Automated and Rapid Easy-to-Use Magnetic Solid-Phase Extraction System for Five Heavy Metals in Cereals and Feeds
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Samples
2.2. Apparatus and Materials
2.3. Synthesis and Characterization of Functional-Group-Modified MBs
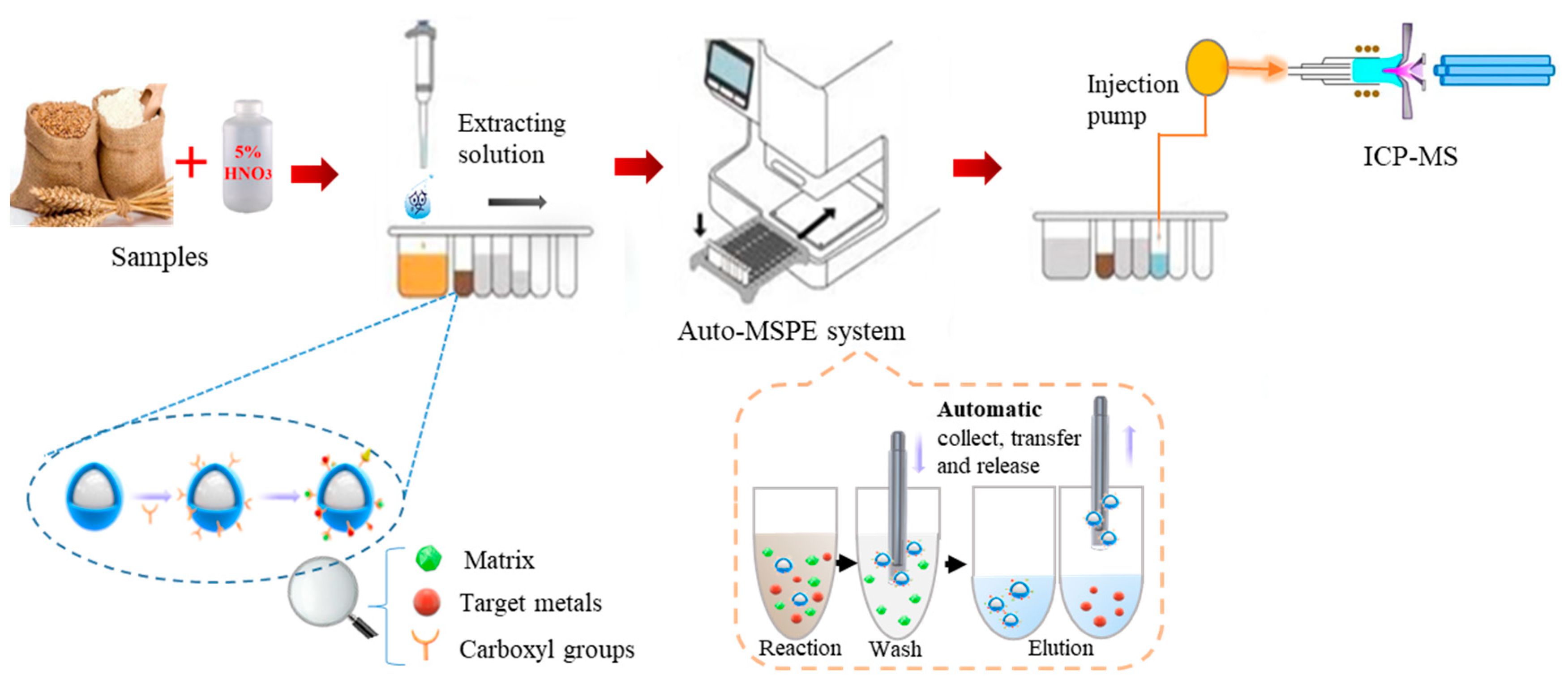
2.4. Automated Magnet-Controlled Pretreatment System
2.5. Optimization of the Experimental Variables
2.6. Application to Cereals and Their Products
3. Results and Discussion
3.1. Physical and Chemical Properties of Materials
3.2. Effect of Initial pH
3.3. Effect of Adsorption Time
3.4. Influences of the Eluent Condition
3.5. Clean-Up Performance
3.6. Method Validation
3.7. Application to Real Samples
3.8. Comparison with Other MSPE Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feist, B.; Sitko, R. Method for the determination of Pb, Cd, Zn, Mn and Fe in rice samples using carbon nanotubes and cationic complexes of batophenanthroline. Food Chem. 2018, 249, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.P.; Cerveira, C.; Miceli, T.M.; Moraes, D.P.; Mesko, M.F.; Pereira, J.S. Evaluation of sample preparation methods for cereal digestion for subsequent As, Cd, Hg and Pb determination by AAS-based techniques. Food Chem. 2020, 321, 126715. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, J.; Ye, J.; Wang, L.; Sun, X. One-step extraction and simultaneous quantitative fluorescence immunochromatography strip for AFB1 and Cd detection in grain. Food Chem. 2022, 374, 131684. [Google Scholar] [CrossRef]
- Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jiang, R.; Zhu, F.; Liu, H.; Ouyang, G. Application of functionalized magnetic nanoparticles in sample preparation. Anal. Bioanal. Chem. 2013, 406, 377–399. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Ping, J. Simultaneous determination of Cd(II) and Pb(II) ions in honey and milk samples using a single-walled carbon nanohorns modified screen-printed electrochemical sensor. Food Chem. 2019, 274, 8–15. [Google Scholar] [CrossRef]
- Runge, J.; Heringer, O.A.; Ribeiro, J.S.; Biazati, L.B. Multi-element rice grains analysis by ICP OES and classification by processing types. Food Chem. 2019, 271, 419–424. [Google Scholar] [CrossRef]
- Cheng, S.; Qin, C.; Xie, H.; Wang, W.; Zhang, J.; Hu, Z.; Liang, S. Comprehensive evaluation of manganese oxides and iron oxides as metal substrate materials for constructed wetlands from the perspective of water quality and greenhouse effect. Ecotoxicol. Environ. Saf. 2021, 221, 112451. [Google Scholar] [CrossRef]
- Studer, J.M.; Schweer, W.P.; Gabler, N.K.; Ross, J.W. Functions of manganese in reproduction. Anim. Reprod. Sci. 2022, 238, 106924. [Google Scholar] [CrossRef]
