Co-Pigmentation Mechanism and Thermal Reaction Kinetics of Mulberry Anthocyanins with Different Phenolic Acids
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Co-pigmented Anthocyanin Solution
2.2.1. The Effect of the Anthocyanin/Co-pigment Molar Ratio
2.2.2. The Effect of the pH on Co-pigmentation
2.2.3. The Effect of the Temperature on Co-pigmentation
2.3. Thermal Degradation Kinetic Calculation
2.4. Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry
2.5. FTIR and UV–Vis Spectroscopy
2.6. Molecular Docking
2.7. Molecular Dynamics
3. Results and Discussion
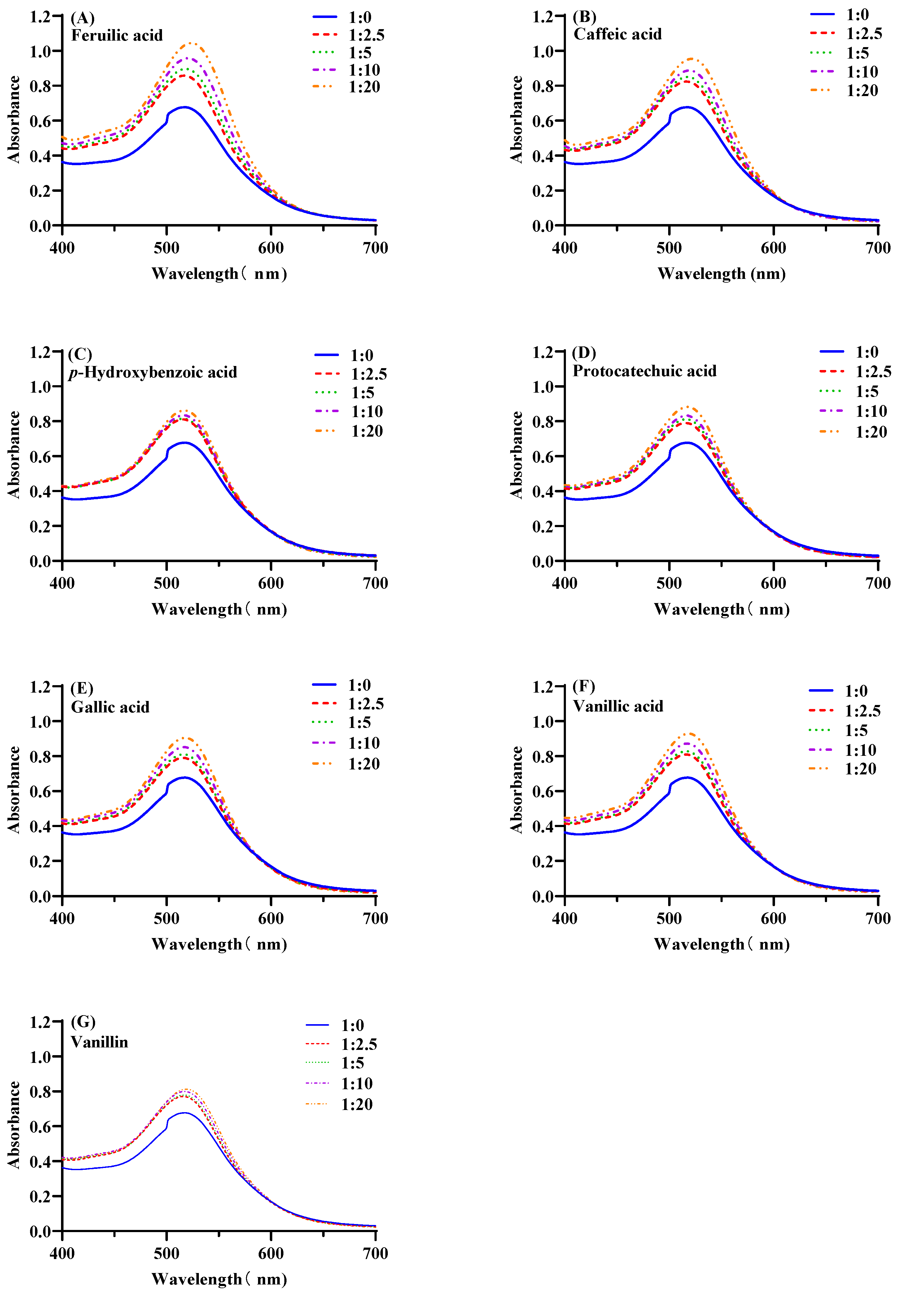
3.1. The Influence of Anthocyanins/Co-Pigmentation Molar Ratio on Co-Pigmentation
3.2. The Influence of pH on Co-Pigmentation
3.3. The Influence of Temperature on Co-pigmentation
3.4. The Co-Pigmentation Influence on the Thermal Degradation Kinetics of Mulberry Anthocyanins
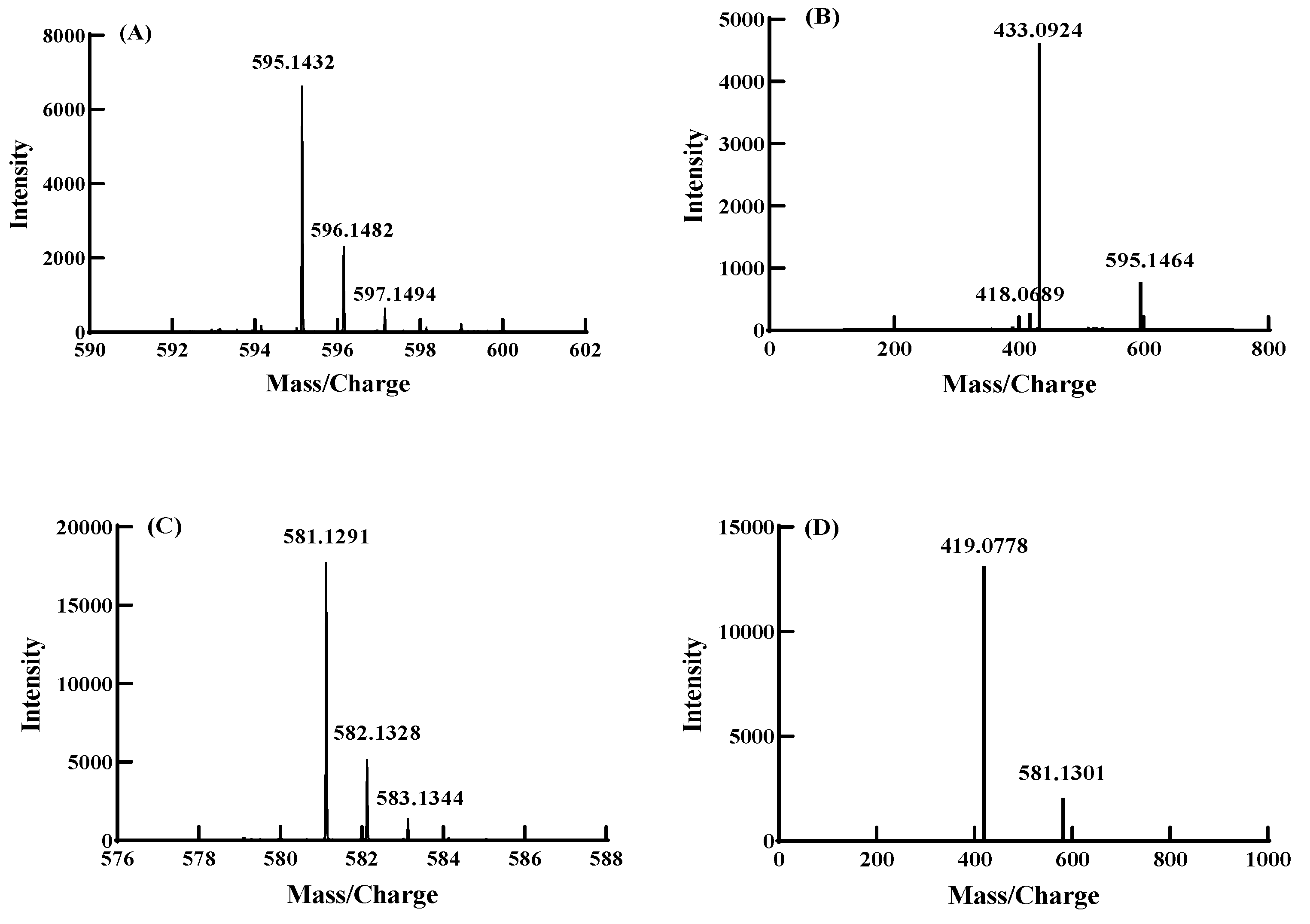
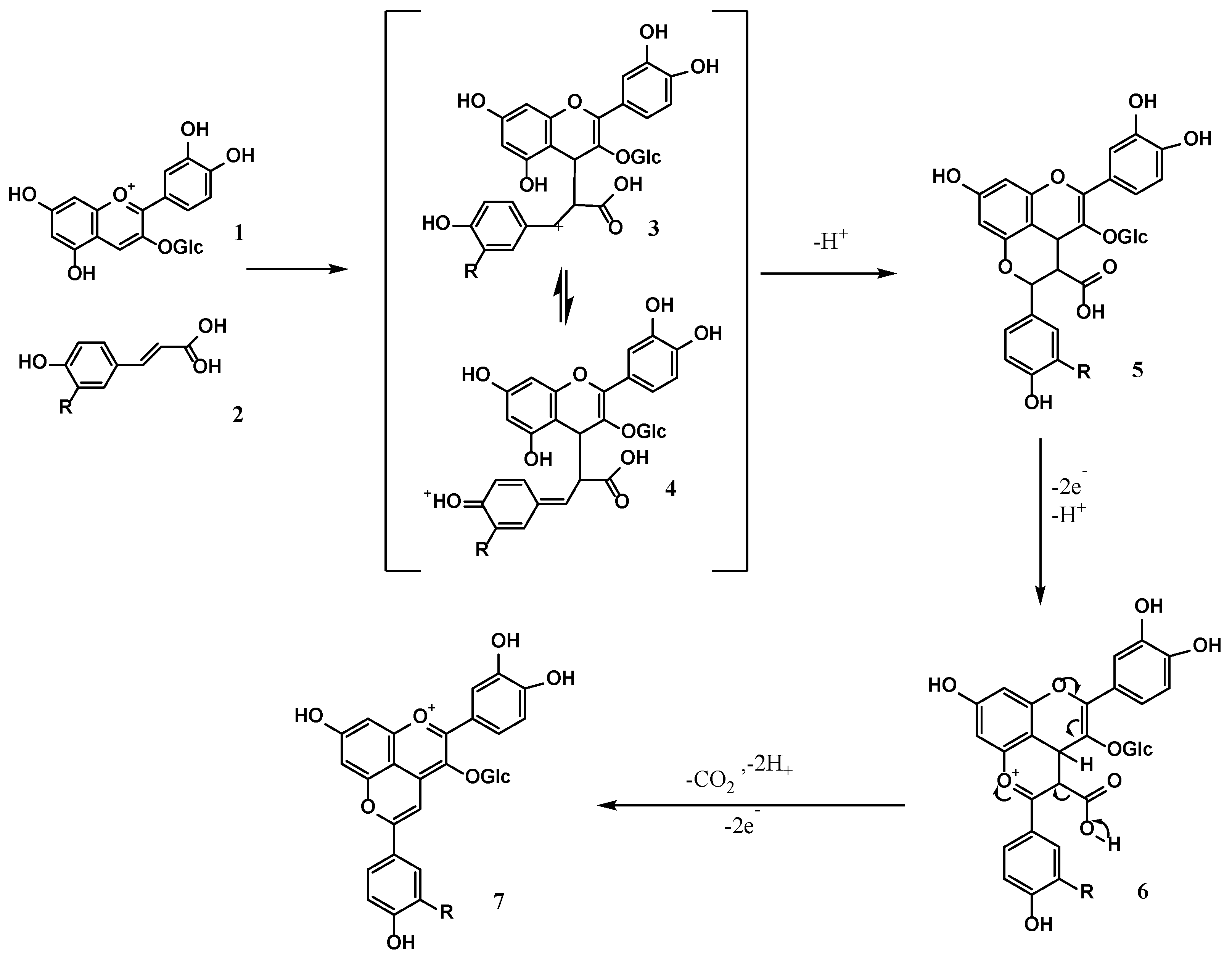
3.5. Component Analysis of Anthocyanin Derivatives by UPLC-Q-TOF-MS/MS
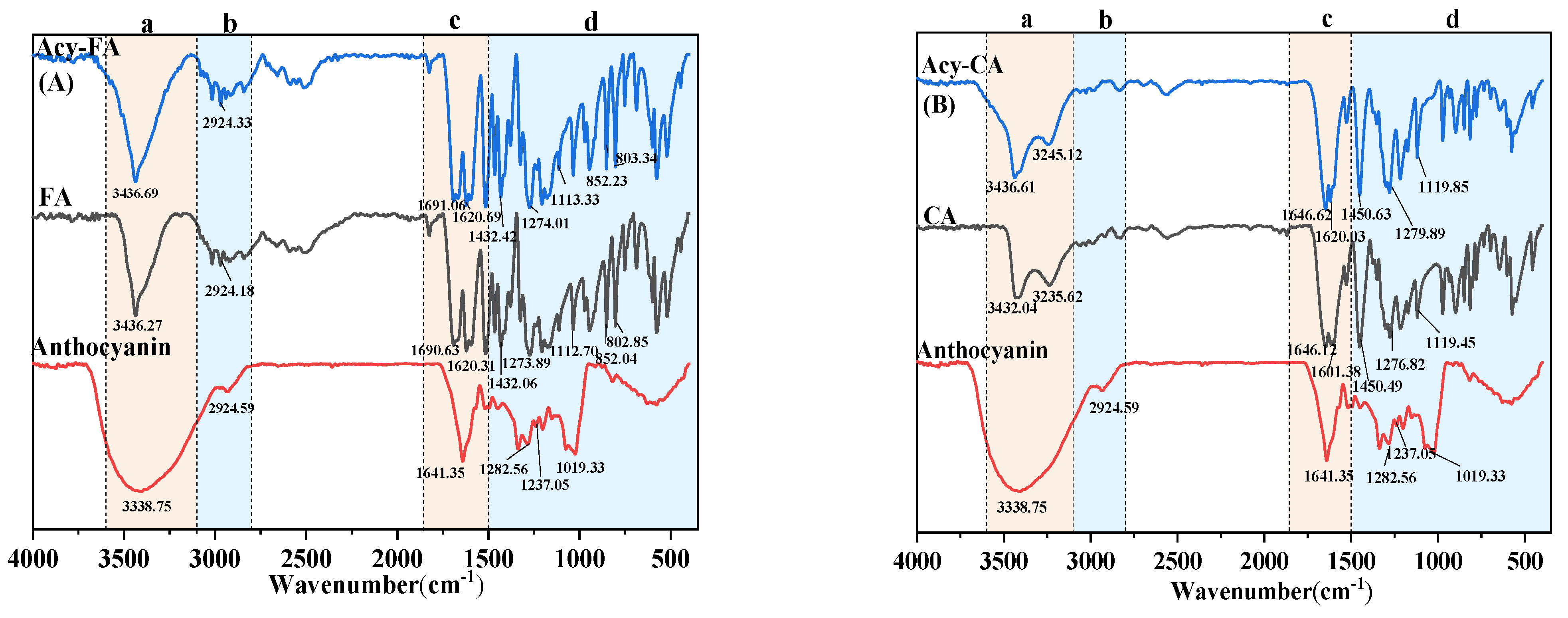
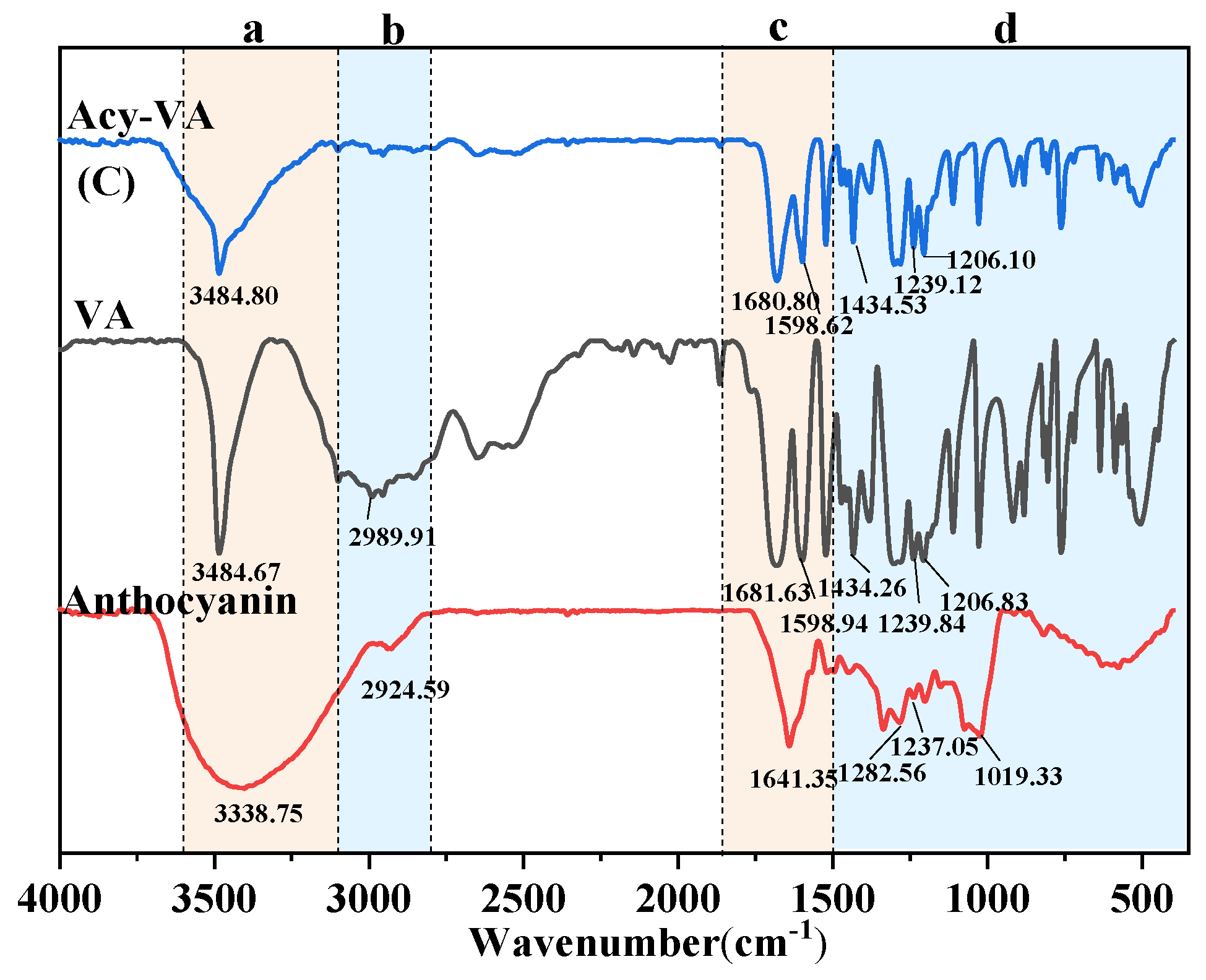
3.6. FTIR Analysis
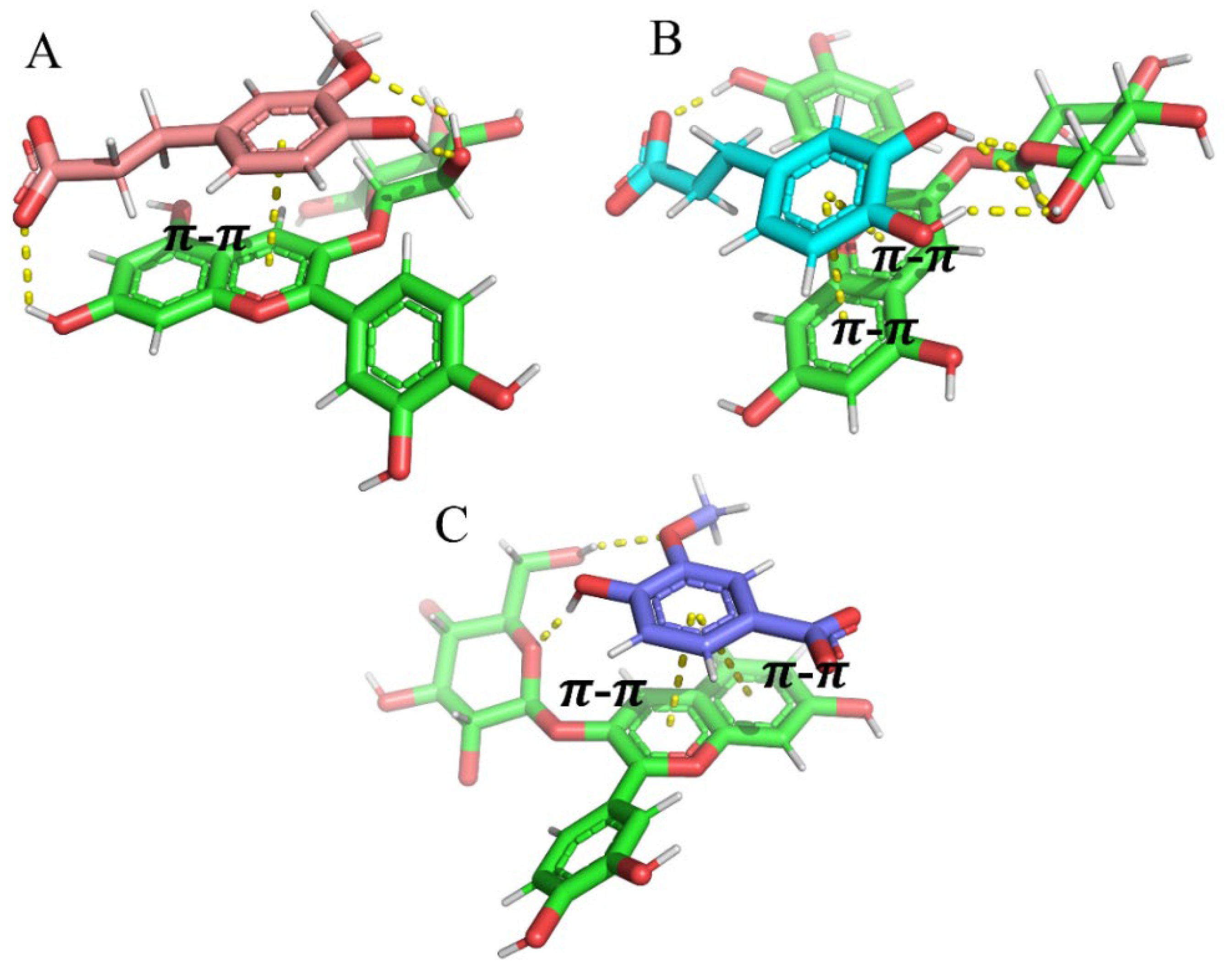
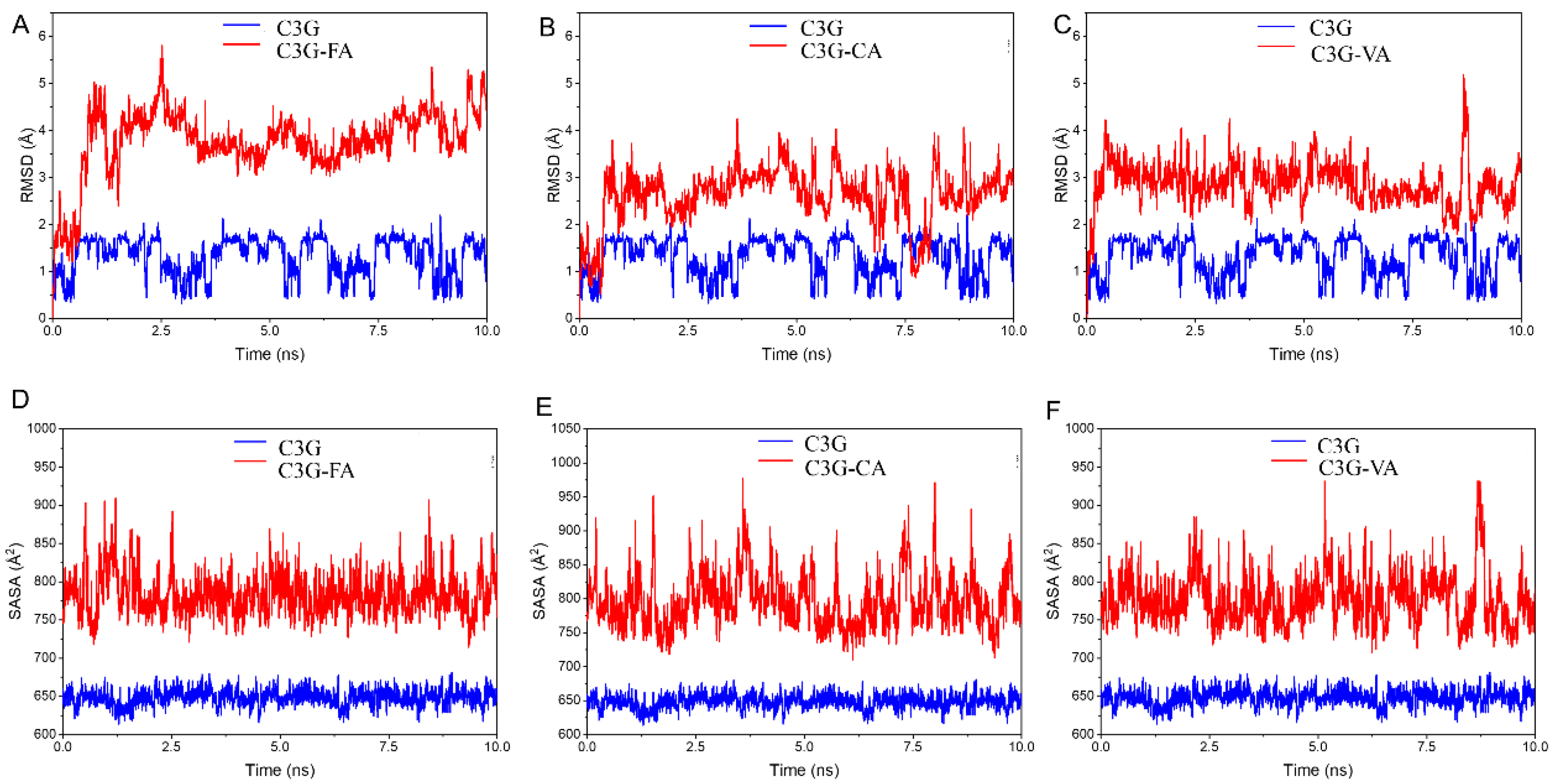
3.7. Molecular Dynamics Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318, 126449. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Jiang, Y.-W.; Wang, L.-T.; Niu, L.-J.; Liu, Z.-M.; Fu, Y.-J. Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Jiang, L.; Zhang, J.-G.; Deng, W.; Wang, H.-L.; Wei, Z.-J. Quantitative determination of 1-deoxynojirimycin in mulberry leaves from 132 varieties. Ind. Crops Prod. 2013, 49, 782–784. [Google Scholar] [CrossRef]
