Assessment of the Effect of Cold Atmospheric Plasma (CAP) on the Hairtail (Trichiurus lepturus) Quality under Cold Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection and Preparation
2.3. Plasma Treatment
2.4. Quality Analysis
2.4.1. Determination of TBC
2.4.2. Determination of TVB-N and pH
2.4.3. Determination of TBARS
2.4.4. Color Analysis
2.4.5. Texture Profile Analysis (TPA)
2.4.6. Sensory Evaluation
2.5. Statistical Analyses
3. Results and Discussion
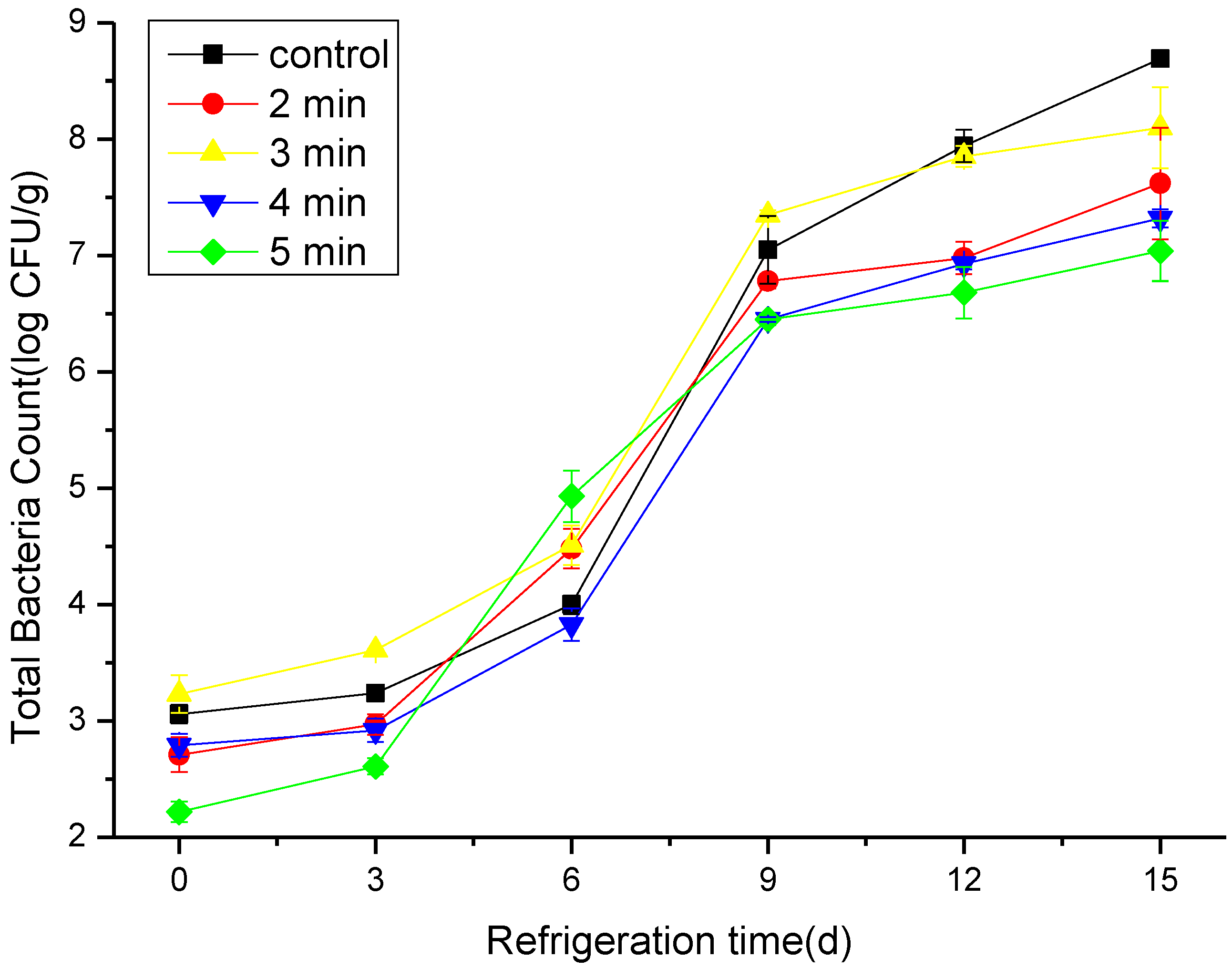
3.1. The Effect of CAP on the TBC
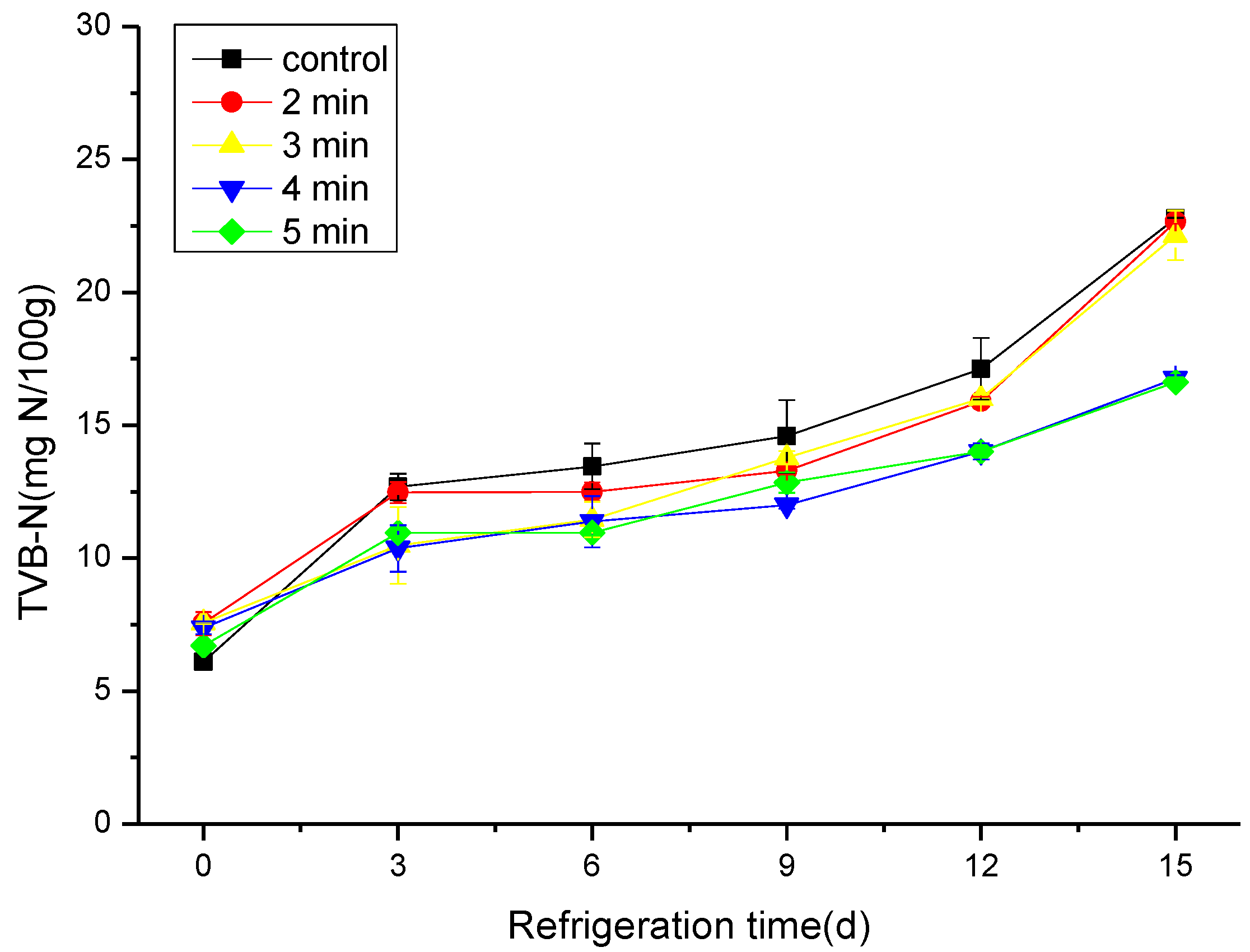
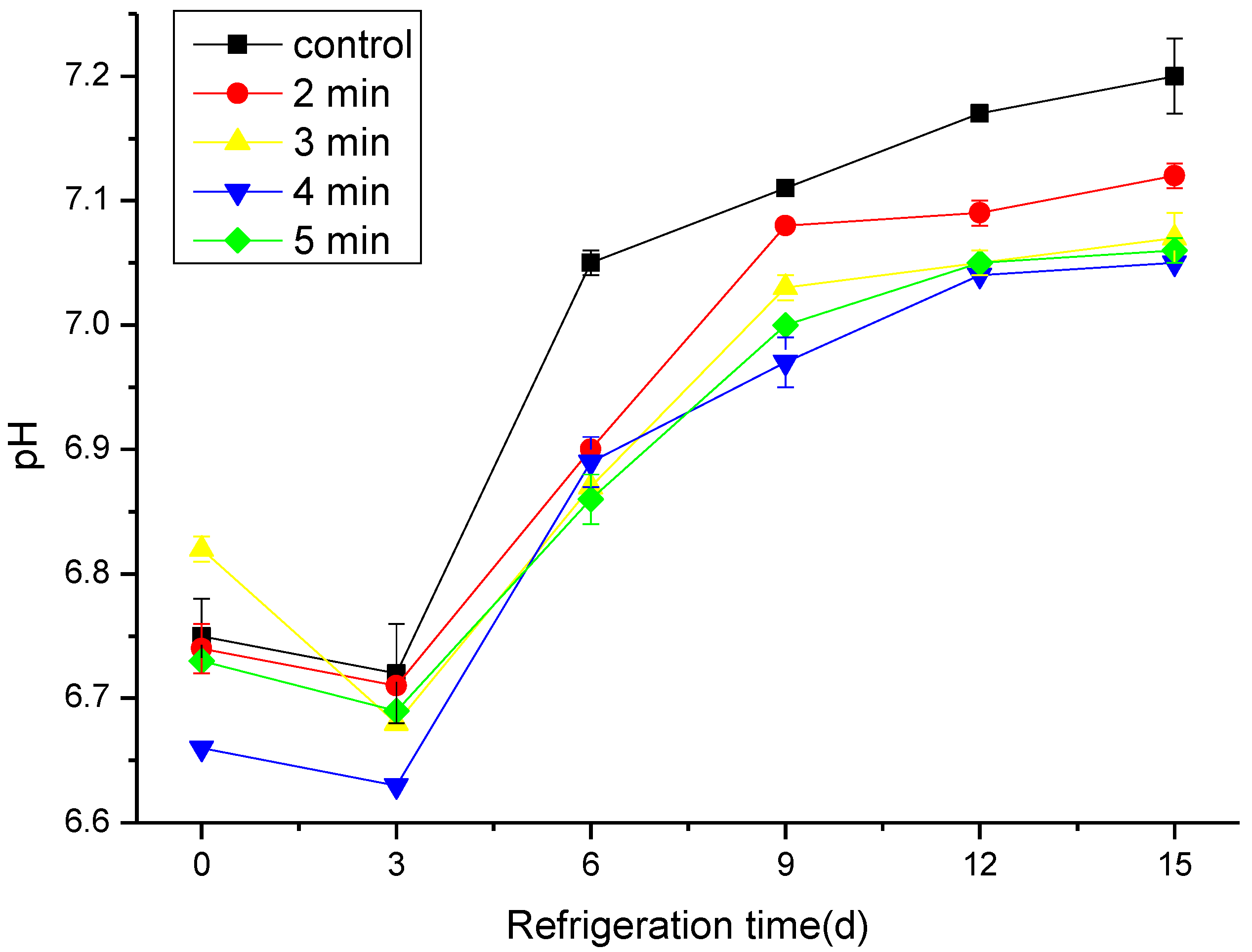
3.2. The Effect of CAP on the TVB-N and pH
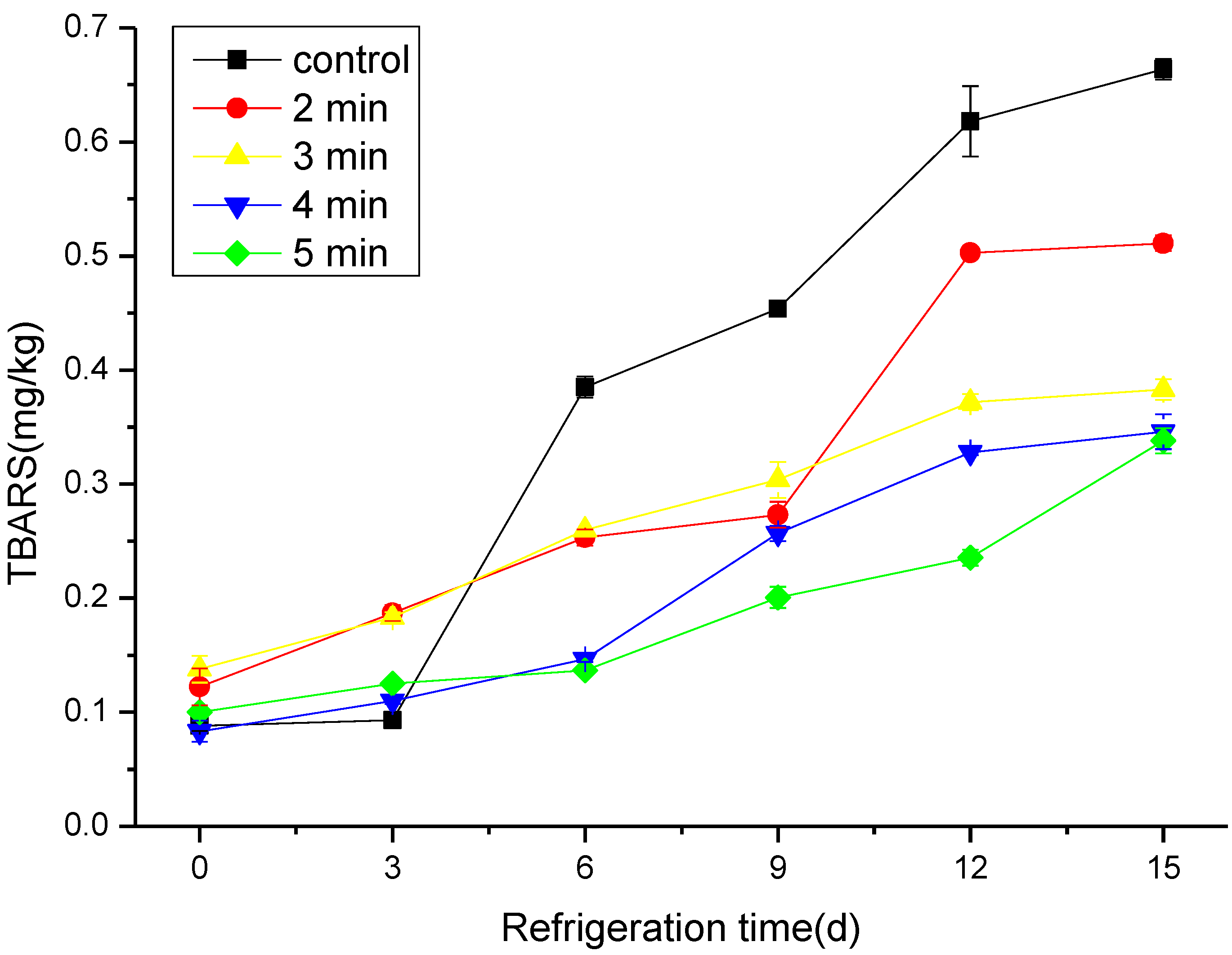
3.3. The Effect of CAP on the TBARS
3.4. The Effect of CAP on the Color Difference
3.5. The Effect of CAP on the Texture
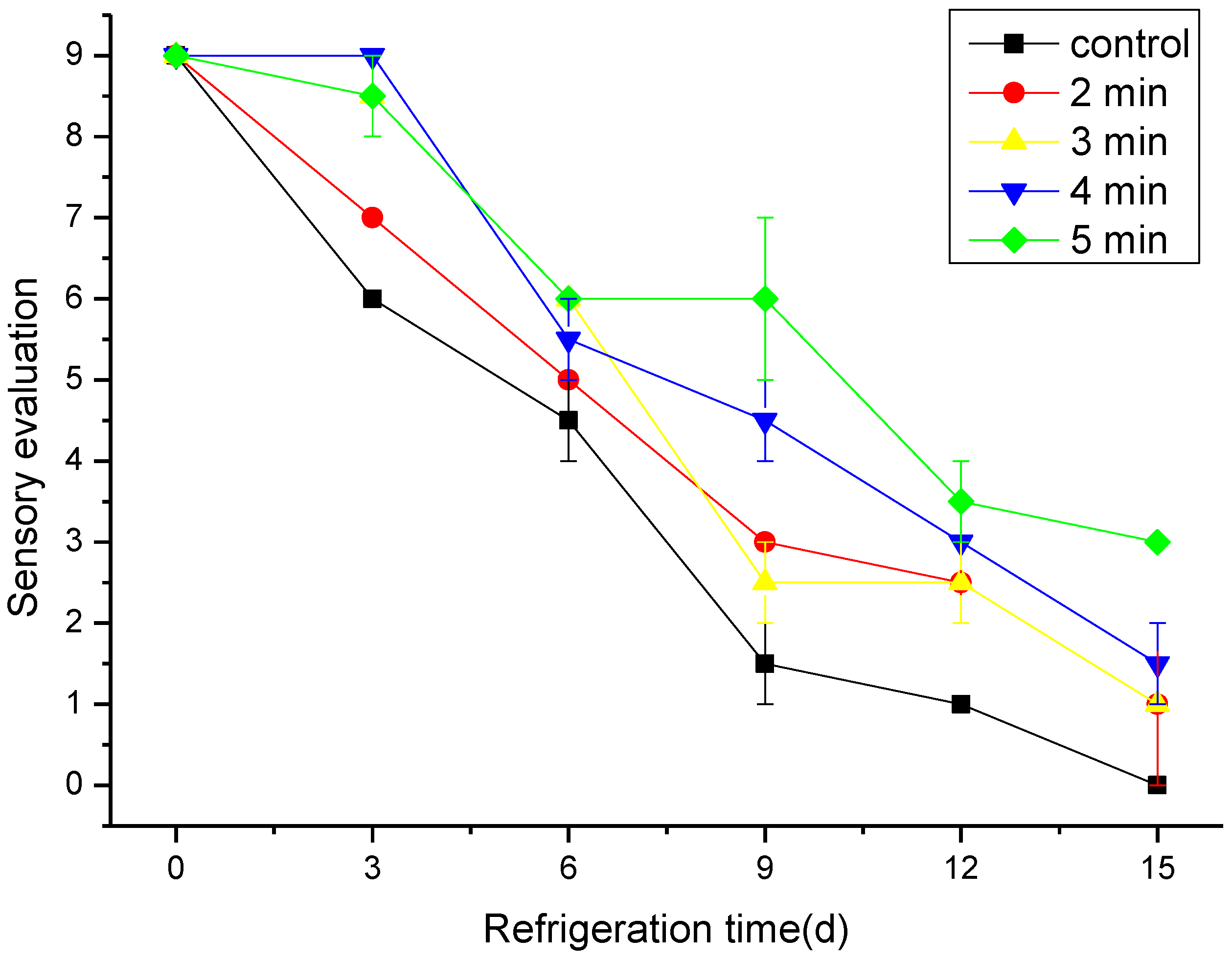
3.6. The Effect of CAP on the Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kwok, K.; Ni, I. Age and growth of cutlassfi shes, Trichiurus spp., from the South China Sea. Fish. Bull. 2000, 98, 748–758. [Google Scholar]
- Li, Y.; Yu, H.X.; Cai, Y.; Yuan, C.H.; Chen, S.G.; Ding, T.; Liu, D.H.; Hu, Y.Q. Ferulic acid-β-cyclodextrin inclusion complexes: Application on the preservation of hairtail (Trichiurus lepturus). Int. J. Food Prop. 2020, 23, 282–296. [Google Scholar] [CrossRef]
- Melika, M.; Salma, B.; Nejla, B.M.; Mokthar, K.; Ahmed, S.; Moncef, C.; Hassouna, M. Electron-beam irradiation effect on the microbiological, physicochemical, and sensory parameters of refrigerated raspberries. J. Food Process. Preserv. 2021, 45, e15828. [Google Scholar]
