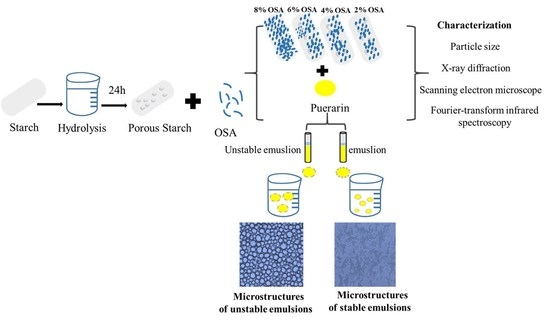
Enhanced Bioaccessibility of Microencapsulated Puerarin Delivered by Pickering Emulsions Stabilized with OSA-Modified Hydrolyzed Pueraria montana Starch: In Vitro Release, Storage Stability, and Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification and Characterization of Pueraria montana Starch
2.2.1. Pretreatment of PMS with Acid Hydrolysis
2.2.2. OSA Modification of PMS
2.3. Determination of DS
2.4. Particle Size Determination of Modified PMS
2.5. X-ray Diffraction (XRD) Patterns of Starches
2.6. FTIR Spectroscopy
2.7. Morphological Analysis Using SEM (SEM)
2.8. Preparation of Pickering Emulsions Stabilized by Modified Pueraria montana Starches
2.8.1. Formulation of Pickering Emulsions
2.8.2. Determination of the Emulsification Index
2.8.3. Droplet Size Distribution and Confocal Laser Scanning Microscopy
2.8.4. Measurement of ζ-Potential
2.8.5. Retention Degree and Microencapsulation Efficiency (MEE)
2.8.6. Measurement of In Vitro Release Profile of Puerarin
2.8.7. HPLC Measurement
2.9. Statistical Analyses
3. Results and Discussion
3.1. Acid Hydrolysis Pretreatment and Degree of Substitution of Modified Starches
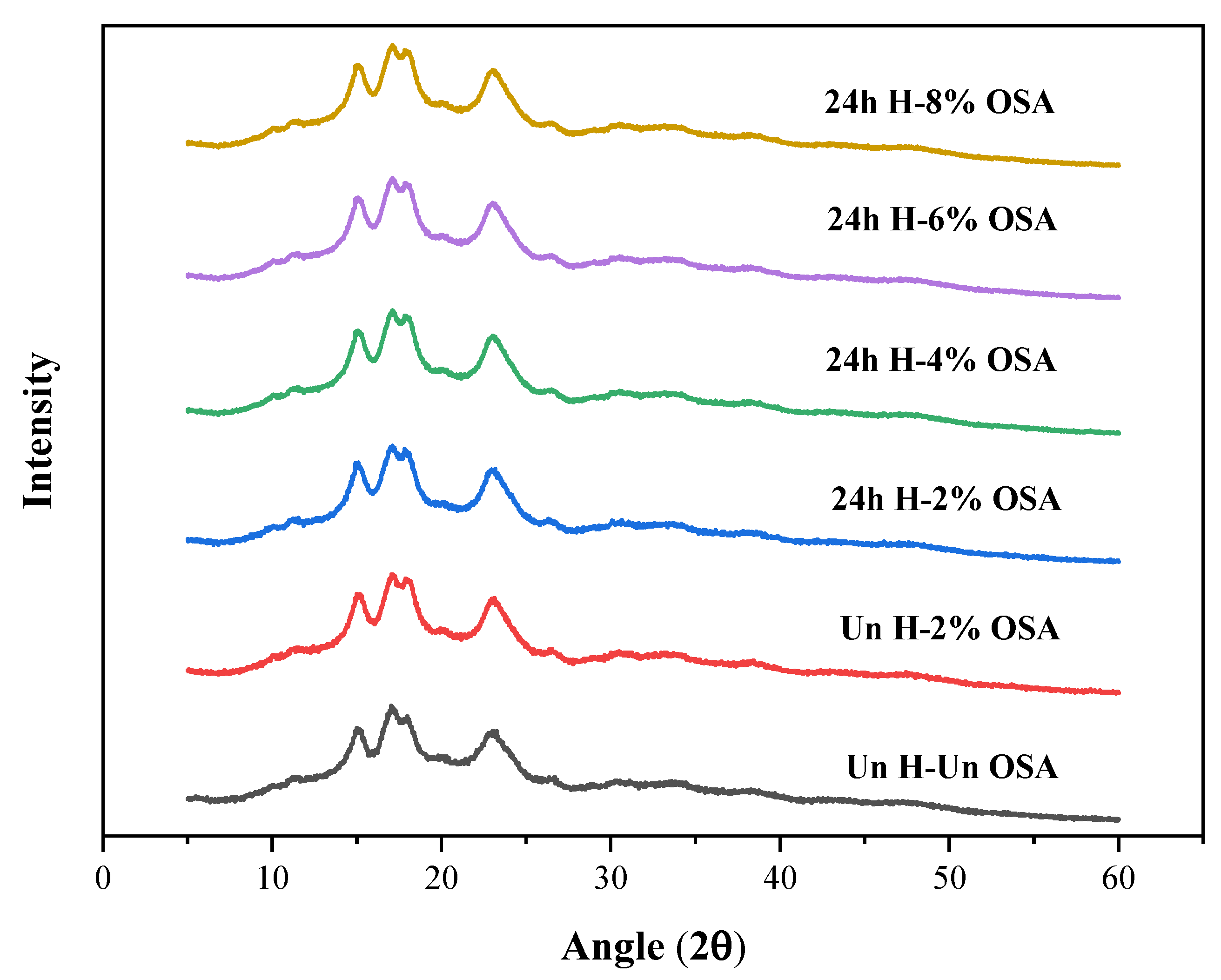
3.2. X-ray Diffraction Analysis
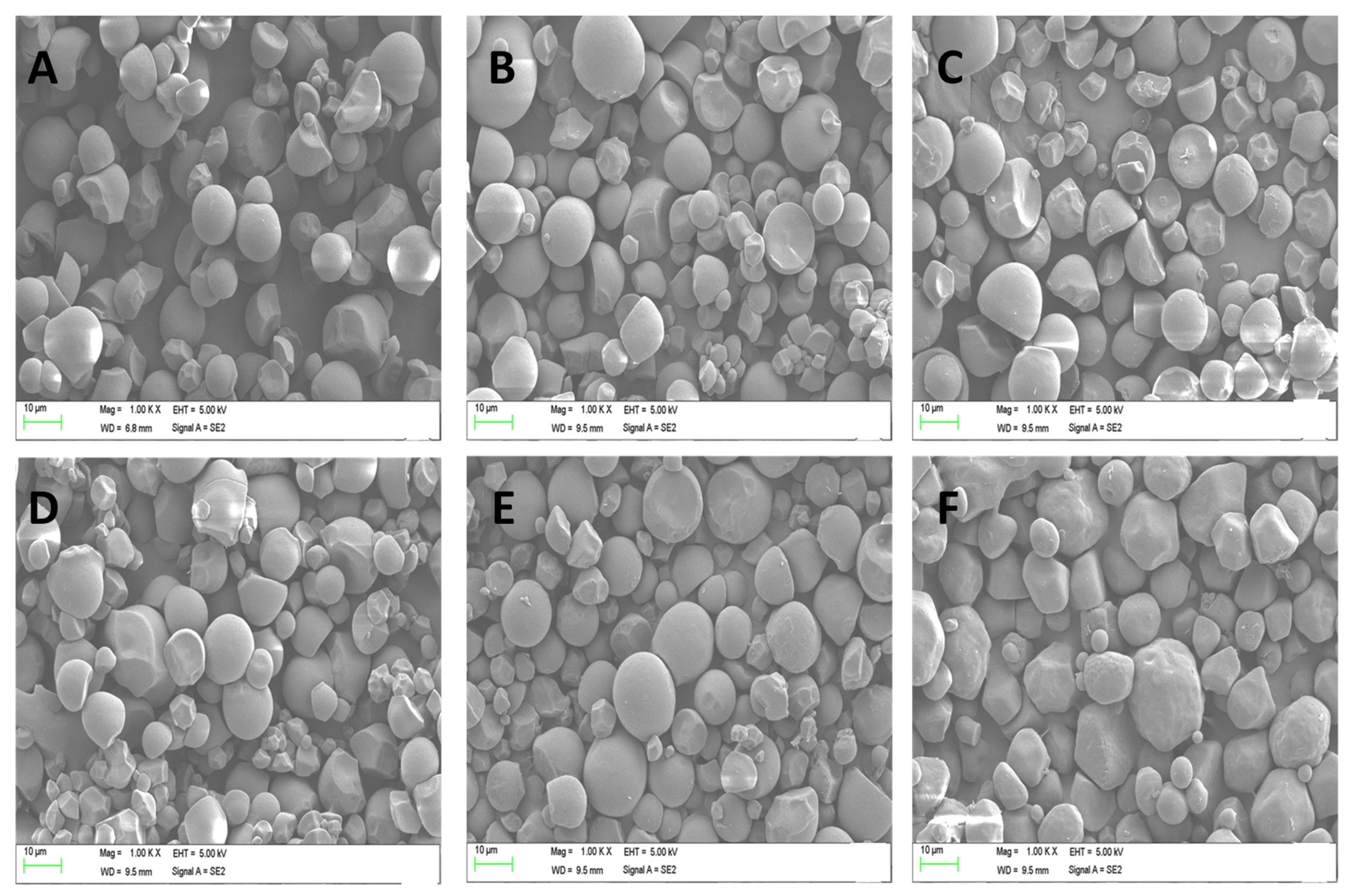
3.3. Scanning Electron Microscopic (SEM) Analysis
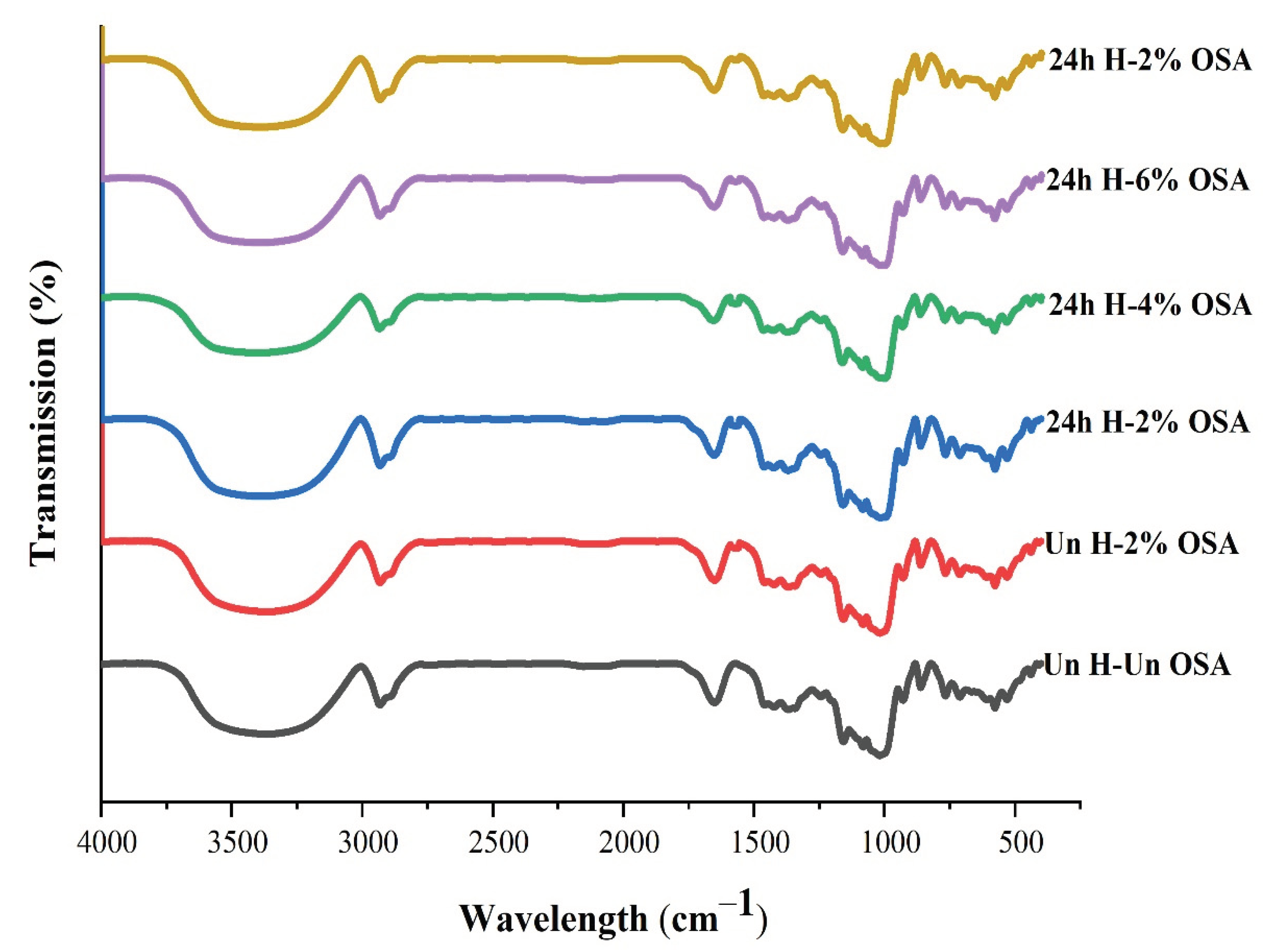
3.4. FT-IR Analysis
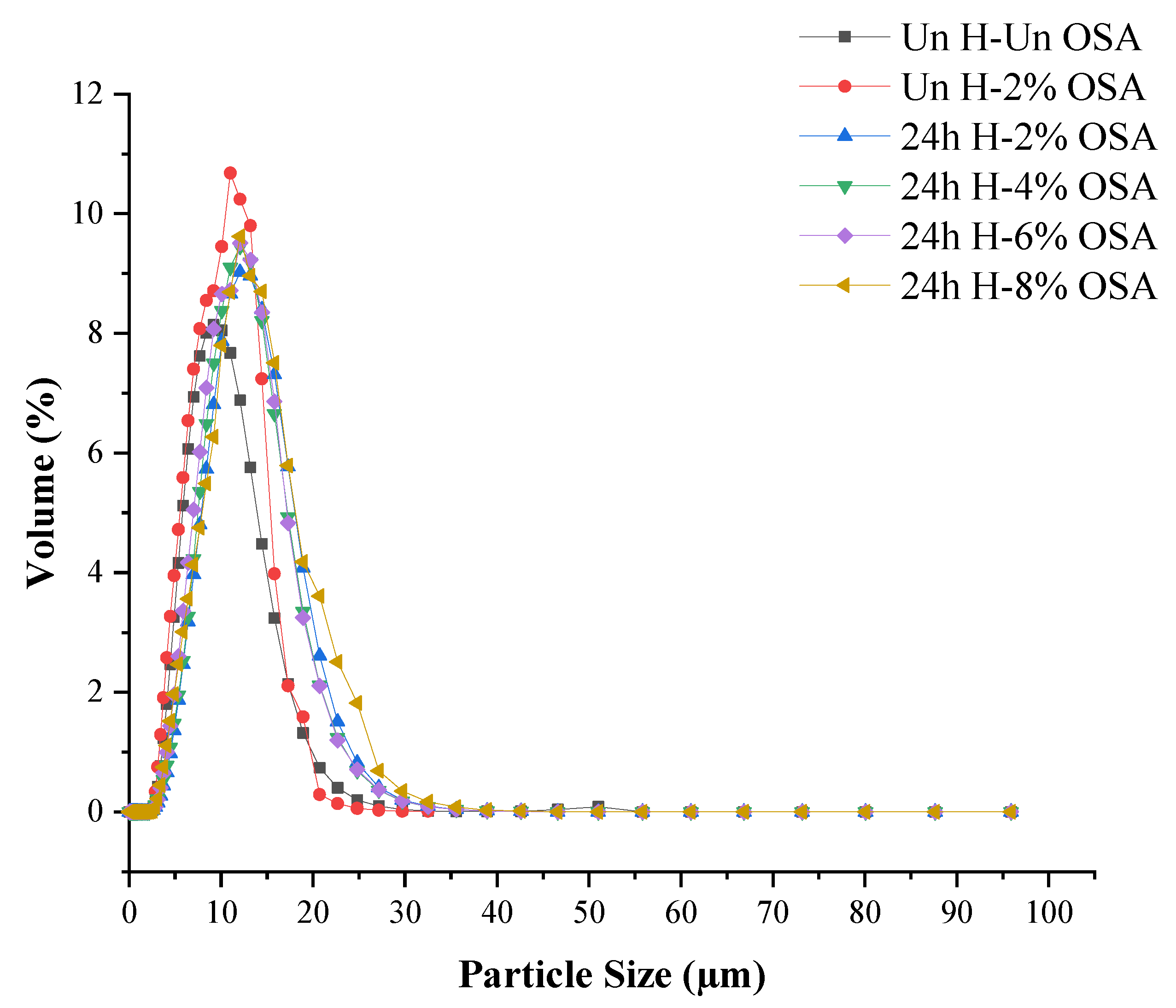
3.5. Distribution of Particle Size
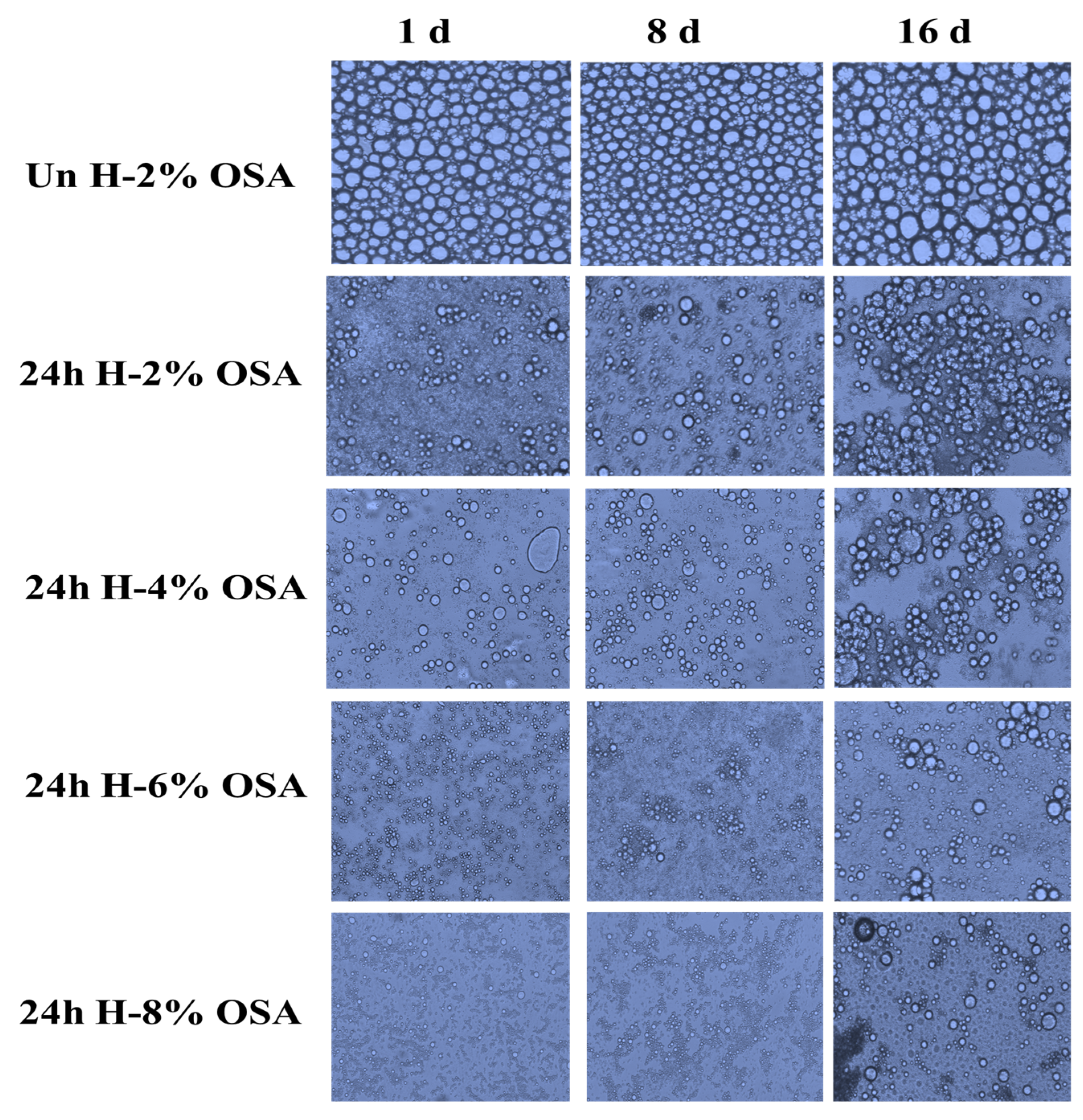
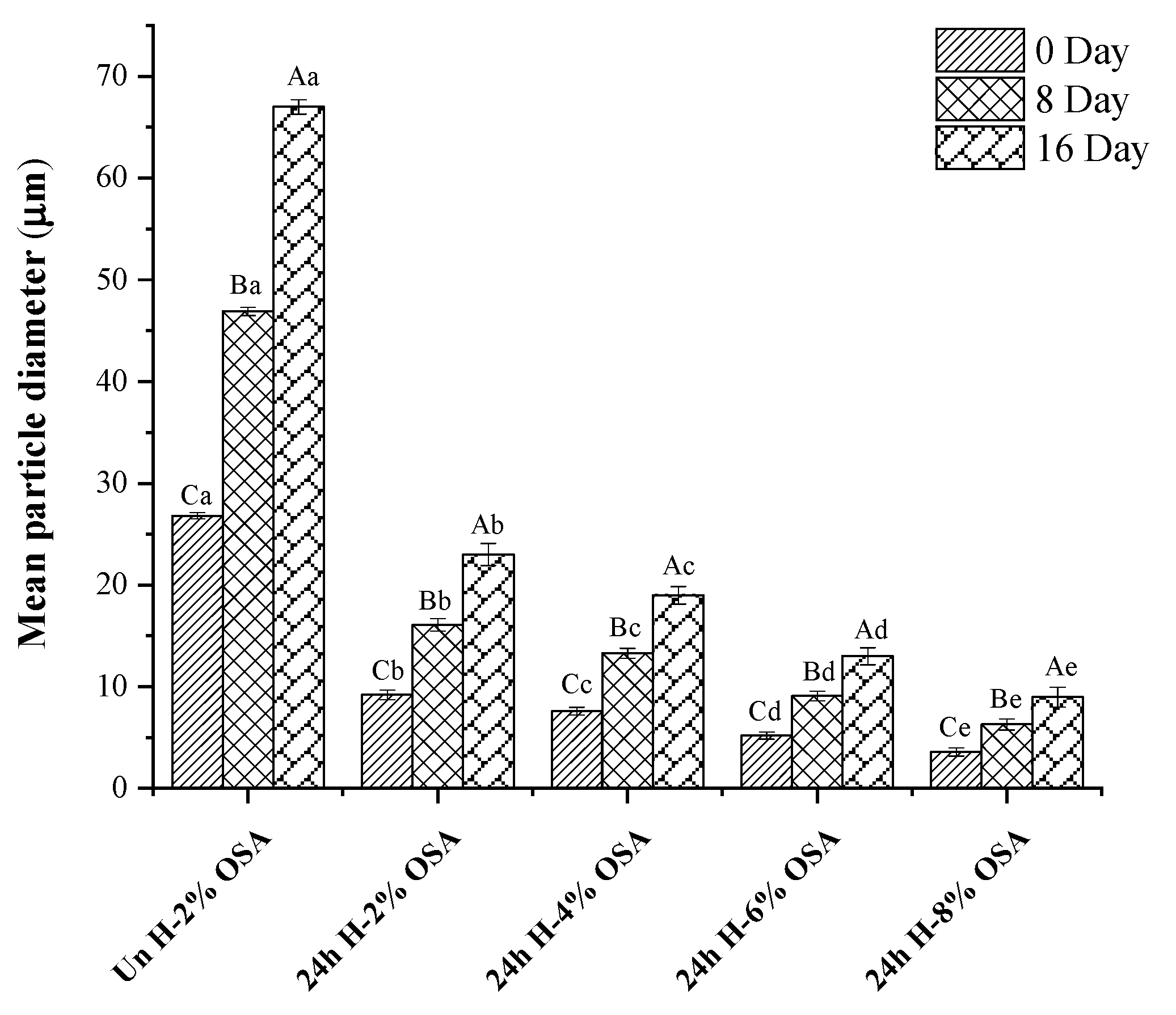
3.6. Stability of Emulsions
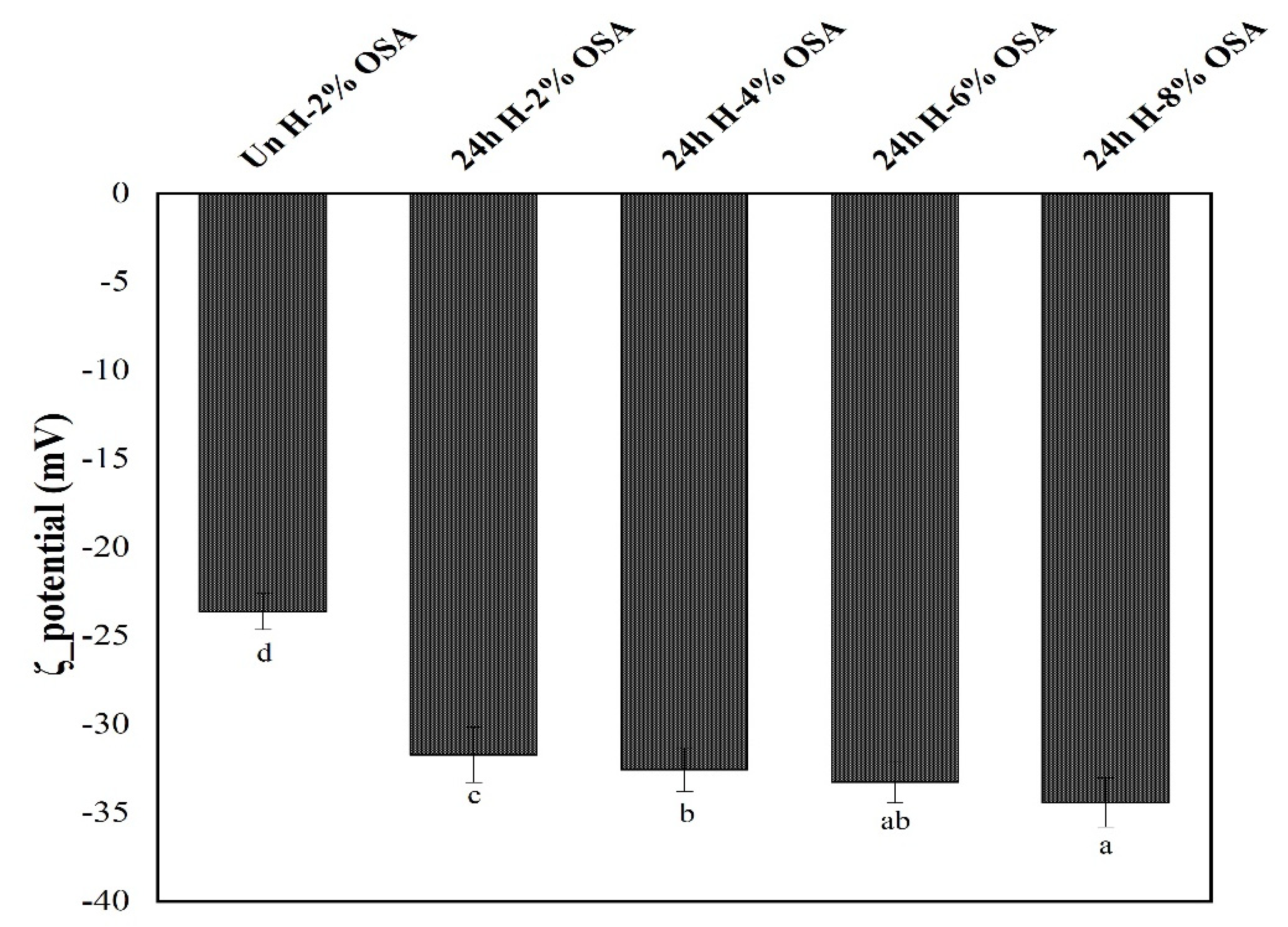
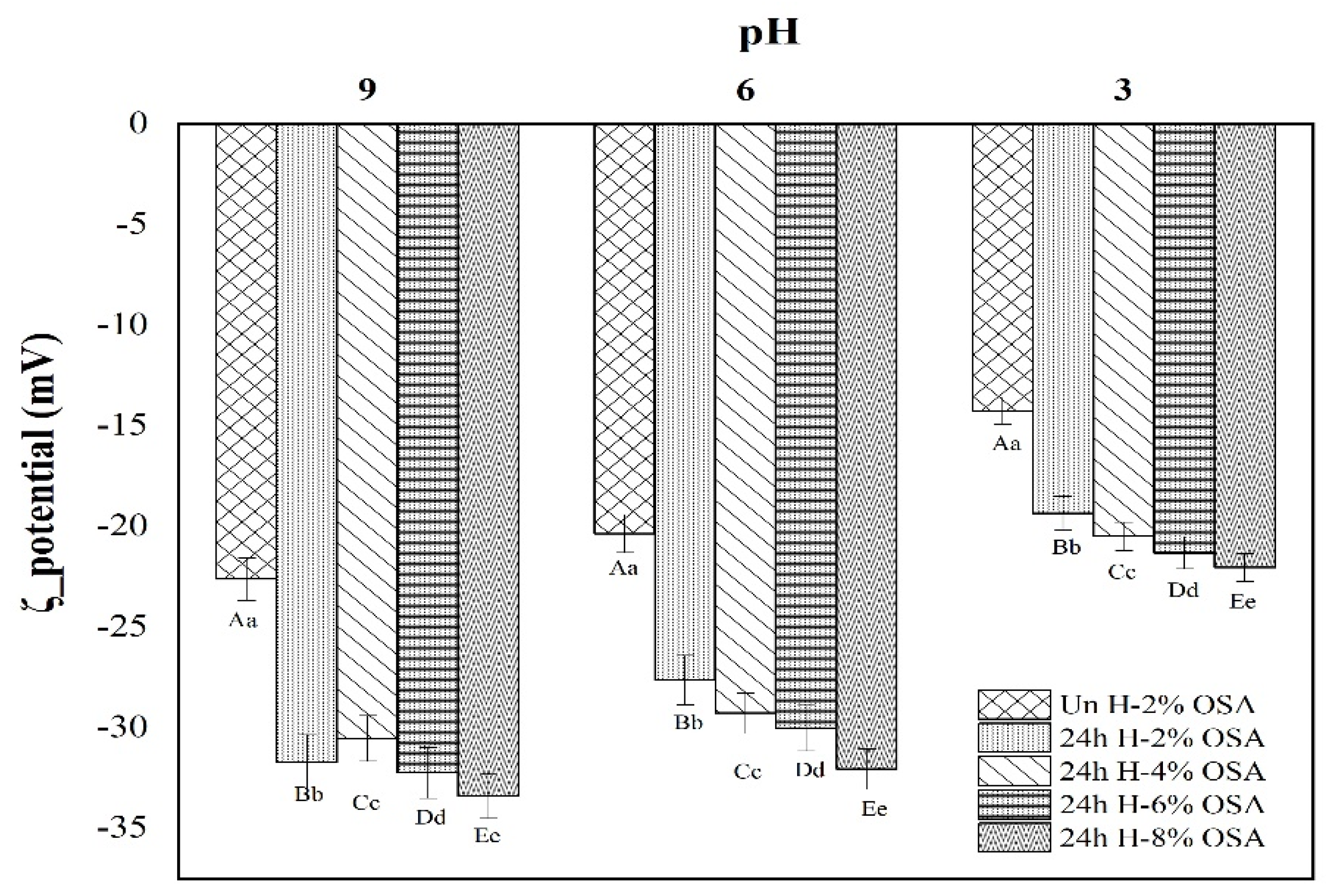
3.7. ζ-Potential of Emulsions
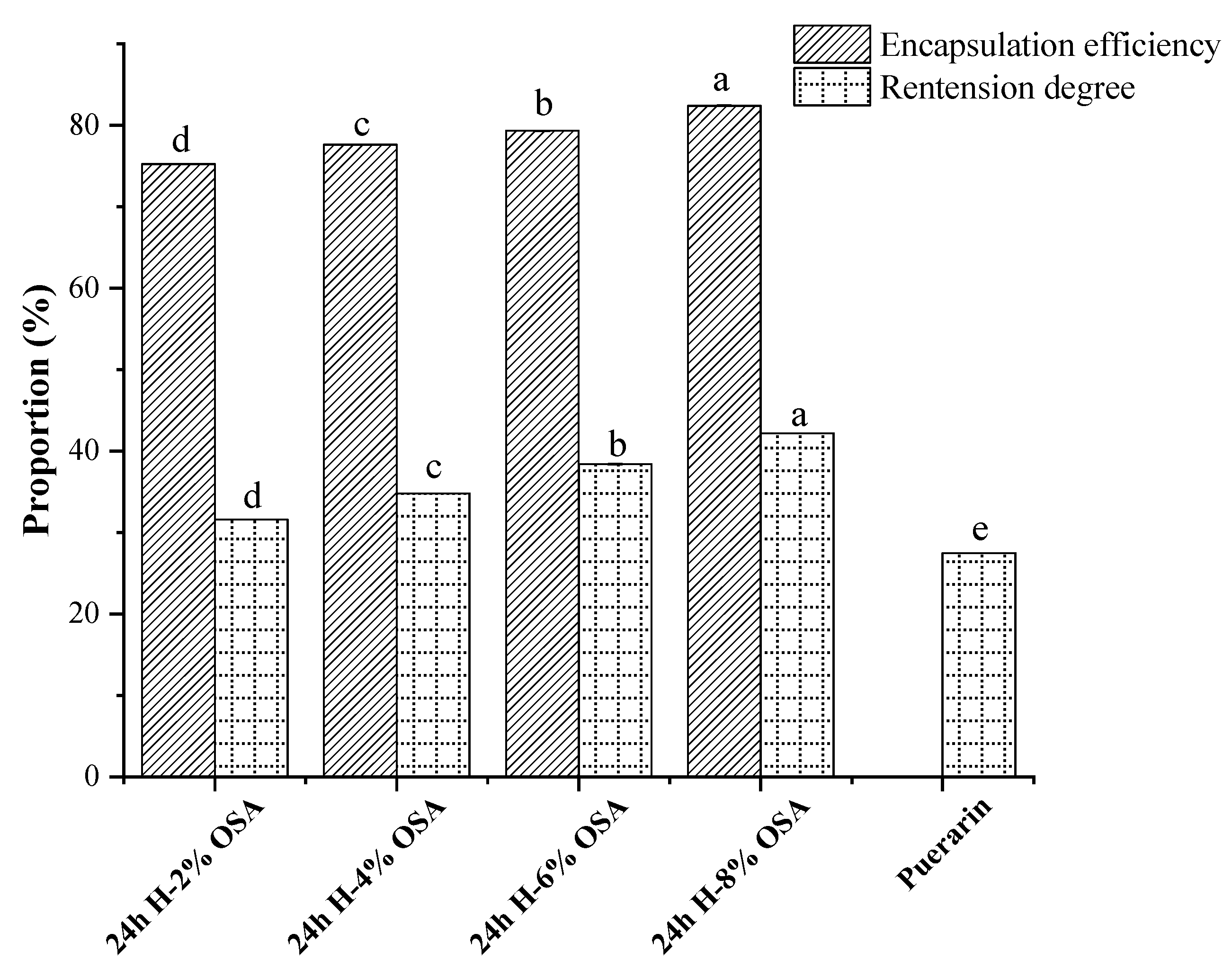
3.8. Retention Degree and Microencapsulation Efficiency (MEE) of Puerarin
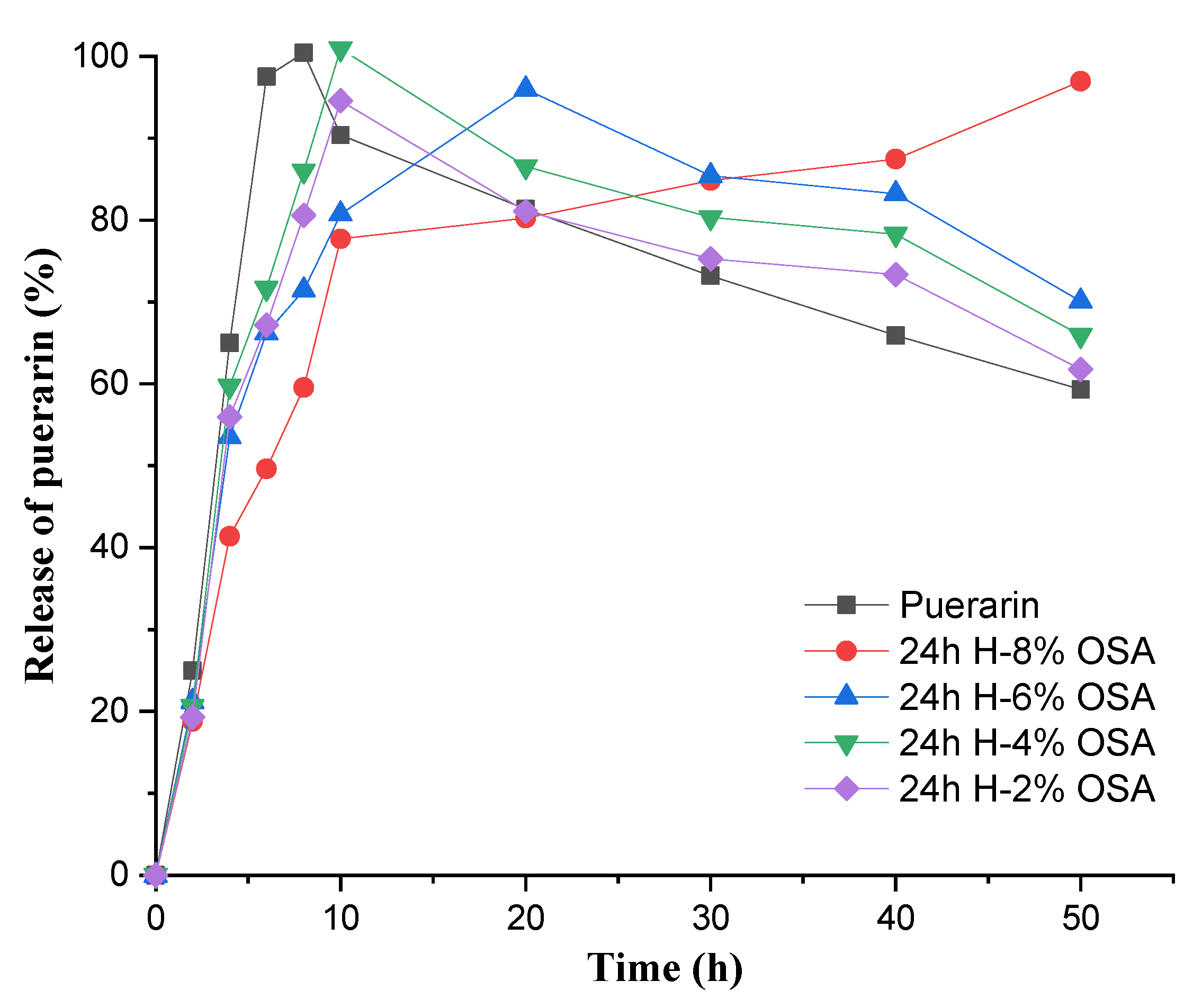
3.9. In Vitro Release Profile of Puerarin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phyther. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zhao, G.L.; Li, X.F.; Xiao, X.L.; He, N.; Xu, H.X.; Yu, Y.G. Green synthesis of puerarin acid esters and their oral absorption evaluation in vivo. J. Drug Deliv. Sci. Technol. 2022, 67, 102882. [Google Scholar] [CrossRef]
- Li, R.; Yuan, G.; Li, D.; Xu, C.; Du, M.; Tan, S.; Liu, Z.; He, Q.; Rong, L.; Li, J. Enhancing the bioaccessibility of puerarin through the collaboration of high internal phase Pickering emulsions with β-carotene. Food Funct. 2022, 13, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Raines, J.M.; Martin, J.M.; Rodriguez, J.M. Thermal stability of kudzu root (Pueraria Radix) isoflavones as additives to beef patties. J. Food Sci. Technol. 