- Zhou, Q.-Z.; Wang, L.-Y.; Ma, G.-H.; Su, Z.-G. Multi-stage premix membrane emulsification for preparation of agarose microbeads with uniform size. J. Membr. Sci. 2008, 322, 98–104. [Google Scholar] [CrossRef]
- Yıldız, H.; Tokalıoğlu, S.; Soykan, C. Preparation of polyacrylonitrile/polyindole conducting polymer composite and its use for solid phase extraction of copper in a certified reference material. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 244, 118826. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.Z.; Afzali, D.; Pourtalebi, D. Flame atomic absorption spectrometric determination of trace amounts of lead, cadmium and nickel in different matrixes after solid phase extraction on modified multiwalled carbon nanotubes. Open Chem. 2010, 8, 662–668. [Google Scholar] [CrossRef]
- Daşbaşı, T.; Soykan, C. A synthesized ternary polymer composite and its use for SPE of trace metals in Coffee, tea and legumes. Food Chem. 2021, 365, 130518. [Google Scholar] [CrossRef]
- Londonio, A.; Morzan, E.; Smichowski, P. Simultaneous on-line preconcentration and determination of toxic elements in rice and rice-based products by SPE–ICP–MS: Multiple response optimization. J. Food Compos. Anal. 2022, 107, 104388. [Google Scholar] [CrossRef]
- Cobelo-García, A.; Mulyani, M.E.; Schäfer, J. Ultra-trace interference-free analysis of palladium in natural waters by ICP-MS after on-line matrix separation and pre-concentration. Talanta 2021, 232, 122289. [Google Scholar] [CrossRef]
- Losev, V.N.; Didukh-Shadrina, S.L.; Orobyeva, A.S.; Metelitsa, S.I.; Borodina, E.V.; Ondar, U.V.; Nesterenko, P.N.; Maznyak, N.V. A new method for highly efficient separation and determination of arsenic species in natural water using silica modified with polyamines. Anal. Chim. Acta 2021, 1178, 338824. [Google Scholar] [CrossRef]
- Su, S.; Chen, B.; He, M.; Hu, B.; Xiao, Z. Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta 2014, 119, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, S.; Mohamedsaid, S.A.; Kılınç, E.; Soylak, M. Magnetic solid phase extractions of Co(II) and Hg(II) by using magnetized C. micaceus from water and food samples. Food Chem. 2019, 271, 232–238. [Google Scholar] [CrossRef]
- Zhou, D.-B.; Xiao, Y.-B.; Han, F.; Lv, Y.-N.; Ding, L.; Song, W.; Liu, Y.-X.; Zheng, P.; Chen, D. Magnetic solid-phase extraction based on sulfur-functionalized magnetic metal-organic frameworks for the determination of methylmercury and inorganic mercury in water and fish samples. J. Chromatogr. A 2021, 1654, 462465. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Y.; Zhang, J.; Zhang, Y.; Chen, X.; Ye, J.; Wang, S. Development and Collaborative Study of a Diluted Acid Mild Extraction Method for Determination of Cadmium in Grain by Graphite Furnace Atomic Absorption Spectrometry. Anal. Sci. 2019, 35, 283–287. [Google Scholar] [CrossRef]