- Yıkmış, S. New Approaches in Non-thermal Processes in the Food Industry. Int. J. Nutr. Food Sci. 2016, 5, 344–351. [Google Scholar] [CrossRef][Green Version]
- Jennifer, R.; Fiona, L.; Swapan, K.B. Emerging Seafood Preservation Techniques to Extend Freshness and Minimize Vibrio Contamination. Front. Microbiol. 2016, 7, 350. [Google Scholar]
- Damyeh, M.S.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef]
- Tang, L.L.; Hatab, S.; Yan, J.H.; Miao, W.H.; Nyaisaba, B.M.; Piao, X.Y.; Zheng, B.; Deng, S.G. Changes in Biochemical Properties and Activity of Trypsin-like Protease (Litopenaeus vannamei) Treated by Atmospheric Cold Plasma (ACP). Foods 2022, 11, 1277. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.Z.; Chen, J.Y.; Chen, D.Z.; Deng, S.G.; Xu, B. Effect of cold plasma on maintaining the quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. J. Sci. Food Agric. 2018, 99, 39–46. [Google Scholar] [CrossRef]
- Miao, W.H.; Nyaisaba, B.M.; Koddy, J.K.; Chen, M.L.; Hatab, S.; Deng, S.G. Effect of cold atmospheric plasma on the physicochemical and functional properties of myofibrillar protein from Alaska pollock (Theragra chalcogramma). Int. J. Food Sci. Technol. 2020, 55, 517–525. [Google Scholar] [CrossRef]
- Piotr, K.; Carlos, A.; Patrick, J.C.; Ramon, A.R.; Anne, M.M.; Brijesh, T. The effect of non-thermal plasma on the lipid oxidation and microbiological quality of sushi. Innov. Food Sci. Emerg. Technol. 2018, 45, 412–417. [Google Scholar]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Thompson, A. The inactivation of Salmonella by cold atmospheric plasma treatment. Food Res. Int. 2012, 45, 678–684. [Google Scholar] [CrossRef]