- Bi, Y.; Chi, X.; Zhang, R.; Lu, Y.; Wang, Z.; Dong, Q.; Ding, C.; Yang, R.; Jiang, L. Highly efficient extraction of mulberry anthocyanins in deep eutectic solvents: Insights of degradation kinetics and stability evaluation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, J.-R.; Liu, X.-M.; Zhu, M.-J. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. LWT 2019, 100, 62–68. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, Z.; Wu, Y.; Qie, X.; Chen, Y.; Zeng, M.; Wang, Z.; Chen, J.; He, Z. Inhibitory effects of soy protein and its hydrolysate on the degradation of anthocyanins in mulberry extract. Food Biosci. 2021, 40, 100911. [Google Scholar] [CrossRef]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, north Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Dong, K.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Mulberry fruit powder enhanced the antioxidant capacity and gel properties of hammered minced beef: Oxidation degree, rheological, and structure. LWT 2022, 154, 112648. [Google Scholar] [CrossRef]
- Chen, X.; Guan, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Effect of whey protein isolate and phenolic copigments in the thermal stability of mulberry anthocyanin extract at an acidic ph. Food Chem. 2022, 377, 132005. [Google Scholar] [CrossRef]
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; Freitas, V.d. Synthesis of a new bluish pigment from the reaction of a methylpyranoanthocyanin with sinapaldehyde. Tetrahedron Lett. 2011, 52, 1996–2000. [Google Scholar] [CrossRef]
- Molaeafard, S.; Jamei, R.; Poursattar Marjani, A. Co-pigmentation of anthocyanins extracted from sour cherry (Prunus cerasus L.) with some organic acids: Color intensity, thermal stability, and thermodynamic parameters. Food Chem. 2020, 339, 128070. [Google Scholar] [CrossRef]
