Structural Characterization and Biological Activity of Polysaccharides from Stems of Houttuynia cordata
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction, Isolation, and Purification of Polysaccharides
2.2.1. Extraction of HCPS
2.2.2. Multiple Stepwise Regression Analysis of Extraction Factors
2.2.3. Pearson Correlation Analysis of Extraction Factors
2.2.4. Isolation and Purification of HCPS
2.3. Homogeneity and Molecular Weight Determination
2.4. Monosaccharide Identification
2.5. FT-IR and NMR Analyses
2.6. Periodate Oxidation
2.7. Methylation Analysis
2.8. Congo-Red Test
2.9. Scanning Electron Microscopy (SEM)
2.10. In Vitro Anti-Oxidant Activity
2.10.1. The Scavenging Efficiency of DPPH •
2.10.2. The Scavenging Efficiency of •OH
2.10.3. The Scavenging Efficiency of ABTS+
2.11. In Vitro α-Amylase Inhibitory Activity Assay
2.12. In Vitro α-Glucosidase Inhibitory Activity Assay
2.13. Statistical Analysis
3. Results
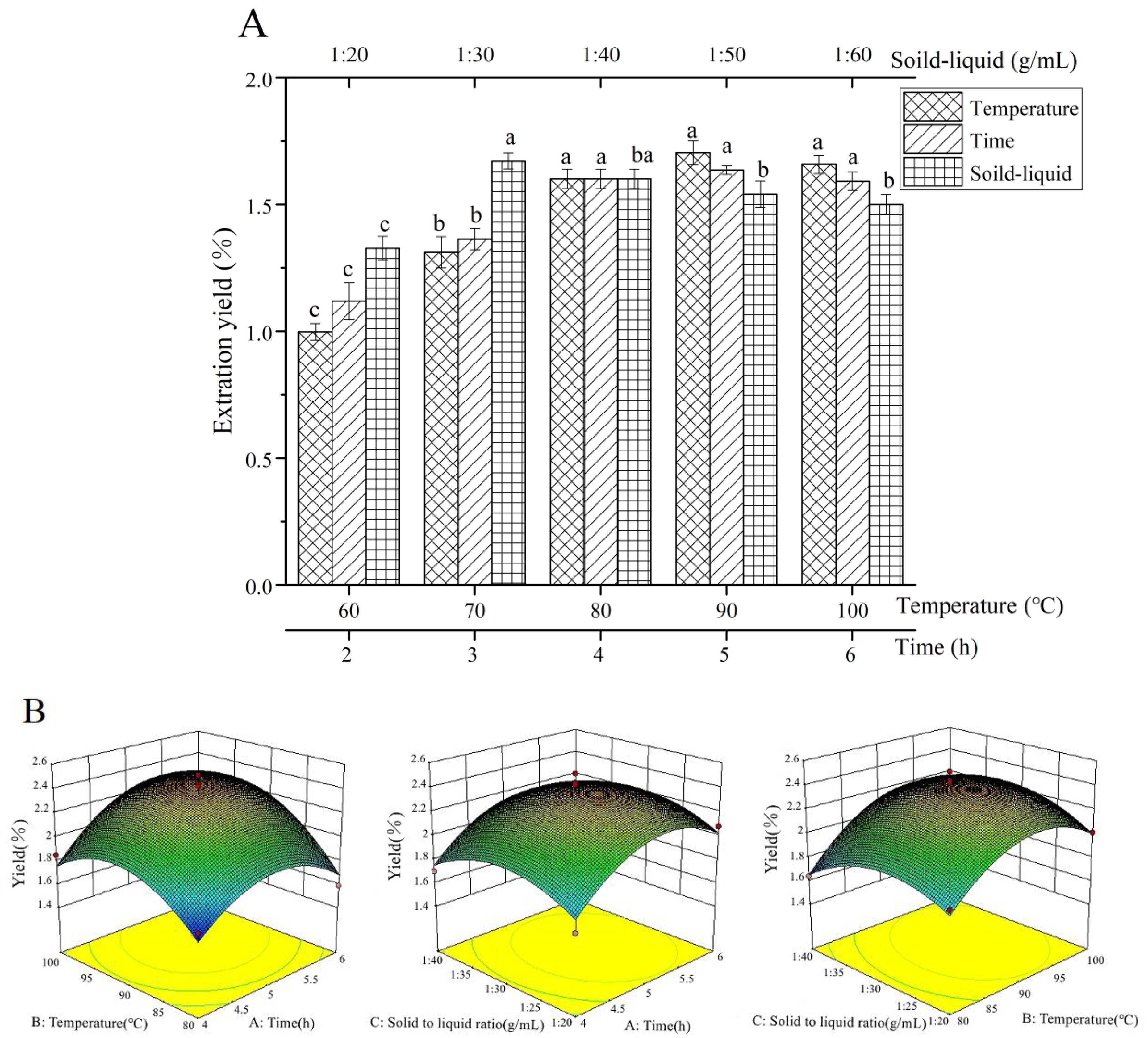
3.1. Polysaccharide Extraction
3.1.1. Single-Factor Experiment
3.1.2. RSM Experiment
3.1.3. Multiple Stepwise Regression Analysis
3.1.4. Pearson Correlation Analysis
3.2. Isolation and Purification of Polysaccharides
3.3. Homogeneity and Molecular Weight Determination of HCPS
3.4. Structure Characterization
3.4.1. Monosaccharide Composition of HCPS
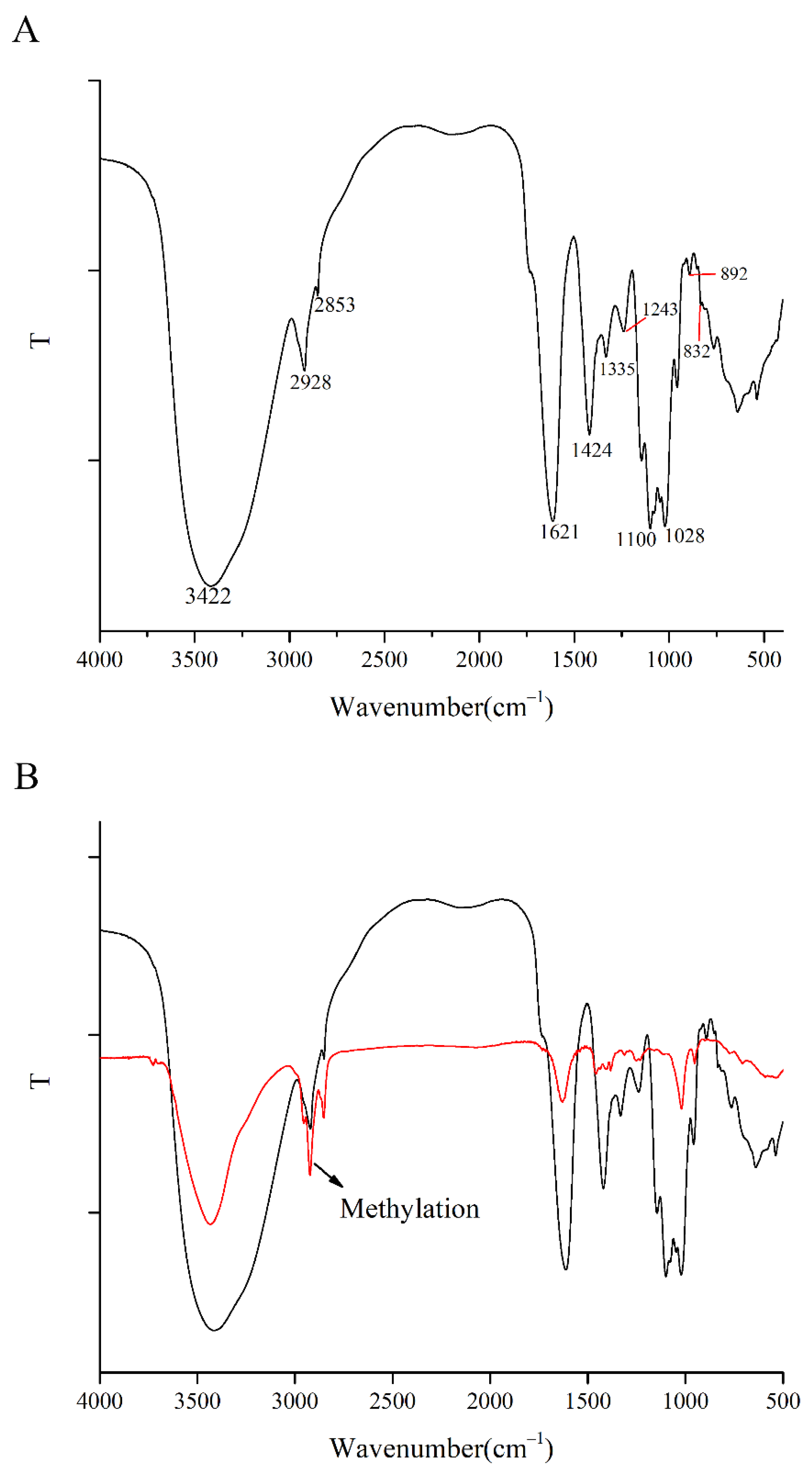
3.4.2. FT-IR Spectrometric Analysis
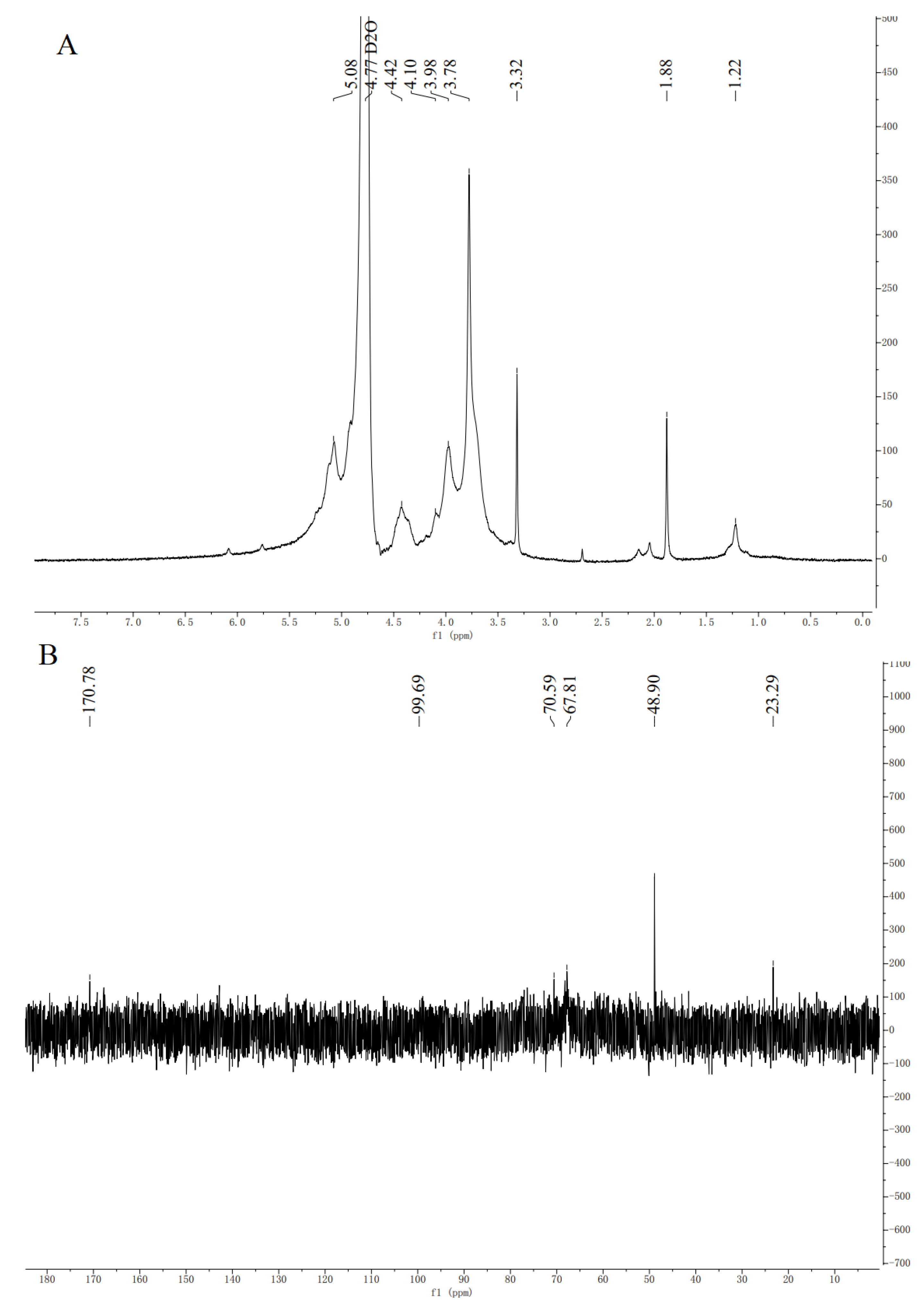
3.4.3. NMR Analysis
3.4.4. Periodate Oxidation
3.4.5. Methylation Analysis
3.4.6. Congo-Red Test
3.4.7. Scanning Electron Microscopy (SEM) Analysis
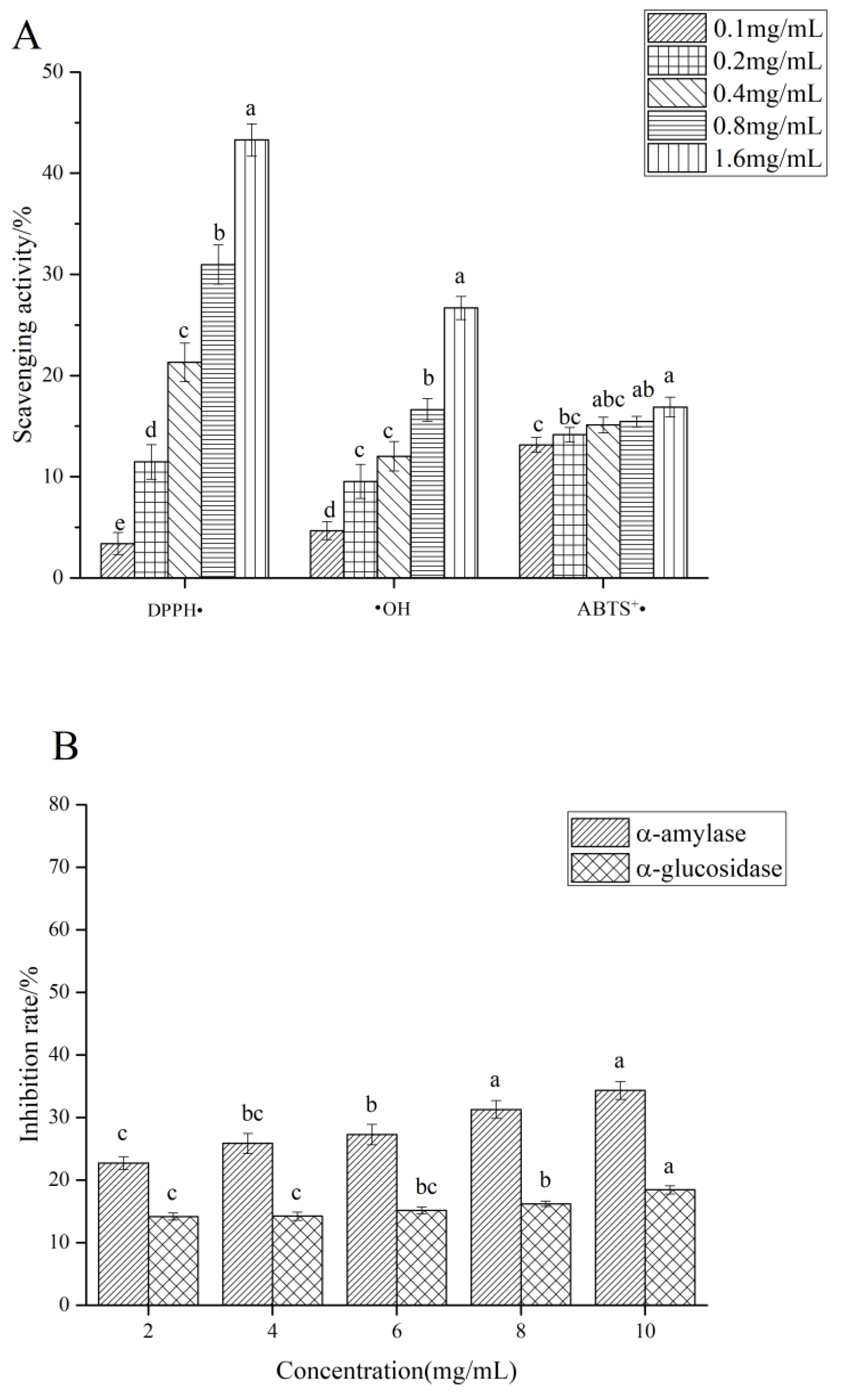
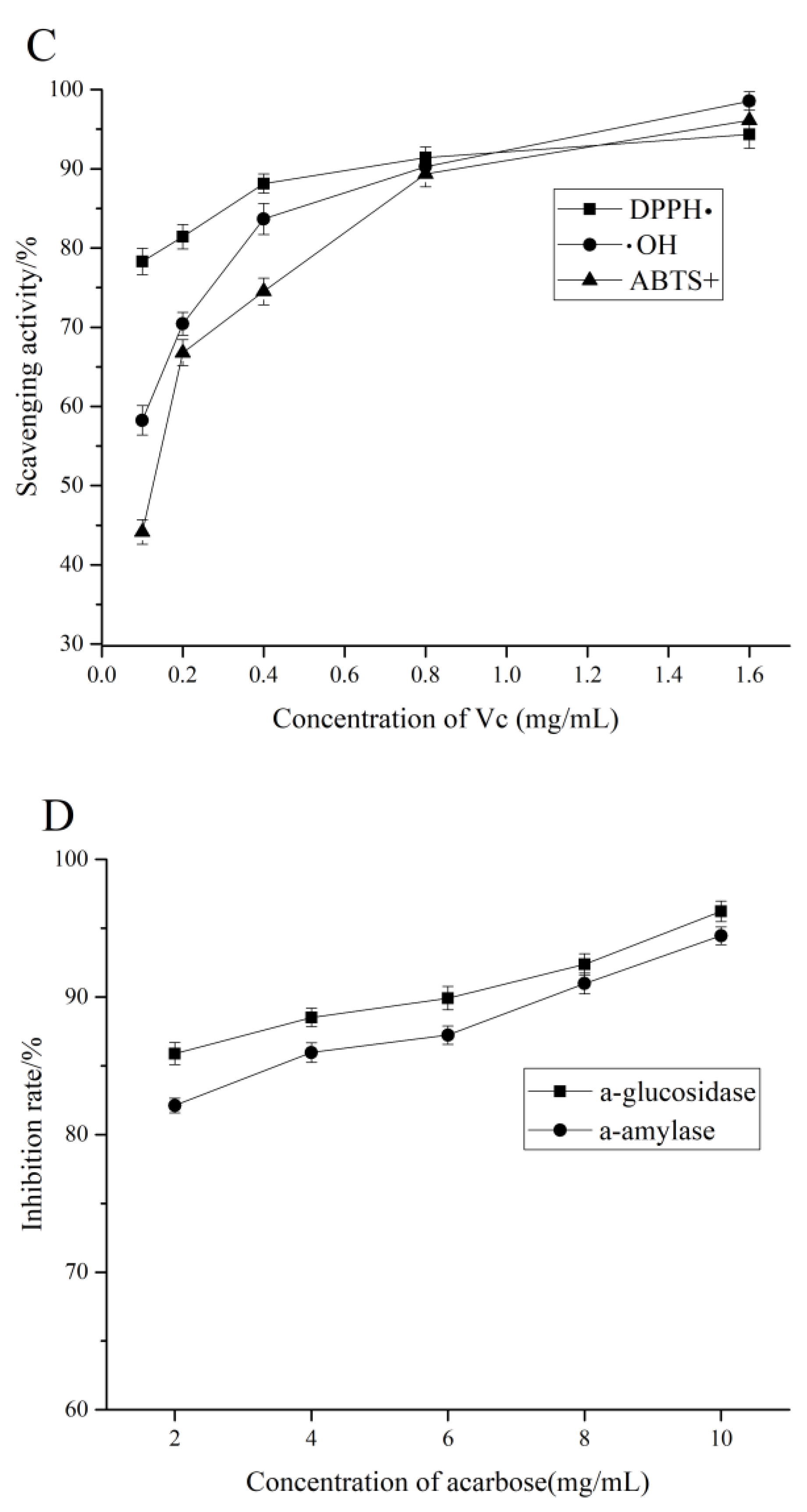
3.5. Anti-Oxidant Activity In Vitro
3.6. α-Amylase and α-Glucosidase Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ezuruike, U.F.; Prieto, J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Kant, R.; Kumar, V. alpha-D-Glucosidase inhibitory activity of polysaccharide isolated from Acacia tortilis gum exudate. Int. J. Biol. Macromol. 2013, 59, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Karayannakidis, P.D.; Kwon, Y.-I.; Lee, C.M.; Seeram, N.P. Seasonal Variation of Phenolic Antioxidant-mediated alpha-glucosidase Inhibition of Ascophyllum nodosum. Plant Foods Hum. Nutr. 2011, 66, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ding, H.; Hu, X.; Zhang, G.; Gong, D. Galangin inhibits alpha-glucosidase activity and formation of non-enzymatic glycation products. Food Chem. 2019, 271, 70–79. [Google Scholar] [CrossRef] [PubMed]
