Hypoglycemic Effect of Exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on Streptozotocin and High-Fat Diet-Induced Type 2 Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals and Experiment Design
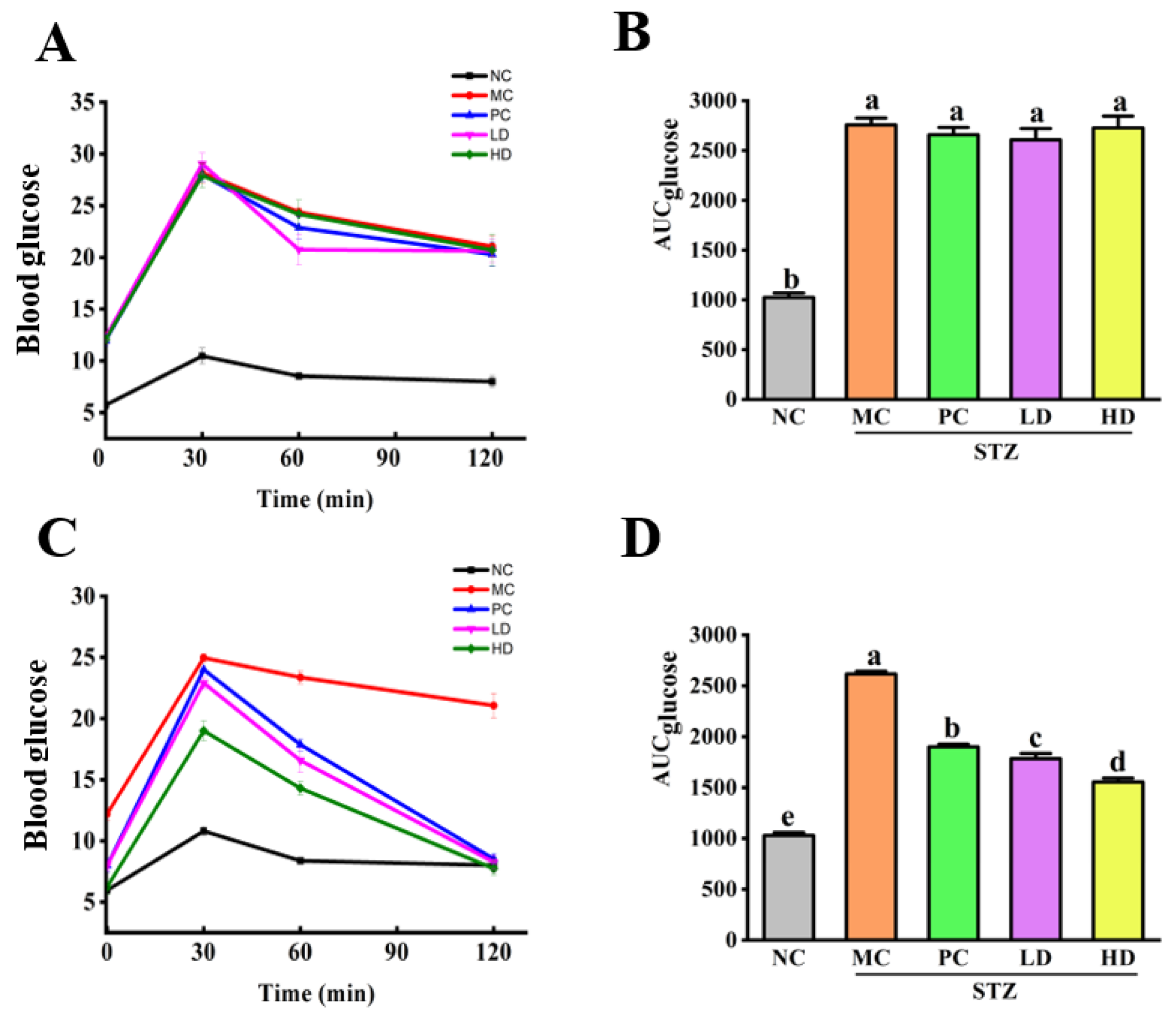