- Koddy, J.K.; Miao, W.H.; Hatab, S.; Tang, L.L.; Xu, H.Q.; Nyaisaba, B.M.; Chen, M.L.; Deng, S.G. Understanding the role of atmospheric cold plasma (ACP) in maintaining the quality of hairtail (Trichiurus Lepturus). Food Chem. 2021, 343, 128418. [Google Scholar] [CrossRef] [PubMed]
- Smet, C.; Noriega, E.; Rosier, F.; Walsh, J.L.; Valdramidis, V.P.; Impe, J.F. Impact of food model (micro)structure on the microbial inactivation efficacy of cold atmospheric plasma. Int. J. Food Microbiol. 2017, 240, 47–56. [Google Scholar] [CrossRef]
- Athanasios, L.; Namrata, P.; Graziele, G.B.; Antje Fröhling Valdramidis, V.P.; Taoukis, P.S.; Oliver, S. Effect of cold atmospheric pressure plasma processing on quality and shelf life of red currants. LWT 2021, 151, 112213. [Google Scholar]
- Liao, X.Y.; Liu, D.H.; Xiang, Q.S.; Ahn, J.; Chen, S.G.; Ye, X.Q.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Wang, X.; Shan, J.J.; Han, S.Q.; Zhao, J.B.; Zhang, Y.T. Optimization of Fish Quality by Evaluation of Total Volatile Basic Nitrogen (TVB-N) and Texture Profile Analysis (TPA) by Near-Infrared (NIR) Hyperspectral Imaging. Anal. Lett. 2019, 52, 1845–1859. [Google Scholar] [CrossRef]
- Hatab, S.; Lin, K.H.; Miao, W.H.; Chen, M.L.; Lin, J.H.; Deng, S.G. Potential Utilization of Green Tea Leaves and Fenugreek Seeds Extracts as Natural Preservatives for Pacific White Shrimp During Refrigerated Storage. Foodborne Pathog. Dis. 2018, 15, 498–505. [Google Scholar] [CrossRef]
- Zheng, R.H.; Xu, X.R.; Xing, J.L.; Cheng, H.; Zhang, S.F.; Shen, J.; Li, H.S. Quality Evaluation and Characterization of Specific Spoilage Organisms of Spanish Mackerel by High-Throughput Sequencing during 0 °C Cold Chain Logistics. Foods 2020, 9, 312. [Google Scholar] [CrossRef]