- He, X.-F.; Zhang, X.-K.; Geng, C.-A.; Hu, J.; Zhang, X.-M.; Guo, Y.-Q.; Chen, J.-J. Tsaokopyranols A-M, 2,6-epoxydiarylheptanoids from Amomum tsao-ko and their alpha-glucosidase inhibitory activity. Bioorg. Chem. 2020, 96, 103638. [Google Scholar] [CrossRef]
- Xu, X.; Shan, B.; Liao, C.-H.; Xie, J.-H.; Wen, P.-W.; Shi, J.-Y. Anti-diabetic properties of Momordica charantia L. polysaccharide in alloxan-induced diabetic mice. Int. J. Biol. Macromol. 2015, 81, 538–543. [Google Scholar] [CrossRef]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. alpha-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 2001, 49, 1948–1951. [Google Scholar] [CrossRef]
- Hameed, S.; Kanwal; Seraj, F.; Rafique, R.; Chigurupati, S.; Wadood, A.; Rehman, A.U.; Venugopal, V.; Salar, U.; Taha, M.; et al. Synthesis of benzotriazoles derivatives and their dual potential as alpha-amylase and alpha-glucosidase inhibitors in vitro: Structure-activity relationship, molecular docking, and kinetic studies. Eur. J. Med. Chem. 2019, 183, 111677. [Google Scholar]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef]
- Fu, J.; Fu, J.; Liu, Y.; Li, R.; Gao, B.; Zhang, N.; Wang, B.; Cao, Y.; Guo, K.; Tu, Y. Modulatory effects of one polysaccharide from Acanthopanax senticosus in alloxan-induced diabetic mice. Carbohydr. Polym. 2012, 87, 2327–2331. [Google Scholar] [CrossRef]
- Liao, X.; Yang, L.; Chen, M.; Yu, J.; Zhang, S.; Ju, Y. The hypoglycemic effect of a polysaccharide (GLP) from Gracilaria lemaneiformis and its degradation products in diabetic mice. Food Funct. 2015, 6, 2542–2549. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.L.; Laloo, D.; Prasad, S.K.; Hemalatha, S. Aldose reductase inhibitory potential of different fractions of Houttuynia cordata Thunb. J. Acute Dis. 2014, 3, 64–68. [Google Scholar] [CrossRef][Green Version]
- Banjerdpongchai, R.; Kongtawelert, P. Ethanolic Extract of Fermented Houttuynia cordata Thunb Induces Human Leukemic HL-60 and Molt-4 Cell Apoptosis Via Oxidative Stress and a Mitochondrial Pathway. Asian Pac. J. Cancer Prev. 2011, 12, 2871–2874. [Google Scholar] [PubMed]
- Chen, Y.-F.; Yang, J.-S.; Chang, W.-S.; Tsai, S.-C.; Peng, S.-F.; Zhou, Y.-R. Houttuynia cordata Thunb extract modulates G(0)/G(1) arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J. Biomed. Sci. 2013, 20, 18. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, S.K.; Krishnamurthy, S.; Hemalatha, S. Antihyperglycemic Activity of Houttuynia cordata Thunb. in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Sci. 2014, 2014, 809438. [Google Scholar]