- Zhou, M.-H.; Tian, W.; Zhang, J.-Q.; Chen, X.; Wu, Y.-X.; Wang, S.-X. A rapid on-site analysis method for the simultaneous extraction and determination of Pb2+ and Cd2+ in cereals. RSC Adv. 2019, 9, 32839–32847. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, B.; Rezania, S. Carbon-based sorbents and their nanocomposites for the enrichment of heavy metal ions: A review. Mikrochim. Acta 2019, 186, 578. [Google Scholar] [CrossRef] [PubMed]
- Giakisikli, G.; Anthemidis, A.N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Taher, M.A.; Behzadi, M.; Moghaddam, F.H. Simultaneous preconcentration of bismuth and lead ions on modified magnetic core–shell nanoparticles and their determination by ETAAS. Chem. Eng. J. 2015, 281, 444–452. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Ma, J.; Jin, Z.; Chai, M.; Xiao, X.; Zhang, L.; Zhang, Y. A polyethyleneimine-modified attapulgite as a novel solid support in matrix solid-phase dispersion for the extraction of cadmium traces in seafood products. Talanta 2018, 180, 254–259. [Google Scholar] [CrossRef]
- Abudiab, T.; Beitle, R.R. Preparation of magnetic immobilized metal affinity separation media and its use in the isolation of proteins. J. Chromatogr. A 1998, 795, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Li, N.; Cui, L.; Wang, X.; Zhao, R.-S. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120, 115632. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Hu, L.; Li, Y.; Luo, D.; Mei, S. Recent applications of magnetic composites as extraction adsorbents for determination of environmental pollutants. TrAC Trends Anal. Chem. 2019, 119, 115611. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, J.; Huang, X. Allylthiourea functionalized magnetic adsorbent for the extraction of cadmium, copper and lead ions prior to their determination by atomic absorption spectrometry. Mikrochim. Acta 2019, 186, 51. [Google Scholar] [CrossRef]
- Molaei, K.; Bagheri, H.; Asgharinezhad, A.A.; Ebrahimzadeh, H.; Shamsipur, M. SiO2-coated magnetic graphene oxide modified with polypyrrole–polythiophene: A novel and efficient nanocomposite for solid phase extraction of trace amounts of heavy metals. Talanta 2017, 167, 607–616. [Google Scholar] [CrossRef]