2015, 52, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Y.; Chen, Y.; Xu, X.Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Wang, Z.K.; Chen, R.R.; Li, J.H.; Chen, J.Y.; Li, W.; Niu, X.L.; Wang, F.F.; Wang, J.; Yang, J.X. Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: The role of the SIRT1/NF-κB pathway. Int. Immunopharmacol. 2020, 89, 107086. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Cui, X.; Qu, P.; Shang, C.; Xiang, M.; Wang, J. Roles and mechanisms of puerarin on cardiovascular disease: A review. Biomed. Pharmacother. 2022, 147, 112655. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Effects of Puerarin on the Prevention and Treatment of Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 771793. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Wang, M. Effects of puerarin on chronic inflammation: Focus on the heart, brain, and arteries. Aging Med. 2021, 4, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, Y.; Chen, Q.; Han, X.; Cai, M.; Hao, L. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG2 cells. Biomed. Pharmacother. 2021, 137, 111325. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, Y.; Wu, Y.; Wu, T.; Feng, H.; Xu, Z.; Liu, Y.; Ruan, Z.; Zhou, S. Puerarin improves intestinal barrier function through enhancing goblet cells and mucus barrier. J. Funct. Foods 2020, 75, 104246. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, J.; Dai, T.; McClements, D.J.; Liu, C. The effect of whey protein-puerarin interactions on the formation and performance of protein hydrogels. Food Hydrocoll. 2021, 113, 106444. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Huang, C.; Liu, Q.; Huang, M.; Chen, B.; Peng, Z.; Zhu, W.; Ding, B. Efficacy and safety of Puerarin injection on acute heart failure: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 934598. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, C.; Sun, Y.; Pang, L.; Zhu, S.; Liu, Y.; Zhu, L.; Zhang, S.; Wang, L.; Du, L. Comparative study of oral and intranasal puerarin for prevention of brain injury induced by acute high-altitude hypoxia. Int. J. Pharm. 2020, 591, 120002. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.Q.; Tan, T.W. Modeling and prediction of the mixed-mode retention mechanisms for puerarin and its analogues on n-octylamine modified poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) monoliths. Process Biochem. 2009, 44, 1225–1230. [Google Scholar] [CrossRef]