- Miyata, M.; Koyama, T.; Yazawa, K. Water Extract of Houttuynia cordata Thunb. Leaves Exerts Anti-Obesity Effects by Inhibiting Fatty Acid and Glycerol Absorption. J. Nutr. Sci. Vitaminol. 2010, 56, 150–156. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, H.-S.; Kim, G.-J.; Jeon, C.Y.; Park, J.H.; Jang, B.-H.; Park, S.-J.; Shin, Y.-C.; Ko, S.-G. Houttuynia cordata Thunb inhibits the production of pro-inflammatory cytokines through inhibition of the NF kappa B signaling pathway in HMC-1 human mast cells. Mol. Med. Rep. 2013, 8, 731–736. [Google Scholar] [CrossRef]
- Han, E.H.; Park, J.H.; Kim, J.Y.; Jeong, H.G. Houttuynia cordata water extract suppresses anaphylactic reaction and IgE-mediated allergic response by inhibiting multiple steps of Fc epsilon RI signaling in mast cells. Food Chem. Toxicol. 2009, 47, 1659–1666. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Y.; Guo, C.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Yang, Z.; Wang, J.; Xu, Y.; Tan, R.-x.; Li, E. Houttuynia cordata blocks HSV infection through inhibition of NF-kappa B activation. Antivir. Res. 2011, 92, 341–345. [Google Scholar] [CrossRef]
- Lau, K.-M.; Lee, K.-M.; Koon, C.-M.; Cheung, C.S.-F.; Lau, C.-P.; Ho, H.-M.; Lee, M.Y.-H.; Au, S.W.-N.; Cheng, C.H.-K.; Lau, C.B.-S.; et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008, 118, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.-H.; Chan, J.Y.-W.; Chan, B.C.-L.; Lin, H.-Q.; Han, X.-Q.; Zhou, X.; Wan, D.C.-C.; Wang, Y.-F.; Leung, P.-C.; Fung, K.-P.; et al. Structural characterization and immunomodulatory effect of a polysaccharide HCP-2 from Houttuynia cordata. Carbohydr. Polym. 2014, 103, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Sun, L.; Zou, S.; Chen, J.; Mao, H.; Zhang, Y.; Liao, N.; Zhang, R. Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1) A Human Norovirus Surrogate. Molecules 2019, 24, 1835. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jin, C.; Chen, H.; Wang, P.; Yu, M.; Ding, K. Structural characterization and anti-A549 lung cancer cells bioactivity of a polysaccharide from Houttuynia cordata. Int. J. Biol. Macromol. 2018, 120, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.-J.; Huo, J.-Y.; Chen, D.-F. Structural Characterization and Anti-complementary Activities of Two Polysaccharides from Houttuynia cordata. Planta Med. 2019, 85, 1098–1106. [Google Scholar] [CrossRef]
- Li, T.; Sang, T.; Song, Y.-H.; Hu, X.-J.; Wu, Q.; Yao, Y.-F.; Li, W.-J. Houttuynia cordata polysaccharide alleviates chronic vascular inflammation by suppressing calcium-sensing receptor in rats. J. Funct. Foods 2022, 95, 105172. [Google Scholar] [CrossRef]
- Zou, M.; Hu, X.; Wang, Y.; Wang, J.; Tang, F.; Liu, Y. Structural characterization and anti-inflammatory activity of a pectin polysaccharide HBHP-3 from Houttuynia cordata. Int. J. Biol. Macromol. 2022, 210, 161–171. [Google Scholar] [CrossRef]