- Suo, L.; Zhao, J.; Dong, X.; Gao, X.; Li, X.; Xu, J.; Lu, X.; Zhao, L. Functionalization of a SiO2-coated magnetic graphene oxide composite with polyaniline–polypyrrole for magnetic solid phase extraction of ultra-trace Cr(iii) and Pb(ii) in water and food samples using a Box–Behnken design. New J. Chem. 2019, 43, 12126–12136. [Google Scholar] [CrossRef]
- Xu, C.; He, M.; Chen, B.; Hu, B. Magnetic porous coordination networks for preconcentration of various metal ions from environmental water followed by inductively coupled plasma mass spectrometry detection. Talanta 2022, 245, 123470. [Google Scholar] [CrossRef] [PubMed]
- Sricharoen, P.; Limchoowong, N.; Areerob, Y.; Nuengmatcha, P.; Techawongstien, S.; Chanthai, S. Fe3O4/hydroxyapatite/graphene quantum dots as a novel nano-sorbent for preconcentration of copper residue in Thai food ingredients: Optimization of ultrasound-assisted magnetic solid phase extraction. Ultrason. Sonochem. 2017, 37, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magied, A.F.; Abdelhamid, H.N.; Ashour, R.M.; Fu, L.; Dowaidar, M.; Xia, W.; Forsberg, K. Magnetic metal-organic frameworks for efficient removal of cadmium(II), and lead(II) from aqueous solution. J. Environ. Chem. Eng. 2022, 10, 107467. [Google Scholar] [CrossRef]




| Statistical Parameters | Cu | Zn | Mn | Cd | Pb |
|---|---|---|---|---|---|
| Linear ranges (μg L−1) | 0~200 | 0~400 | 0~400 | 0~400 | 0~400 |
| Regression equations | y = 0.930x − 2.07 | y = 1.068x + 0.98 | y = 0.955x + 0.54 | y = 1.077x − 2.03 | y = 1.066x − 2.18 |
| Coefficients (R2) | 0.9983 | 0.9995 | 0.9992 | 0.9998 | 0.9991 |
| RSD (%) | 1.5 | 1.7 | 2.1 | 2.5 | 1.2 |
| LOD (ng L−1) | 11.54 | 2.71 | 3.84 | 0.16 | 6.01 |
| LOQ (ng L−1) | 38.08 | 8.94 | 12.67 | 0.53 | 19.83 |
| Sample | Mn | Cu | Zn | Cd | Pb |
|---|---|---|---|---|---|
| Rice | 88.8 ± 2.1 | 88.7 ± 0.8 | 82.8 ± 2.5 | 91.7 ± 1.6 | 82.7 ± 2.4 |
| 86.3 ± 2.4 | 83.6 ± 1.2 | 84.7 ± 1.4 | 97.8 ± 1.4 | 89.0 ± 1.6 | |
| 82.0 ± 3.0 | 89.8 ± 2.3 | 83.4 ± 1.3 | 92.7 ± 1.5 | 88.8 ± 1.7 | |
| 88.5 ± 1.9 | 86.0 ± 1.5 | 81.1 ± 2.1 | 89.8 ± 2.1 | 86.8 ± 2.5 | |
| 88.9 ± 1.0 | 89.0 ± 1.2 | 83.8 ± 2.0 | 90.1 ± 2.8 | BDL | |
| 88.0 ± 0.9 | 92.1 ± 2.5 | 81.2 ± 1.6 | 94.2 ± 3.2 | 98.9 ± 3.4 | |
| Wheat | 88.3 ± 2.3 | 85.6 ± 3.4 | 89.8 ± 3.5 | 88.0 ± 3.5 | 91.8 ± 3.2 |
| 85.0 ± 3.0 | 91.7 ± 3.2 | 87.7 ± 3.6 | 90.8 ± 3.7 | 95.8 ± 1.5 | |
| 95.3 ± 1.5 | 98.6 ± 1.8 | 87.1 ± 2.9 | 80.1 ± 2.9 | 85.2 ± 3.8 | |
| 88.3 ± 2.4 | 91.0 ± 2.7 | 81.8 ± 3.2 | 89.2 ± 2.5 | 91.2 ± 2.5 | |
| 92.4 ± 3.0 | 92.9 ± 1.9 | 88.2 ± 3.7 | 89.8 ± 1.8 | 93.8 ± 2.4 | |
| 82.8 ± 3.1 | 98.8 ± 1.5 | 85.7 ± 2.8 | 86.2 ± 1.5 | 91.1 ± 1.7 | |
| Corn | 83.4 ± 2.6 | 91.5 ± 2.8 | 82.4 ± 3.1 | 87.6 ± 3.3 | 91.2 ± 1.1 |
| 80.3 ± 3.8 | 91.8 ± 3.2 | 85.8 ± 3.4 | 94.6 ± 2.1 | 94.1 ± 1.4 | |