- Anukunwithaya, T.; Poo, P.; Hunsakunachai, N.; Rodsiri, R.; Malaivijitnond, S.; Khemawoot, P. Absolute oral bioavailability and disposition kinetics of puerarin in female rats. BMC Pharmacol. Toxicol. 2018, 19, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, Y.; Ma, P.; Fan, L.; Zhao, L.; Wang, M.; Li, X. An integrated strategy for anti-inflammatory quality markers screening of traditional Chinese herbal medicine Mume Fructus based on phytochemical analysis and anti-colitis activity. Phytomedicine 2022, 99, 154002. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Wang, X.; Shi, R.; Zhao, Y.; Cheng, J.; Yan, X.; Liu, X.; Wang, Y.; Zhang, M.; Wang, Q.; et al. Pharmacokinetics and tissue distribution kinetics of puerarin in rats using indirect competitive ELISA. Molecules 2017, 22, 939. [Google Scholar] [CrossRef]
- Nakamura, K.; Zhu, S.; Komatsu, K.; Hattori, M.; Iwashima, M. Deglycosylation of the isoflavone C-glucoside puerarin by a combination of two recombinant bacterial enzymes and 3-oxo-glucose. Appl. Environ. Microbiol. 2020, 86, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.M.; Wan, X.H.; Zou, B.; Wu, W.T.; Chen, L.H.; Zhu, W.F. Effect of isoflavones from Puerariae Lobatae Radix on solubility and permeability of puerarin based on “self-consistent regulation”. Chin. Tradit. Herb. Drugs 2021, 52, 7138–7147. [Google Scholar] [CrossRef]
- Mo, L.; Zhao, G.L.; Li, X.F.; Xiao, X.L.; He, N.; Ma, J.J.; Yu, Y.G. Evaluation of the digestion and transport profiles and potential immunocompetence of puerarin and its acylated derivatives. Food Funct. 2021, 12, 5949–5958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. 2019, 26, 860–869. [Google Scholar] [CrossRef]
- Yan, J.; Guan, Z.Y.; Zhu, W.F.; Zhong, L.Y.; Qiu, Z.Q.; Yue, P.F.; Wu, W.T.; Liu, J.; Huang, X. Preparation of puerarin chitosan oral nanoparticles by ionic gelation method and its related kinetics. Pharmaceutics 2020, 12, 216. [Google Scholar] [CrossRef]
- Saari, H.; Rayner, M.; Wahlgren, M. Effects of starch granules differing in size and morphology from different botanical sources and their mixtures on the characteristics of Pickering emulsions. Food Hydrocoll. 2019, 89, 844–855. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Yu, L.; Xue, H.; Wang, Y.; Chen, J.; Zhang, S. Construction of octenyl succinic anhydride modified porous starch for improving bioaccessibility of β-carotene in emulsions. RSC Adv. 2020, 10, 8480–8489. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hadi, N.; Marefati, A.; Matos, M.; Wiege, B.; Rayner, M. Characterization and stability of short-chain fatty acids modified starch Pickering emulsions. Carbohydr. Polym. 2020, 240, 116264. [Google Scholar] [CrossRef]