- Zhu, H.; Lu, X.; Ling, L.; Li, H.; Ou, Y.; Shi, X.; Lu, Y.; Zhang, Y.; Chen, D. Houttuynia cordata polysaccharides ameliorate pneumonia severity and intestinal injury in mice with influenza virus infection. J. Ethnopharmacol. 2018, 218, 90–99. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Ling, L.; Zhang, Y.; Li, H.; Chen, D. Beneficial effects of Houttuynia cordata polysaccharides on “two-hit” acute lung injury and endotoxic fever in rats associated with anti-complementary activities. Acta Pharm. Sin. B 2018, 8, 218–227. [Google Scholar] [CrossRef]
- Zhu, Z.-y.; Liu, R.-q.; Si, C.-l.; Zhou, F.; Wang, Y.-x.; Ding, L.-n.; Jing, C.; Liu, A.-j.; Zhang, Y.-m. Structural analysis and anti-tumor activity comparison of polysaccharides from Astragalus. Carbohydr. Polym. 2011, 85, 895–902. [Google Scholar] [CrossRef]
- Zhao, G.H.; Kan, J.Q.; Li, Z.X.; Chen, Z.D. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr. Polym. 2005, 61, 125–131. [Google Scholar] [CrossRef]
- Yan, J.-K.; Li, L.; Wang, Z.-M.; Wu, J.-Y. Structural elucidation of an exopolysaccharide from mycelial fermentation of a Tolypocladium sp fungus isolated from wild Cordyceps sinensis. Carbohydr. Polym. 2010, 79, 125–130. [Google Scholar] [CrossRef]
- Van Leeuwen, S.S.; Leeflang, B.R.; Gerwig, G.J.; Kamerling, J.P. Development of a H-1 NMR structural-reporter-group concept for the primary structural characterisation of alpha-D-glucans. Carbohydr. Res. 2008, 343, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Linker, A.; Evans, L.R.; Impallomeni, G. The structure of a polysaccharide from infectious strains of Burkholderia cepacia. Carbohydr. Res. 2001, 335, 45–54. [Google Scholar] [CrossRef]
- Sahragard, N.; Jahanbin, K. Structural elucidation of the main water-soluble polysaccharide from Rubus anatolicus roots. Carbohydr. Polym. 2017, 175, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Yu, P.; Zhao, T.; Chen, Y.; Feng, W.; Zhang, Q.; Yang, L.; Wu, X. Aqueous two-phase simultaneous extraction and purification of a polysaccharide from Grifola frondosa: Process optimization, structural characteristics and antioxidant activity. Ind. Crops Prod. 2022, 184, 114962. [Google Scholar] [CrossRef]
- Lai, L.-S.; Yang, D.-H. Rheological properties of the hot-water extracted polysaccharides in Ling-Zhi (Ganoderma lucidum). Food Hydrocoll. 2007, 21, 739–746. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, X.; McClements, D.J.; Huang, Q.; Tang, H.; Yu, K.; Xiang, X.; Chen, P.; Wang, X.; Deng, Q. Effect of flaxseed polyphenols on physical stability and oxidative stability of flaxseed oil-in-water nanoemulsions. Food Chem. 2019, 301, 125207. [Google Scholar] [CrossRef]