- Okutan, G.; Boran, G. Effect of gelatin based edible coatings on quality of surimi from pearl mullet (Alburnus tarichi, Güldenstdt, 1814) during cold storage. Ciênc. Tecnol. Aliment. 2020, 42, e34520. [Google Scholar] [CrossRef]
- Maggie, A. Review of Textural Characteristics of World Foods. J. Agric. Food Inf. 2021, 22, 142. [Google Scholar]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO Fisheries Technical Paper No. 348; FAO: Rome, Italy, 1995. [Google Scholar]
- Cao, R.; Lin, R.H.; Sun, H.H.; Liu, Q. Microbiota and Shelf Life of Whole and Gutted Pacific Saury (Cololabis saira) during Refrigerated Storage. J. Ocean Univ. China 2020, 19, 473–478. [Google Scholar] [CrossRef]
- Albertos, I.; Martín-Diana, A.; Cullen, P.; Tiwari, B.; Ojha, S.; Bourke, P.; Álvarez, C.; Rico, D. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Choi, E.H.; Uhm, H.S.; Kaushik, N.K. Plasma bioscience and its application to medicine. AAPPS Bull. 2021, 31, 10. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Li, C.Z.; Cui, H.Y.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Yadav, D.; Kumar, S.; Choi, E.; Kim, M. Electric-field-induced electroporation and permeation of reactive oxygen species across a skin membrane. J. Biomol. Struct. Dyn. 2021, 39, 1343–1353. [Google Scholar] [CrossRef]
- Luan, L.L.; Fu, S.L.; Yuan, C.H.; Ishimura, G.; Chen, S.G.; Chen, J.C.; Hu, Y.Q. Combined effect of superchilling and tea polyphenols on the preservation quality of hairtail (Trichiurus haumela). Int. J. Food Prop. 2017, 20, S992–S1001. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, W.J.; Long, Y.; Peng, Y.K.; Li, Y.Y.; Chao, K.L.; Tang, X.Y. A feasibility study of rapid nondestructive detection of total volatile basic nitrogen (TVB-N) content in beef based on airflow and laser ranging technique. Meat Sci. 2018, 145, 367–374. [Google Scholar] [CrossRef]