- Lu, X.; Liu, H.; Huang, Q. Fabrication and characterization of resistant starch stabilized Pickering emulsions. Food Hydrocoll. 2020, 103, 105703. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.; Yin, Z.; Wu, J.; Zhu, L.; Gao, M.; Zhan, X. Emulsifying properties and bioavailability of clove essential oil Pickering emulsions stabilized by octadecylaminated carboxymethyl curdlan. Int. J. Biol. Macromol. 2022, 216, 629–642. [Google Scholar] [CrossRef]
- Calabrese, V.; Courtenay, J.C.; Edler, K.J.; Scott, J.L. Pickering emulsions stabilized by naturally derived or biodegradable particles. Curr. Opin. Green Sustain. Chem. 2018, 12, 83–90. [Google Scholar] [CrossRef]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Khalid, N.; Nakajima, M. Fabrication and Characterization of Dodecenyl Succinic Anhydride Modified Kudzu Starch. Starch-Staerke 2022, 74, 2100188. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, Y.; Lu, Y.; Xu, Y.; Li, W.; Yan, W. Sodium caseinate and OSA-modified starch as carriers for the encapsulation of lutein using spray drying to improve its water solubility and stability. Int. J. Food Sci. Technol. 2022, 57, 6409–6421. [Google Scholar] [CrossRef]
- Matos, M.; Marefati, A.; Barrero, P.; Rayner, M.; Gutiérrez, G. Resveratrol loaded Pickering emulsions stabilized by OSA modified rice starch granules. Food Res. Int. 2021, 139, 109837. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wu, D.; Singh, H.; Ye, A. Improving solubility and stability of β-carotene by microencapsulation in soluble complexes formed with whey protein and OSA-modified starch. Food Chem. 2021, 352, 129267. [Google Scholar] [CrossRef] [PubMed]
- Ananda; Tariq, N.; Rafiq, N.; Yousaf, S.; Abbas, S. Assessment of oral bioavailability of nanocapsules loaded-curcumin in-vivo. World J. Adv. Res. Rev. 2021, 9, 5–17. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 59–66. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Grossmann, L.; Weiss, J. Pickering emulsion stabilized by hydrolyzed starch: Effect of the molecular weight. J. Colloid Interface Sci. 2022, 612, 525–535. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martinez, M.; Marefati, A.; Sjöö, M.; Rayner, M. Chemical composition, digestibility and emulsification properties of octenyl succinic esters of various starches. Food Res. Int. 2015, 75, 41–49. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of amylose content in morphological, functional and emulsification properties of OSA modified corn starch. Food Hydrocoll. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Jiang, S.W.; Zheng, Z.; Cao, X.M.; Hou, Z.G.; Xu, J.J.; Wang, H.L.; Jiang, S.T.; Pan, L.J. Preparation and properties of OSA-modified taro starches and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2019, 137, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, K.; Pietrzyk, S.; Łabanowska, M.; Kurdziel, M.; Pająk, P. The influence of acid hydrolysis on physicochemical properties of starch-oleic acid mixtures and generation of radicals. Food Hydrocoll. 2021, 118, 320–329. [Google Scholar] [CrossRef]