- De Falco, B.; Fiore, A.; Bochicchio, R.; Amato, M.; Lanzotti, V. Metabolomic analysis by UAE-GC MS and antioxidant activity of Salvia hispanica (L.) seeds grown under different irrigation regimes. Ind. Crops Prod. 2018, 112, 584–592. [Google Scholar] [CrossRef]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Antidiabetic potential of marine algae by inhibiting key metabolic enzymes. Front. Life Sci. 2015, 8, 148–159. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; Zhu, Z.-Y.; Sun, H.-Q.; Chen, L.-J. Structural characterization and inhibition on alpha-glucosidase activity of acidic polysaccharide from Annona squamosa. Carbohydr. Polym. 2017, 174, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Wiboonsirikul, J.; Hata, S.; Tsuno, T.; Kimura, Y.; Adachi, S. Production of functional substances from black rice bran by its treatment in subcritical water. LWT-Food Sci. Technol. 2007, 40, 1732–1740. [Google Scholar] [CrossRef]
- Shen, W.W.; Ren, J.M. Multiple Stepwise Regression Analysis Crack Open Degree Data in Gravity Dam. Appl. Mech. 2013, 477–478, 888–891. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Zhang, Y.-Y.; Ou, Y.-Y.; Lu, X.-X.; Pan, L.-Y.; Li, H.; Lu, Y.; Chen, D.-F. Houttuynia cordata Thunb. polysaccharides ameliorates lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2015, 173, 81–90. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Zeng, H.; Zhang, Y.; Zheng, B. Structural characterization of a novel neutral polysaccharide from Lentinus giganteus and its antitumor activity through inducing apoptosis. Carbohydr. Polym. 2016, 154, 231–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural characterization and immunomodulatory activity of exopolysaccharides from submerged culture of Auricularia auricula-judae. Int. J. Biol. Macromol. 2018, 115, 978–984. [Google Scholar] [CrossRef]
- Bian, C.; Wang, Z.; Shi, J. Extraction Optimization, Structural Characterization, and Anticoagulant Activity of Acidic Polysaccharides from Auricularia auricula-judae. Molecules 2020, 25, 710. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X. Ultrasonic degradation effects on the physicochemical, rheological and antioxidant properties of polysaccharide from Sargassum pallidum. Carbohydr. Polym. 2020, 239, 116230. [Google Scholar] [CrossRef]
