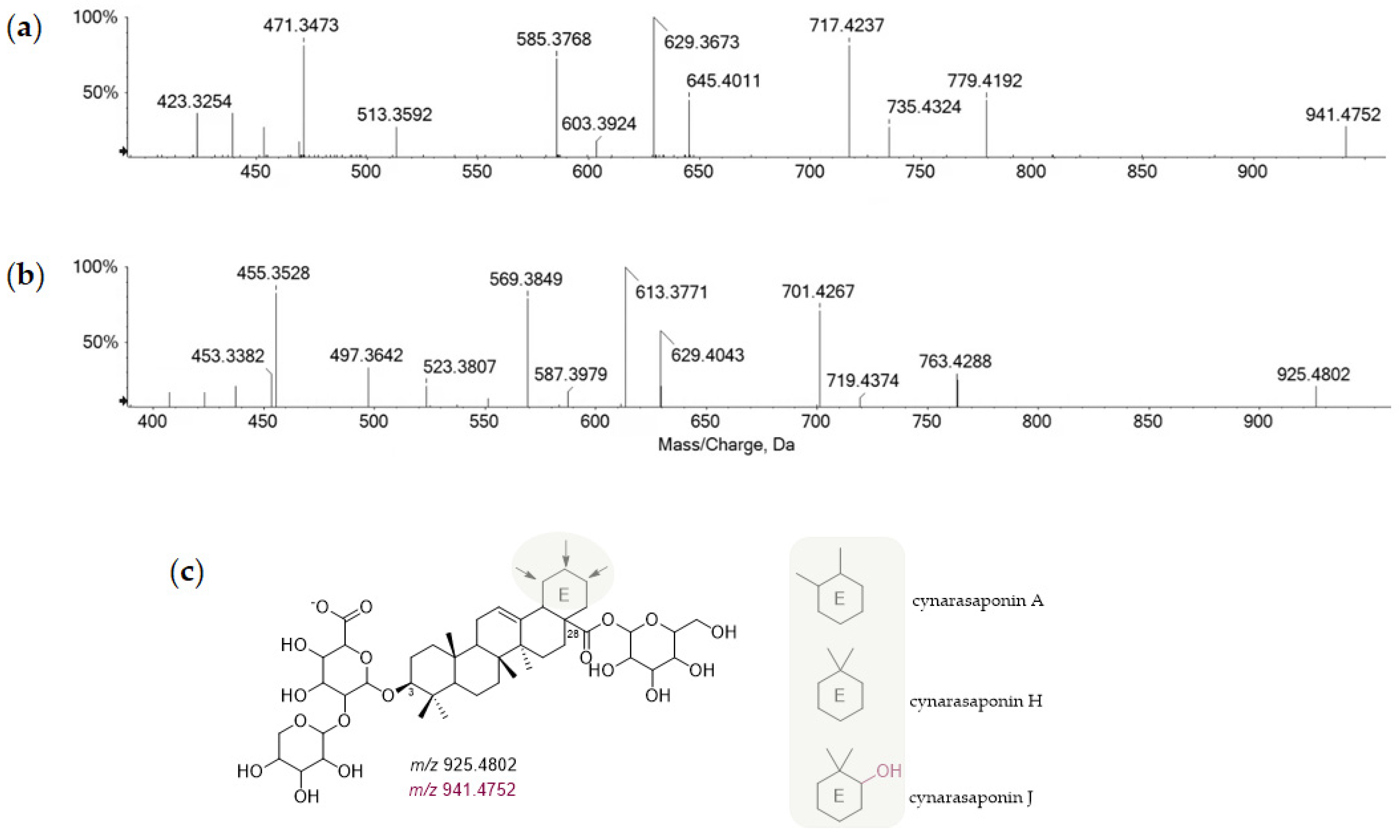
| 37 | 14.125 | Cynarasaponin A (or H) | C47H74O19 | 941.4752 | 941.4766 | 1.5 | 11 | 941.4752 *; 779.4192; 735.4324; 717.4237; 645.4011; 629.3673; 603.3924; 585.3768; 513.3592; 471.3473; 423.3254 | × | ||
| 38 | 14.433 | Cynarasaponin J | C47H74O18 | 925.4802 | 925.4813 | 1.1 | 11 | 925.4802 *; 763.4288; 719.4374; 701.4267; 629.4043; 613.3771; 587.3979; 569.3849; 523.3807; 497.3642; 455.3528; 453.3382; 407.3288 | × |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahmi-Chendouh, N.; Piccolella, S.; Gravina, C.; Fiorentino, M.; Formato, M.; Kheyar, N.; Pacifico, S. Ready-to-Use Nutraceutical Formulations from Edible and Waste Organs of Algerian Artichokes. Foods 2022, 11, 3955. https://doi.org/10.3390/foods11243955
Brahmi-Chendouh N, Piccolella S, Gravina C, Fiorentino M, Formato M, Kheyar N, Pacifico S. Ready-to-Use Nutraceutical Formulations from Edible and Waste Organs of Algerian Artichokes. Foods. 2022; 11(24):3955. https://doi.org/10.3390/foods11243955
Chicago/Turabian StyleBrahmi-Chendouh, Nabila, Simona Piccolella, Claudia Gravina, Marika Fiorentino, Marialuisa Formato, Naoual Kheyar, and Severina Pacifico. 2022. "Ready-to-Use Nutraceutical Formulations from Edible and Waste Organs of Algerian Artichokes" Foods 11, no. 24: 3955. https://doi.org/10.3390/foods11243955
APA StyleBrahmi-Chendouh, N., Piccolella, S., Gravina, C., Fiorentino, M., Formato, M., Kheyar, N., & Pacifico, S. (2022). Ready-to-Use Nutraceutical Formulations from Edible and Waste Organs of Algerian Artichokes. Foods, 11(24), 3955. https://doi.org/10.3390/foods11243955