- Lu, X.; Huang, Q. Bioaccessibility of polymethoxyflavones encapsulated in resistant starch particle stabilized Pickering emulsions: Role of fatty acid complexation and heat treatment. Food Funct. 2019, 10, 5969–5980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, H.; Liu, P.; Wang, W.; Dong, H. Effects of acid hydrolysis on the physicochemical properties of pea starch and its film forming capacity. Food Hydrocoll. 2019, 87, 173–179. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y. Effects of acid hydrolysis on the evolution of starch fine molecular structures and gelatinization properties. Food Chem. 2021, 353, 129449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Khalid, N.; Shu, G.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Formulation and characterization of O/W emulsions stabilized using octenyl succinic anhydride modified kudzu starch. Carbohydr. Polym. 2017, 176, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cheng, L.; Li, Z.; Li, C.; Hong, Y.; Gu, Z. The degree of substitution of OSA-modified starch affects the retention and release of encapsulated mint flavour. Carbohydr. Polym. 2022, 119781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, Z.; Wang, Q.; Chen, H.; Wang, C.; Hou, J.; Shi, L.; Liu, D.; Zhang, L. Effect of Fe (III) Species on the Stability of a Water-Model Oil Emulsion with an Anionic Sulfonate Surfactant as an Emulsifier. ACS Omega 2022, 41, 36343–36353. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, Z.; Gu, Z.; Wang, Y.; Pang, Y. Structure and emulsification properties of octenyl succinic anhydride starch using acid-hydrolyzed method. Starch-Staerke 2017, 68, 1–9. [Google Scholar] [CrossRef]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Junges, A.; Monteiro, W.F.; Diz, F.M.; Ligabue, R.A.; Morrone, F.B.; Severino, P.; et al. Chitosan and chitosan/PEG nanoparticles loaded with indole-3-carbinol: Characterization, computational study and potential effect on human bladder cancer cells. Mater. Sci. Eng. C 2021, 124, 112089. [Google Scholar] [CrossRef] [PubMed]