- Cai, Z.-N.; Li, W.; Mehmood, S.; Pan, W.-J.; Wang, Y.; Meng, F.-J.; Wang, X.-F.; Lu, Y.-M.; Chen, Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydr. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Huang, L.; Lv, W.; Tong, H.; Song, L.; Hu, X.; Yu, R. Structural Characterization of a Novel Polysaccharide from Pulp Tissues of Litchi chinensis and Its Immunomodulatory Activity. J. Agric. Food Chem. 2014, 62, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ji, P.; Cheng, J.; Wang, Y.; Qian, H.; Li, W.; Gong, X.; Wang, Z. Structural characterization and immunostimulatory activity of a novel protein-bound polysaccharide produced by Hirsutella sinensis Liu, Guo, Yu & Zeng. Food Chem. 2013, 141, 946–953. [Google Scholar]
- Wu, D.-T.; Feng, K.-L.; Huang, L.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves. Foods 2021, 10, 2330. [Google Scholar] [CrossRef]
- Li, F.; Jin, Y.; Wang, J.; Xu, H. Structure Identification of Two Polysaccharides from Morchella sextelata with Antioxidant Activity. Foods 2022, 11, 982. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, M.; Ma, L.; Ye, H.; Zeng, X. A comparative study of the neutral and acidic polysaccharides from Allium macrostemon Bunge. Carbohydr. Polym. 2015, 117, 980–987. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Peng, X.; Lee, K.-L.D.; Tang, J.C.-o.; Cheung, P.C.-K.; Wu, J.-Y. Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011, 125, 637–643. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.-W.; Liao, S.-T.; Zhang, R.-F.; Deng, Y.-Y.; Wei, Z.-C.; Yang, B. Effects of alkali dissociation on the molecular conformation and immunomodulatory activity of longan pulp polysaccharide (LPI). Carbohydr. Polym. 2012, 87, 1311–1317. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Chen, G.-T.; Yuan, B.; Wang, H.-X.; Qi, G.-H.; Cheng, S.-J. Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int. J. Biol. Macromol. 2019, 139, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Sorourian, R.; Khajehrahimi, A.E.; Tadayoni, M.; Azizi, M.H.; Hojjati, M. Ultrasound-assisted extraction of polysaccharides from Typha domingensis: Structural characterization and functional properties. Int. J. Biol. Macromol. 2020, 160, 758–768. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.T.; Henson, C.A. A quantitative assessment of the importance of barley seed alpha-amylase, beta-amylase, debranching enzyme, and alpha-glucosidase in starch degradation. Arch. Biochem. Biophys. 1991, 284, 298–305. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential alpha-amylase/alpha-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of a-glucosidase, alpha-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Tian, J.; Wang, Y.; Xing, L.; Wang, J. Chemical modification, antioxidant and alpha-amylase inhibitory activities of corn silk polysaccharides. Carbohydr. Polym. 2013, 98, 428–437. [Google Scholar] [CrossRef]