- Du, L.H.; Fan, X.R.; Liu, F.; Zhou, Q.; Yuan, J.; Ju, X.R. Changes of dominant spoilage bacteria and biogenic amines of Taihu White Prawn (Exopalaemon modestus) during ice storage. J. Food Prot. 2017, 80, 2099–2104. [Google Scholar] [CrossRef]
- Szymczak, M.; Kołakowski, E. Total Volatile Basic Nitrogen in Meat and Brine during Marinating of Herring. J. Aquat. Food Prod. Technol. 2016, 25, 373–387. [Google Scholar] [CrossRef]
- Liu, H.; Ji, Z.T.; Liu, X.L.; Shi, C.; Yang, X.T. Non-destructive determination of chemical and microbial spoilage indicators of beef for freshness evaluation using front-face synchronous fluorescence spectroscopy. Food Chem. 2020, 321, 126628. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.W.; Nicholas, P.; Mark, J.R. The effects of pre-harvest stress and harvest method on the stress response, rigor onset, muscle pH and drip loss in barramundi (Lates calcarifer). Aquaculture 2008, 282, 26–32. [Google Scholar]
- Zhu, X.S.; Ruusunen, M.; Gusella, M.; Zhou, G.H.; Puolanne, E. High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles. Meat Sci. 2011, 89, 181–188. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Wang, H.; Xiao, S.; Wang, J.H.; Feng, L. Sensitivity enhancement of pH indicator and its application in the evaluation of fish freshness. Talanta 2015, 143, 127–131. [Google Scholar] [CrossRef]