- Maji, A.K.; Banerjee, D.; Maity, N.; Banerji, P. A validated RP-HPLC-UV method for quantitative determination of puerarin in Pueraria tuberosa DC tuber extract. Pharm. Methods 2012, 3, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Singh, H. Effect of degree of octenyl succinic anhydride (OSA) substitution on the digestion of emulsions and the bioaccessibility of β-carotene in OSA-modified-starch-stabilized-emulsions. Food Hydrocoll. 2018, 84, 303–312. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, Z.; Zhang, B.; Li, X.M.; Meng, R.; Chen, H.Q.; Jin, Z.Y. Preparation and characterization of emulsion stabilized by octenyl succinic anhydride-modified dextrin for improving storage stability and curcumin encapsulation. Food Chem. 2019, 294, 326–332. [Google Scholar] [CrossRef]
- Zheng, W.; Ren, L.; Hao, W.; Wang, L.; Liu, C.; Zheng, L. Encapsulation of indole-3-carbinol in Pickering emulsions stabilized by OSA-modified high amylose corn starch: Preparation, characterization and storage stability properties. Food Chem. 2022, 386, 132846. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Khatkar, B.S. Thermal, structural and textural properties of amaranth and buckwheat starches. J. Food Sci. Technol. 2018, 55, 5153–5160. [Google Scholar] [CrossRef] [PubMed]
- Han, J.A.; Chung, H.J.; Lim, S.T. Physical and emulsifying properties of OSA-corn dextrin with various manufacturing methods. Food Hydrocoll. 2019, 89, 563–569. [Google Scholar] [CrossRef]
- Javadian, N.; Mohammadi Nafchi, A.; Bolandi, M. The effects of dual modification on functional, microstructural, and thermal properties of tapioca starch. Food Sci. Nutr. 2021, 9, 5467–5476. [Google Scholar] [CrossRef]
- Chen, L.; Ma, R.; Zhang, Z.; Huang, M.; Cai, C.; Zhang, R.; McClements, D.J.; Tian, Y.; Jin, Z. Comprehensive investigation and comparison of surface microstructure of fractionated potato starches. Food Hydrocoll. 2019, 89, 11–19. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Sun, B.; Wang, J.; Tong, Q.; Jin, Z. Rapid, accurate, and simultaneous measurement of water and oil contents in the fried starchy system using low-field NMR. Food Chem. 2017, 233, 525–529. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Chen, L.; Xie, F.; Li, L.; Li, B. Structure and colon-targeted releasing property of resistant octenyl succinate starch. Food Res. Int. 2012, 47, 905–920. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple ultrasound method to obtain starch micro- and nanoparticles from cassava, corn and yam starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, B.; Li, J.; Shu, Z.; Wang, P.; Zeng, X. Structure and properties of octenyl succinic anhydride-modified high-amylose japonica rice starches. Polymers 2021, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