- Wang, B.-H.; Cao, J.-J.; Zhang, B.; Chen, H.-Q. Structural characterization, physicochemical properties and alpha-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. The effect of ultrasound irradiation on the physicochemical properties and alpha-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]


| Polysaccharide Name | Molecular Weight | Monosaccharide Composition | Glycosidic Bond | Biological Activity | Reference |
|---|---|---|---|---|---|
| HCP | - | Man:Rha:GlcA:GalA:Glc:Xyl:Gal:Ara = 1.8:17.2:6.8:29.4:5.3:2.1:24:13.5 | - | anti-oxidative | [19] |
| HP | 43kDa | GalA:Gal:Glc:Xyl = 1.56:1.49:1.26:1.11 | α-1,4-linked GalpA, β-1,4-linked Galp, β-1,4-linked Glcp, β-1,4-linked Xylp | anti-viral | [23] |
| HCA4S1 | 21.7 kDa | Rha:GalA:Gal:Ara = 15.6:17.5:41.5:25.7 | 1,4-linked-α-d-GalA; 1,2,4-linked-α-L-Rha | anti-cancer | [24] |
| HC-PS1 | 274.53 kDa | Man, Rha, GlcA, GalA, Glc, Xyl, Gal, Ara | terminal Rhap; 1,5-linked Araf; 1,3,6-linked and 1,4,6-linked Manp; 1,4-linked, 1,3-linked, 1,3,6-linked, 1,4,6-linked and 1,3,4,6-linked Glcp; 1,4-linked and 1,6-linked Galp | immune regulation | [26] |
| HC-PS3 | 216.38 kDa | HC-PS3 was similar to HC-PS1. HC-PS3 did not have 1,3,6-linked Glcp but had additional 1,3,4-linked Manp | |||
| HCP-2 | 60 kDa | GalA | 1,4-linked-α-d-galacturonic acid | immune regulation | [22] |
| HCP | 387 kDa | GalA, Gal, Glc | - | anti-inflammatory | [27] |
| HBHP-3 | 397.4 kDa | Rha:Ara:Glc:Gal:GalA = 16.0:12.6:4.6:18.1:15.6 | →2)-α-l-Rhap-(1→, →4)-α-d-GalpA-(1→ and →4)-β-D-Galp-(1→ | anti-inflammatory | [28] |
| HCP | - | Man, Rha, Glc, Xyl, Gal, Ara | - | Intestinal protection | [29] |
| Level | X1 Extraction Time (h) | X2 Extraction Temperature (°C) | X3 Solid-Liquid Ratio (g/mL) |
|---|---|---|---|
| −1 | 4 | 80 | 1:20 |
| 0 | 5 | 90 | 1:30 |
| 1 | 6 | 100 | 1:40 |
| No. | X1 | X2 | X3 | Extraction Yield (%) |
|---|---|---|---|---|
| 1 | 1 | 0 | 1 | 2.11 ± 0.09 |
| 2 | −1 | 0 | 1 | 1.70 ± 0.04 |
| 3 | 1 | 0 | −1 | 2.08 ± 0.06 |
| 4 | 0 | 0 | 0 | 2.44 ± 0.08 |
| 5 | 0 | 0 | 0 | 2.42 ± 0.04 |
| 6 | 0 | 0 | 0 | 2.12 ± 0.04 |
| 7 | 0 | −1 | 1 | 1.64 ± 0.02 |
| 8 | 1 | −1 | 0 | 1.59 ± 0.08 |
| 9 | 0 | 1 | −1 | 2.01 ± 0.07 |
| 10 | −1 | 0 | −1 | 1.66 ± 0.09 |
| 11 | 1 | 1 | 0 | 2.12 ± 0.06 |
| 12 | −1 | −1 | 0 | 1.67 ± 0.03 |
| 13 | 0 | −1 | −1 | 1.82 ± 0.01 |
| 14 | 0 | 0 | 0 | 2.28 ± 0.09 |
| 15 | 0 | 0 | 0 | 2.51 ± 0.04 |
| 16 | 0 | 1 | 1 | 2.05 ± 0.07 |
| 17 | −1 | 1 | 0 | 1.85 ± 0.08 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.27 | 9 | 0.14 | 6.39 | 0.0115 * |
| X1 | 0.13 | 1 | 0.13 | 5.89 | 0.0456 * |
| X2 | 0.21 | 1 | 0.21 | 9.72 | 0.0169 * |
| X3 | 0.001 | 1 | 0.001 | 0.028 | 0.8724 |
| X1X2 | 0.031 | 1 | 0.031 | 1.39 | 0.2774 |