- Hatab, S.; Koddy, J.K.; Miao, W.H.; Tang, L.L.; Xu, H.Q.; Deng, S.G.; Zheng, B. Atmospheric Cold Plasma: A New Approach to Modify Protein and Lipid Properties of Myofibrillar Protein Isolate from Hairtail (Trichiurus lepturus) Fish. J. Sci. Food Agric. 2021, 102, 2041–2049. [Google Scholar] [CrossRef]
- Danielly, G.; Daína, L.; Danilo, G.H.D.S.; Eduardo Alves, D.A. Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: Evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water. Ecotoxicol. Environ. Saf. 2020, 190, 110107. [Google Scholar]
- Bashir, S.; Arshad, M.S.; Khalid, W.; Nayik, G.A.; Al, O.S.; Ansari, M.J.; Moreno, A.; Karabagias, I.K. Effect of Antimicrobial and Antioxidant Rich Pomegranate Peel Based Edible Coatings on Quality and Functional Properties of Chicken Nuggets. Molecules 2022, 27, 4500. [Google Scholar] [CrossRef]
- Luan, L.L.; Sun, Y.S.; Chen, S.G.; Wu, C.H.; Hu, Y.Q. A study of fractal dimension as a quality indicator of hairtail (Trichiurus haumela) samples during frozen storage. Sci. Rep. 2018, 8, 16468. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2006, 103, 891–899. [Google Scholar] [CrossRef]
- Anna, L.K.; Annika, B.; Sylvia, B.; Li, Y.; Corinna, K.; Louise, Z.J.; Günter, K.; Birte, A. Inactivation of Salmonella Typhimurium and Listeria monocytogenes on ham with nonthermal atmospheric pressure plasma. PLoS ONE 2018, 13, e0197773. [Google Scholar]
- Mohamed, E.E.; Younis, E.R.; Mohamed, E.A. Impact of atmospheric cold plasma (ACP) on maintaining bolti fish (Tilapia nilotica) freshness and quality criteria during cold storing. J. Food Process. Preserv. 2021, 45, e15442. [Google Scholar] [CrossRef]