- Yener, I.; Kocakaya, S.O.; Ertas, A.; Erhan, B.; Kaplaner, E.; Oral, E.V.; Yilmaz-Ozden, T.; Yilmaz, M.A.; Ozturk, M.; Kolak, U. Selective in vitro and in silico enzymes inhibitory activities of phenolic acids and flavonoids of food plants: Relations with oxidative stress. Food Chem. 2020, 327, 127045. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoon, W.-J.; Kim, S.S.J.M. Phytochemical compositions of immature wheat bran, and its antioxidant capacity, cell growth inhibition, and apoptosis induction through tumor suppressor gene. Molecules 2016, 21, 1292. [Google Scholar] [CrossRef]
- Cheng, F.; Sheng, J.; Cai, T.; Jin, J.; Liu, W.; Lin, Y.; Du, Y.; Zhang, M.; Shen, L. A protease-insensitive feruloyl esterase from china holstein cow rumen metagenomic library: Expression, characterization, and utilization in ferulic acid release from wheat straw. J. Agric. Food Chem. 2012, 60, 2546–2553. [Google Scholar] [CrossRef]
- Boz, H. Ferulic acid in cereals—A review. Czech J. Food Sci. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Turner, A.L.; Michaelson, L.V.; Shewry, P.R.; Lovegrove, A.; Spencer, J.P. Increased bioavailability of phenolic acids and enhanced vascular function following intake of feruloyl esterase-processed high fibre bread: A randomized, controlled, single blind, crossover human intervention trial. Clin. Nutr. 2021, 40, 788–795. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Iloki Assanga, S.B.; Lewis Luján, L.; O’keefe, J.H.; DiNicolantonio, J.J. Nutraceutical strategies for suppressing nlrp3 inflammasome activation: Pertinence to the management of COVID-19 and beyond. Nutrients 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X.J. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S.J. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Díaz, A.N.; Algarra, M.; Feria, L.S.; Sanchez, F.G. Fluorimetric determination of p-hydroxybenzoic acid in beer as α-cyclodextrin inclusion complex. Anal. Lett. 2008, 41, 1802–1810. [Google Scholar] [CrossRef]
- Brouillard, R.; Mazza, G.; Saad, Z.; Albrecht-Gary, A.; Cheminat, A. The co-pigmentation reaction of anthocyanins: A microprobe for the structural study of aqueous solutions. J. Am. Chem. Soc. 1989, 111, 2604–2610. [Google Scholar] [CrossRef]
- Lambert, S.G.; Asenstorfer, R.E.; Williamson, N.M.; Iland, P.G.; Jones, G.P. Copigmentation between malvidin-3-glucoside and some wine constituents and its importance to colour expression in red wine. Food Chem. 2011, 125, 106–115. [Google Scholar] [CrossRef]
- Malaj, N.; de Simone, B.C.; Quartarolo, A.D.; Russo, N. Spectrophotometric study of the copigmentation of malvidin 3-o-glucoside with p-co: Umaric, vanillic and syringic acids. Food Chem. 2013, 141, 3614–3620. [Google Scholar] [CrossRef]
- Khalifa, I.; Xia, D.; Dutta, K.; Peng, J.M.; Jia, Y.Y.; Li, C.M. Mulberry anthocyanins exert anti-ages effects by selectively trapping glyoxal and structural-dependently blocking the lysyl residues of beta-lactoglobulins. Bioorganic Chem. 2020, 96, 11. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, D.; Liao, S.; Zou, Y.; Zhou, P.; Li, E.; Wang, W. Synergistic effects of cinnamaldehyde and cinnamic acid in cinnamon essential oil against s. Pullorum. Ind. Crops Prod. 2021, 162, 113296. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Lou, L.; Chen, Y.; Ye, X.; Chen, J. Copigmentation effect of three phenolic acids on color and thermal stability of chinese bayberry anthocyanins. Food Sci. Nutr. 2020, 8, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cao, X.; Bai, w.; Liao, X.; Hu, X. Comparative analyses of copigmentation of cyanidin 3-glucoside and cyanidin 3-sophoroside from red raspberry fruits. Food Chem. 2010, 120, 1131–1137. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, R.; He, F.; Zhou, P.-P.; Duan, C.-Q. Copigmentation of malvidin-3-o-glucoside with five hydroxybenzoic acids in red wine model solutions: Experimental and theoretical investigations. Food Chem. 2015, 170, 226–233. [Google Scholar] [CrossRef]
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Regenstein, J.M.; Laokuldilok, T. Copigmentation of cyanidin 3-o-glucoside with phenolics: Thermodynamic data and thermal stability. Food Biosci. 2019, 30, 100419. [Google Scholar] [CrossRef]
- Chen, L.-J.; Hrazdina, G. Structural aspects of anthocyanin-flavonoid complex formation and its role in plant color. Phytochemistry 1981, 20, 297–303. [Google Scholar] [CrossRef]
- Chatham, L.A.; Howard, J.E.; Juvik, J.A. A natural colorant system from corn: Flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem. 2020, 310, 125734. [Google Scholar] [CrossRef]
- Markovic, J.M.D.; Petranovic, N.A.; Baranac, J.M. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acids. J. Agric. Food Chem. 2000, 48, 5530–5536. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Yan, H.; Zhao, Z.; You, L.; Ho, C.-T. Influence of phenolic acids/aldehydes on color intensification of cyanidin-3-o-glucoside, the main anthocyanin in sugarcane (Saccharum officinarum L.). Food Chem. 2022, 373, 131396. [Google Scholar] [CrossRef]