| X1X3 | 0.00002 | 1 | 0.00002 | 0.001 | 0.9741 |
| X2X3 | 0.0012 | 1 | 0.0012 | 0.55 | 0.4832 |
| X12 | 0.31 | 1 | 0.31 | 13.85 | 0.0074 ** |
| X22 | 0.32 | 1 | 0.32 | 14.63 | 0.0065 ** |
| X32 | 0.16 | 1 | 0.16 | 7.40 | 0.0297 * |
| Residual | 0.15 | 7 | 0.022 | ||
| Lack of Fit | 0.058 | 3 | 0.019 | 0.81 | 0.5526 |
| Pure Error | 0.096 | 4 | 0.024 | ||
| Cor Total | 1.43 | 16 |
| Model | R | R2 | Adjusted R2 | Standard Estimation Error |
|---|---|---|---|---|
| 1 | 0.683 a | 0.466 | 0.425 | 0.16057 |
| 2 | 0.839 b | 0.703 | 0.654 | 0.12461 |
| Model | Sum of Squares | DF | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| 1 | Regression | 0.292 | 1 | 0.292 | 11.342 | 0.005 b |
| Residual | 0.335 | 13 | 0.026 | |||
| Cor Total | 0.628 | 14 | ||||
| 2 | Regression | 0.441 | 2 | 0.221 | 14.209 | 0.001 c |
| Residual | 0.186 | 12 | 0.016 | |||
| Cor Total | 0.628 | 14 | ||||
| Model | Coefficient of Non-Standardization | Standardization Coefficient | Collinearity Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| B | Standard Error | Beta | t | Sig. | Tolerance | VIF | ||
| 1 | (Constant) | 0.088 | 0.304 | 0.290 | 0.776 | |||
| Temperature | 0.018 | 0.004 | 0.794 | 4.705 | 0.000 | 1.000 | 1.000 | |
| 2 | (Constant) | −0.375 | 0.354 | −1.059 | 0.311 | |||
| Temperature | 0.017 | 0.004 | 0.683 | 4.340 | 0.001 | 1.000 | 1.000 | |
| Time | 0.122 | 0.039 | 0.487 | 3.096 | 0.009 | 1.000 | 1.000 | |
| Extraction Temperature | Extraction Time | Solid-Liquid Ratio | |
|---|---|---|---|
| R | 0.913 * | 0.875 | 0.260 |
| Sig. (two-sided) | 0.031 | 0.052 | 0.673 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tian, J.; Pan, Y.; Li, Z.; Zhou, Z.; Pan, Z.; Tai, H.; Xing, Y. Structural Characterization and Biological Activity of Polysaccharides from Stems of Houttuynia cordata. Foods 2022, 11, 3622. https://doi.org/10.3390/foods11223622
Liu X, Tian J, Pan Y, Li Z, Zhou Z, Pan Z, Tai H, Xing Y. Structural Characterization and Biological Activity of Polysaccharides from Stems of Houttuynia cordata. Foods. 2022; 11(22):3622. https://doi.org/10.3390/foods11223622
Chicago/Turabian StyleLiu, Xiaocui, Jin Tian, Yinzhen Pan, Zhongqiao Li, Zhiran Zhou, Zihao Pan, Huazhang Tai, and Yage Xing. 2022. "Structural Characterization and Biological Activity of Polysaccharides from Stems of Houttuynia cordata" Foods 11, no. 22: 3622. https://doi.org/10.3390/foods11223622
APA StyleLiu, X., Tian, J., Pan, Y., Li, Z., Zhou, Z., Pan, Z., Tai, H., & Xing, Y. (2022). Structural Characterization and Biological Activity of Polysaccharides from Stems of Houttuynia cordata. Foods, 11(22), 3622. https://doi.org/10.3390/foods11223622