- Han, Y.X.; Cheng, J.H.; Sun, D.W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- Hematyar, N.; Masilko, J.; Mraz, J.; Sampels, S. Nutritional quality, oxidation, and sensory parameters in fillets of common carp (Cyprinus carpio L.) influenced by frozen storage (−20 °C). J. Food Process. Preserv. 2018, 42, e13589. [Google Scholar] [CrossRef]
- Mekala, M. Assessment of Sensory Qualities of Millet-Based Idlies. Int. J. Farm Sci. 2022, 12, 35–36. [Google Scholar] [CrossRef]
- Zhuang, H.; Rothrock, M.J.; Line, J.E.; Lawrence, K.C.; Gamble, G.R.; Bowker, B.C.; Keener, K.M. Optimization of in-package cold plasma treatment conditions for raw chicken breast meat with response surface methodology. Innov. Food Sci. Emerg. Technol. 2020, 66, 102477. [Google Scholar] [CrossRef]

| Best (3) | Good (2) | Poor (1) | Worst (0) | |
|---|---|---|---|---|
| Odor | Inherent smell | Slight smell | Strong smell | Rancid smell |
| Color | Normally shiny | Dull shiny | Not lustrous | Discoloration |
| Texture | Normal, elastic | Slightly worse, elastic | Poor, less elastic | Worse, nonelastic |
| Refrigeration Time (d) | Treatment Time (min) | ||||
|---|---|---|---|---|---|
| Control Group | 2 | 3 | 4 | 5 | |
| 3 | 3.22 ± 0.10 Ba | 2.57 ± 0.37 BCa | 1.47 ± 0.30 Cb | 1.20 ± 0.19 Cbc | 0.50 ± 0.07 Cc |
| 6 | 3.84 ± 0.38 Ba | 2.33 ± 0.22 Cb | 1.82 ± 0.24 BCb | 1.73 ± 0.47 BCbc | 0.72 ± 0.25 Cc |
| 9 | 5.50 ± 0.17 Aa | 3.40 ± 0.30 Bb | 2.56 ± 0.24 Bbc | 2.08 ± 0.34 ABCcd | 1.19 ± 0.37 BCd |
| 12 | 6.32 ± 0.17 Aa | 5.29 ± 0.14 Aa | 3.89 ± 0.37 Ab | 2.72 ± 0.48 ABbc | 2.05 ± 0.56 ABc |
| 15 | 6.34 ± 0.66 Aa | 5.48 ± 0.31 Aa | 4.18 ± 0.17 Abc | 3.28 ± 0.43 Abc | 2.46 ± 0.30 Ac |
| Refrigeration Time (d) | Treatment Time (min) | ||||
|---|---|---|---|---|---|
| Control Group | 2 | Control Group | 4 | Control Group | |