- Zhang, B.; He, F.; Zhou, P.-P.; Liu, Y.; Duan, C.-Q. The color expression of copigmentation between malvidin-3-o-glucoside and three phenolic aldehydes in model solutions: The effects of ph and molar ratio. Food Chem. 2016, 199, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhuang, Y.; Hu, Y.; Xue, S.; Li, H.; Chen, L.; Fei, P. Improving the color stability and antioxidation activity of blueberry anthocyanins by enzymatic acylation with p-coumaric acid and caffeic acid. LWT 2020, 130, 109673. [Google Scholar] [CrossRef]
- Qian, B.-J.; Liu, J.-H.; Zhao, S.-J.; Cai, J.-X.; Jing, P. The effects of gallic/ferulic/caffeic acids on colour intensification and anthocyanin stability. Food Chem. 2017, 228, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Park, J.E.; Lee, K.-S.; Seo, W.D.; Song, S.-B.; Lee, M.-H.; Kim, S.; Kim, J.-I.; Oh, E.; Pae, S.-B.; et al. Identification of anthocyanin compositions in black seed coated korean adzuki bean (Vigna angularis) by nmr and uplc-q-orbitrap-ms/ms and screening for their antioxidant properties using different solvent systems. Food Chem. 2021, 346, 128882. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; Pascual-Teresa, S.d.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; de Freitas, V. Structural diversity of anthocyanin-derived pigments in port wines. Food Chem. 2002, 76, 335–342. [Google Scholar] [CrossRef]
- Fei, P.; Zeng, F.; Zheng, S.; Chen, Q.; Hu, Y.; Cai, J. Acylation of blueberry anthocyanins with maleic acid: Improvement of the stability and its application potential in intelligent color indicator packing materials. Dye. Pigment. 2021, 184, 108852. [Google Scholar] [CrossRef]