| 87.4 ± 1.5 | 89.3 ± 3.0 | 85.9 ± 2.6 | 90.7 ± 1.1 | 94.0 ± 2.2 | |
| Beans | 84.2 ± 1.2 | 91.0 ± 1.6 | 87.7 ± 2.8 | 93.1 ± 3.3 | 88.1 ± 2.0 |
| 80.8 ± 1.0 | 90.6 ± 2.4 | 94.0 ± 1.9 | 91.2 ± 3.5 | BDL | |
| DDGS | 85.5 ± 2.4 | 87.5 ± 2.2 | 87.2 ± 1.2 | 96.6 ± 3.6 | 92.0 ± 2.3 |
| Sheep forage | 80.8 ± 1.8 | 88.7 ± 3.0 | 82.8 ± 0.8 | 91.7 ± 2.8 | 92.7 ± 1.5 |
| Cattle feed | 86.3 ± 2.2 | 83.6 ± 1.6 | 84.7 ± 3.5 | 97.8 ± 2.7 | 89.0 ± 3.3 |
| Chicken feed | 106.3 ± 1.2 | 92.6 ± 1.6 | 87.4 ± 3.5 | 82.5 ± 2.1 | 102.5 ± 2.6 |
| Detection Technique | Pretreatment Technique | Working Mode | MSPE | Heavy Metal | Pretreatment Total Time (min) | LOD (ng L−1) | Real Samples | Capacity (mg g−1) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| AAS | Digestion | Manual | Fe3O4@AT@ED | Cd, Cu, Pb | 1209 | 1080~1510 | Beans | - | [29] |
| ICP-MS | Digestion | Manual | mGO/SiO2@coPPy-Th | Cu, Pb, Zn, Cr, Cd | 116.5 | 150, 650, 230, 360, 210 | Tomatoes, apples, water | 201, 230, 125, 98, 80 | [30] |
| ICP-MS | Digestion | Manual | MGO/SiO2@PANI-PPy | Cr, Pb | 106.3 | 4.808, 3.401 | Rice | 188.9, 213.3 | [31] |
| ICP-MS | Digestion | Manual | Fe3O4@SiO2@PAR | Cr, Cd, Pb | 109 | 11.9, 0.8, 4.1 | Human fluids | 62.9, 56.6, 43.3 | [32] |
| ICP-AES | Digestion | Manual | Fe3O4/HAP/GQDs | Cu | 120 | 580 | Food | - | [33] |
| ICP-MS | Filter | Manual | Fe3O4@UiO-66–NH2 | Cd, Pb | 300 | - | Water | 714.3, 370 | [34] |
| ICP-MS | Diluted acid extraction | Automatic | Fe3O4@COOH | Cu, Zn, Mn, Cd, Pb | 22 | 11.54, 2.71, 3.84, 0.16, 6.10 | Cereals, feeds | 426, 277, 152, 229, 402 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Liu, Y.; Wang, S.; Ye, J.; Liu, H.; Wang, Y.; Zhou, M. Automated and Rapid Easy-to-Use Magnetic Solid-Phase Extraction System for Five Heavy Metals in Cereals and Feeds. Foods 2022, 11, 3944. https://doi.org/10.3390/foods11243944
Tian W, Liu Y, Wang S, Ye J, Liu H, Wang Y, Zhou M. Automated and Rapid Easy-to-Use Magnetic Solid-Phase Extraction System for Five Heavy Metals in Cereals and Feeds. Foods. 2022; 11(24):3944. https://doi.org/10.3390/foods11243944
Chicago/Turabian StyleTian, Wei, Yonglin Liu, Songxue Wang, Jin Ye, Hongmei Liu, Yue Wang, and Minghui Zhou. 2022. "Automated and Rapid Easy-to-Use Magnetic Solid-Phase Extraction System for Five Heavy Metals in Cereals and Feeds" Foods 11, no. 24: 3944. https://doi.org/10.3390/foods11243944
APA StyleTian, W., Liu, Y., Wang, S., Ye, J., Liu, H., Wang, Y., & Zhou, M. (2022). Automated and Rapid Easy-to-Use Magnetic Solid-Phase Extraction System for Five Heavy Metals in Cereals and Feeds. Foods, 11(24), 3944. https://doi.org/10.3390/foods11243944