| 0 | 88.95 ± 0.09 Ab | 88.95 ± 0.23 Ab | 88.86 ± 0.19 Ab | 90.61 ± 0.32 Aa | 89.54 ± 0.60 Ab |
| 3 | 86.52 ± 0.03 Bc | 86.41 ± 0.22 Bc | 88.17 ± 0.38 Ab | 89.68 ± 0.20 ABa | 89.11 ± 0.64 Aab |
| 6 | 85.30 ± 0.38 Cc | 86.74 ± 0.19 Bb | 87.05 ± 0.06 Bb | 89.02 ± 0.27 BCa | 89.34 ± 0.23 Aa |
| 9 | 83.46 ± 0.19 Dc | 85.67 ± 0.15 Cb | 86.53 ± 0.32 Bb | 88.69 ± 0.54 BCa | 89.38 ± 0.30 Aa |
| 12 | 82.71 ± 0.16 Dc | 83.71 ± 0.22 Dc | 85.51 ± 0.25 Cb | 87.96 ± 0.51 CDa | 88.44 ± 0.67 ABa |
| 15 | 82.62 ± 0.58 Dc | 83.47 ± 0.20 Dc | 84.78 ± 0.03 Cb | 87.43 ± 0.20 Da | 87.27 ± 0.58 Ba |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Miao, W.; Zheng, B.; Deng, S.; Hatab, S. Assessment of the Effect of Cold Atmospheric Plasma (CAP) on the Hairtail (Trichiurus lepturus) Quality under Cold Storage Conditions. Foods 2022, 11, 3683. https://doi.org/10.3390/foods11223683
Xu H, Miao W, Zheng B, Deng S, Hatab S. Assessment of the Effect of Cold Atmospheric Plasma (CAP) on the Hairtail (Trichiurus lepturus) Quality under Cold Storage Conditions. Foods. 2022; 11(22):3683. https://doi.org/10.3390/foods11223683
Chicago/Turabian StyleXu, Huiqian, Wenhua Miao, Bin Zheng, Shanggui Deng, and Shaimaa Hatab. 2022. "Assessment of the Effect of Cold Atmospheric Plasma (CAP) on the Hairtail (Trichiurus lepturus) Quality under Cold Storage Conditions" Foods 11, no. 22: 3683. https://doi.org/10.3390/foods11223683
APA StyleXu, H., Miao, W., Zheng, B., Deng, S., & Hatab, S. (2022). Assessment of the Effect of Cold Atmospheric Plasma (CAP) on the Hairtail (Trichiurus lepturus) Quality under Cold Storage Conditions. Foods, 11(22), 3683. https://doi.org/10.3390/foods11223683