- Ahmad, M.; Ashraf, B.; Gani, A.; Gani, A. Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion. Int. J. Biol. Macromol. 2018, 109, 435–442. [Google Scholar]
- Fan, L.; Wang, Y.; Xie, P.; Zhang, L.; Li, Y.; Zhou, J. Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation. Food Chem. 2019, 275, 299–308. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Hładyszowski, J.; Mitek, M. Stabilization of anthocyanin and skullcap flavone complexes—Investigations with computer simulation and experimental methods. Food Chem. 2013, 138, 491–500. [Google Scholar] [CrossRef]
- Xu, X.-J.; Fang, S.; Li, Y.-H.; Zhang, F.; Shao, Z.-P.; Zeng, Y.-T.; Chen, J.; Meng, Y.-C. Effects of low acyl and high acyl gellan gum on the thermal stability of purple sweet potato anthocyanins in the presence of ascorbic acid. Food Hydrocoll. 2019, 86, 116–123. [Google Scholar] [CrossRef]

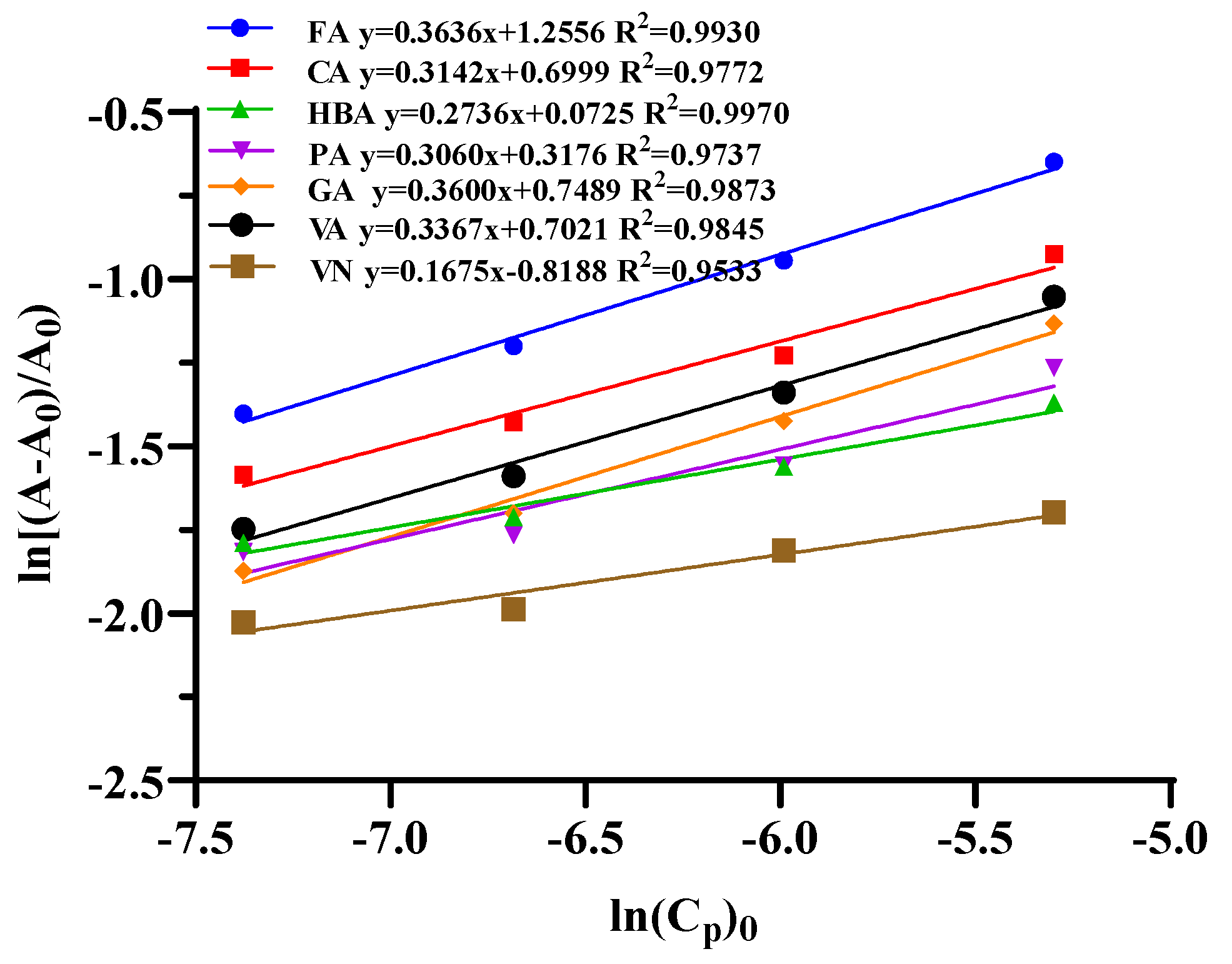



| Co-Pigments | Equation | n | K | ΔG° (KJ/mol) |
|---|---|---|---|---|
| FA | y = 0.3636x + 1.2556 R2 = 0.9930 | 0.36 | 3.51 | −3.06 |
| CA | y = 0.3142x + 0.6999 R2 = 0.9772 | 0.31 | 2.01 | −1.71 |
| HBA | y = 0.2736x + 0.0725 R2 = 0.9970 | 0.27 | 1.08 | −0.18 |
| PA | y = 0.3060x + 0.3176 R2 = 0.9737 | 0.31 | 1.37 | −0.77 |
| GA | y = 0.3600x + 0.7489 R2 = 0.9873 | 0.36 | 2.11 | −1.83 |
| VA | y = 0.3367x + 0.7021 R2 = 0.9845 | 0.34 | 2.02 | −1.71 |
| VN | y = 0.1675x − 0.8188 R2 = 0.9533 | 0.17 | 0.44 | 2.00 |
| Co-pigments | T (℃) | k × 103 (min−1) | T1/2 (h) |
|---|---|---|---|
| Control | 70 °C | 0.66 (0.9749) | 17.5 |
| 80 °C | 0.95 (0.9389) | 12.18 | |
| 90 °C | 1.74 (0.9847) | 6.66 | |
| FA | 70 °C | 0.47 (0.9986) | 24.62 |
| 80 °C | 0.82 (0.9330) | 14.13 | |