- Djori, A.; Moulai-Mostefa, N. Structural, Physicochemical and Thermal Properties of OSA-modified Waxy Maize Starch. Kem. Ind. Časopis Kemičara Kem. Inženjera Hrvat. 2022, 71, 39–47. [Google Scholar] [CrossRef]
- Guo, J.; Tang, W.; Quek, S.Y.; Liu, Z.; Lu, S.; Tu, K. Evaluation of structural and physicochemical properties of octenyl succinic anhydride modified sweet potato starch with different degrees of substitution. J. Food Sci. 2020, 85, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Castaño, V.D.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Lucas-Aguirre, J.C.; Bello-Pérez, L.A.; Rodríguez-Garcia, M.E. Starch from two unripe plantains and esterified with octenyl succinic anhydride (OSA): Partial characterization. Food Chem. 2020, 315, 126241. [Google Scholar] [CrossRef] [PubMed]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Su, Y.; Sun, M.; Zhao, M.; Xu, B.; Li, J.; Zheng, T. Enhancement of the physicochemical and in vitro release properties of lutein by gelatin/octenyl succinic anhydride (OSA)-modified starch composite as vehicles. Int. J. Food Sci. Technol. 2022, 57, 738–750. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Bello-Perez, L.A. Starch as an emulsions stability: The case of octenyl succinic anhydride (OSA) starch. Curr. Opin. Food Sci. 2017, 13, 78–83. [Google Scholar] [CrossRef]
- Saari, H.; Heravifar, K.; Rayner, M.; Wahlgren, M.; Sjöö, M. Preparation and characterization of starch particles for use in pickering emulsions. Cereal Chem. 2016, 93, 116–124. [Google Scholar] [CrossRef]
- Fonseca-Florido, H.A.; Vázquez-García, H.G.; Méndez-Montealvo, G.; Basilio-Cortés, U.A.; Navarro-Cortés, R.; Rodríguez-Marín, M.L.; Castro-Rosas, J.; Gómez-Aldapa, C.A. Effect of acid hydrolysis and OSA esterification of waxy cassava starch on emulsifying properties in Pickering-type emulsions. LWT Food Sci. Technol. 2018, 91, 258–264. [Google Scholar] [CrossRef]
- Li, Y.T.; Wang, R.S.; Liang, R.H.; Chen, J.; He, X.H.; Chen, R.Y.; Liu, W.; Liu, C.M. Dynamic high-pressure microfluidization assisting octenyl succinic anhydride modification of rice starch. Carbohydr. Polym. 2018, 193, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Singh, H. Interactions between octenyl-succinic-anhydride-modified starches and calcium in oil-in-water emulsions. Food Hydrocoll. 2018, 77, 30–39. [Google Scholar] [CrossRef]
- Chen, A.Z.; Li, Y.; Chau, F.T.; Lau, T.Y.; Hu, J.Y.; Zhao, Z.; Mok, D.K.-w. Microencapsulation of puerarin nanoparticles by poly(l-lactide) in a supercritical CO2 process. Acta Biomater. 2009, 5, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
| Samples | Hydrolysis Time (h) | OSA Content (%) | DS (%) |
|---|---|---|---|