| 90 °C | 1.22 (0.9527) | 9.46 | |
| CA | 70 °C | 0.37 (0.9408) | 31.28 |
| 80 °C | 0.71 (0.9598) | 16.21 | |
| 90 °C | 1.05 (0.9547) | 10.98 | |
| HBA | 70 °C | 0.56 (0.9801) | 20.48 |
| 80 °C | 0.91 (0.9884) | 12.63 | |
| 90 °C | 1.38 (0.9981) | 8.35 | |
| PA | 70 °C | 0.60 (0.9829) | 19.27 |
| 80 °C | 0.80 (0.9793) | 14.5 | |
| 90 °C | 1.44 (0.9971) | 8.00 | |
| GA | 70 °C | 0.58 (0.9829) | 19.77 |
| 80 °C | 0.71 (0.9072) | 16.19 | |
| 90 °C | 1.45 (0.9950) | 7.98 | |
| VA | 70 °C | 0.46 (0.9847) | 24.98 |
| 80 °C | 0.77 (0.9824) | 14.99 | |
| 90 °C | 1.45 (0.9902) | 7.95 | |
| VN | 70 °C | 0.47 (0.9596) | 24.55 |
| 80 °C | 0.80 (0.9564) | 14.42 | |
| 90 °C | 1.64 (0.9838) | 7.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Gao, Q.; Liao, S.; Zou, Y.; Yan, J.; Li, Q. Co-Pigmentation Mechanism and Thermal Reaction Kinetics of Mulberry Anthocyanins with Different Phenolic Acids. Foods 2022, 11, 3806. https://doi.org/10.3390/foods11233806
Chen X, Gao Q, Liao S, Zou Y, Yan J, Li Q. Co-Pigmentation Mechanism and Thermal Reaction Kinetics of Mulberry Anthocyanins with Different Phenolic Acids. Foods. 2022; 11(23):3806. https://doi.org/10.3390/foods11233806
Chicago/Turabian StyleChen, Xiangyue, Qunyu Gao, Sentai Liao, Yuxiao Zou, Jiangang Yan, and Qian Li. 2022. "Co-Pigmentation Mechanism and Thermal Reaction Kinetics of Mulberry Anthocyanins with Different Phenolic Acids" Foods 11, no. 23: 3806. https://doi.org/10.3390/foods11233806
APA StyleChen, X., Gao, Q., Liao, S., Zou, Y., Yan, J., & Li, Q. (2022). Co-Pigmentation Mechanism and Thermal Reaction Kinetics of Mulberry Anthocyanins with Different Phenolic Acids. Foods, 11(23), 3806. https://doi.org/10.3390/foods11233806