| 2 h H-2% OSA | 2 | 2 | 0.70 ± 0.03 d |
| 4 h H-2% OSA | 4 | 2 | 0.75 ± 0.02 c |
| 6 h H-2% OSA | 6 | 2 | 0.81 ± 0.04 b |
| 8 h H-2% OSA | 8 | 2 | 0.88 ± 0.05 b |
| 12 h H-2% OSA | 12 | 2 | 0.91 ± 0.01 a |
| 24 h H-2% OSA | 24 | 2 | 0.96 ± 0.06 a |
| Samples | Hydrolysis Time (h) | OSA Content (%) | DS (%) |
|---|---|---|---|
| Un H-Un OSA | 0 | 0 | |
| Un-2% OSA | 0 | 2 | 0.67 ± 0.02 d |
| 24 h H-2% OSA | 24 | 2 | 0.96 ± 0.06 c |
| 24 h H-4% OSA | 24 | 4 | 1.23 ± 0.04 b |
| 24 h H-6% OSA | 24 | 6 | 1.64 ± 0.07 a |
| 24 h H-8% OSA | 24 | 8 | 1.80 ± 0.03 a |
| Samples | Emulsification Index | ||
|---|---|---|---|
| 1 d | 8 d | 16 d | |
| Un H-2% OSA | 0.53 ± 0.02 d | 0.48 ± 0.01 d | 0.45 ± 0.00 d |
| 24 h H-2% OSA | 0.92± 0.06 a | 0.91 ± 0.03 a | 0.90 ± 0.04 c |
| 24 h H-4% OSA | 0.93 ± 0.03 a | 0.92 ± 0.04 a | 0.90 ± 0.02 b |
| 24 h H-6% OSA | 0.93 ± 0.01 a | 0.92 ± 0.02 a | 0.91 ± 0.01 a |
| 24 h H-8% OSA | 0.94 ± 0.05 a | 0.93 ± 0.04 a | 0.91 ± 0.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, Z.; Ramzan, R.; Zhang, R.; Zhao, D.; Khalid, N.; Deng, M.; Dong, L.; Aziz, M.; Batool, R.; Zhang, M. Enhanced Bioaccessibility of Microencapsulated Puerarin Delivered by Pickering Emulsions Stabilized with OSA-Modified Hydrolyzed Pueraria montana Starch: In Vitro Release, Storage Stability, and Physicochemical Properties. Foods 2022, 11, 3591. https://doi.org/10.3390/foods11223591
Muhammad Z, Ramzan R, Zhang R, Zhao D, Khalid N, Deng M, Dong L, Aziz M, Batool R, Zhang M. Enhanced Bioaccessibility of Microencapsulated Puerarin Delivered by Pickering Emulsions Stabilized with OSA-Modified Hydrolyzed Pueraria montana Starch: In Vitro Release, Storage Stability, and Physicochemical Properties. Foods. 2022; 11(22):3591. https://doi.org/10.3390/foods11223591
Chicago/Turabian StyleMuhammad, Zafarullah, Rabia Ramzan, Ruifen Zhang, Dong Zhao, Nazia Khalid, Mei Deng, Lihong Dong, Mahwash Aziz, Rizwana Batool, and Mingwei Zhang. 2022. "Enhanced Bioaccessibility of Microencapsulated Puerarin Delivered by Pickering Emulsions Stabilized with OSA-Modified Hydrolyzed Pueraria montana Starch: In Vitro Release, Storage Stability, and Physicochemical Properties" Foods 11, no. 22: 3591. https://doi.org/10.3390/foods11223591
APA StyleMuhammad, Z., Ramzan, R., Zhang, R., Zhao, D., Khalid, N., Deng, M., Dong, L., Aziz, M., Batool, R., & Zhang, M. (2022). Enhanced Bioaccessibility of Microencapsulated Puerarin Delivered by Pickering Emulsions Stabilized with OSA-Modified Hydrolyzed Pueraria montana Starch: In Vitro Release, Storage Stability, and Physicochemical Properties. Foods, 11(22), 3591. https://doi.org/10.3390/foods11223591