2.3. The Oral Glucose Tolerance Test (OGTT)
2.4. Blood Biochemical Measurement
2.5. Determination of Hepatic Glycogen Synthesis
2.6. Determination of Antioxidant Activity in Hippocampus
2.7. Western Blot Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Effect of EPS103 on FBG, Lipids and the OGTT
3.2. The Effect of EPS103 on HbA1c, LEP, FINS and HOMA-IR
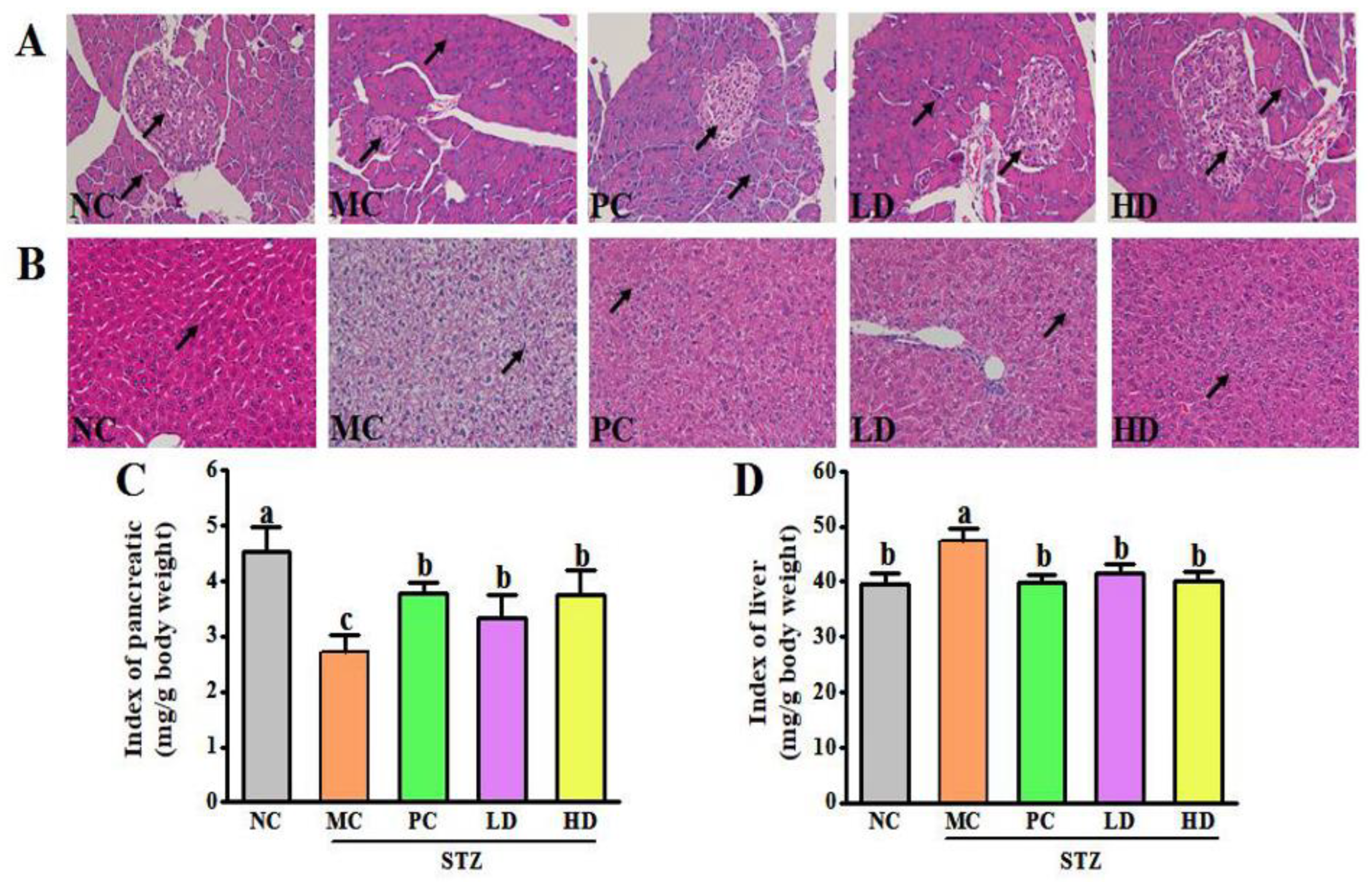
3.3. EPS103 Ameliorates Pancreatic and Hepatic Injury
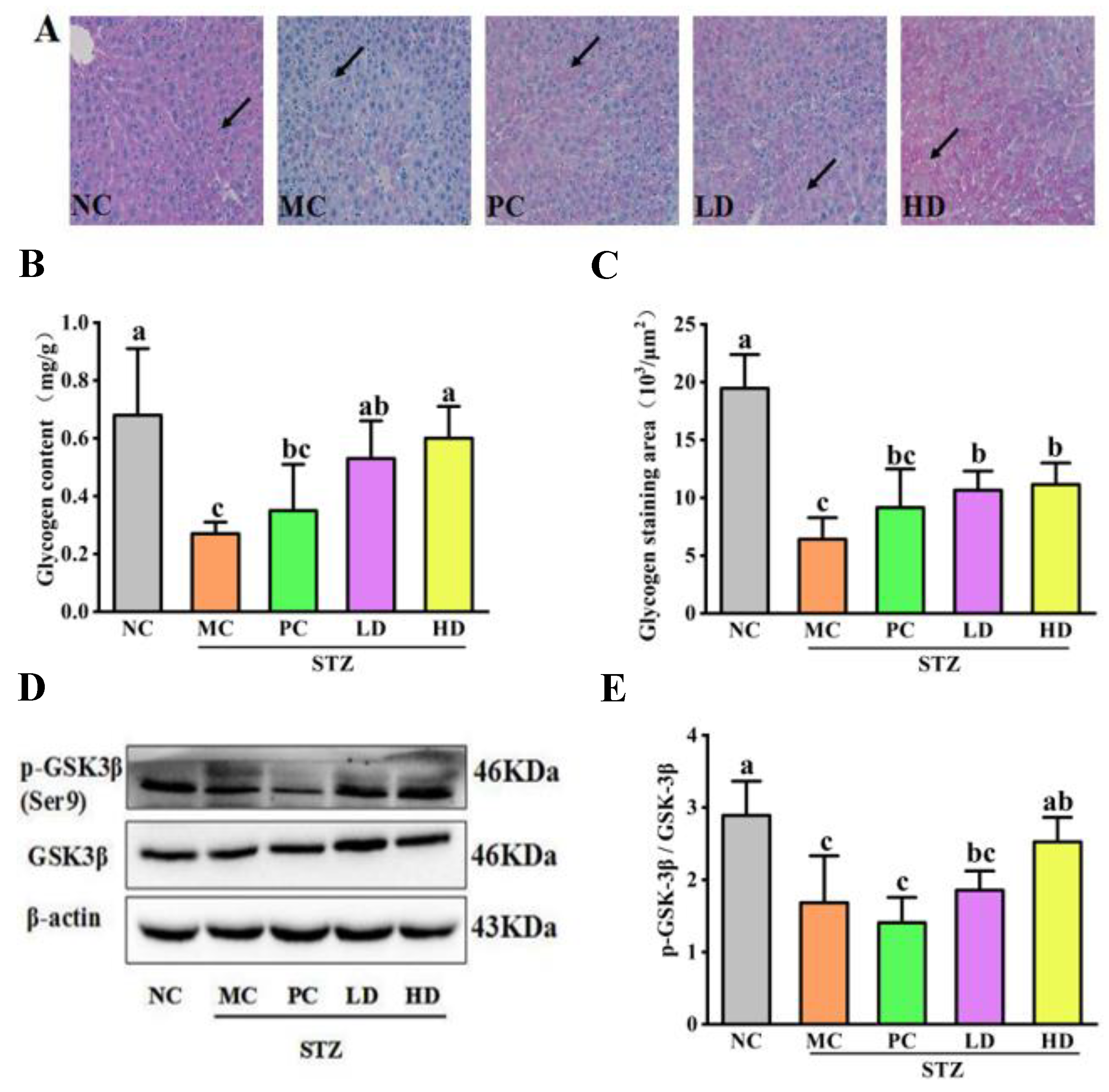
3.4. The Effect of EPS103 on Hepatic Glycogen Synthesis
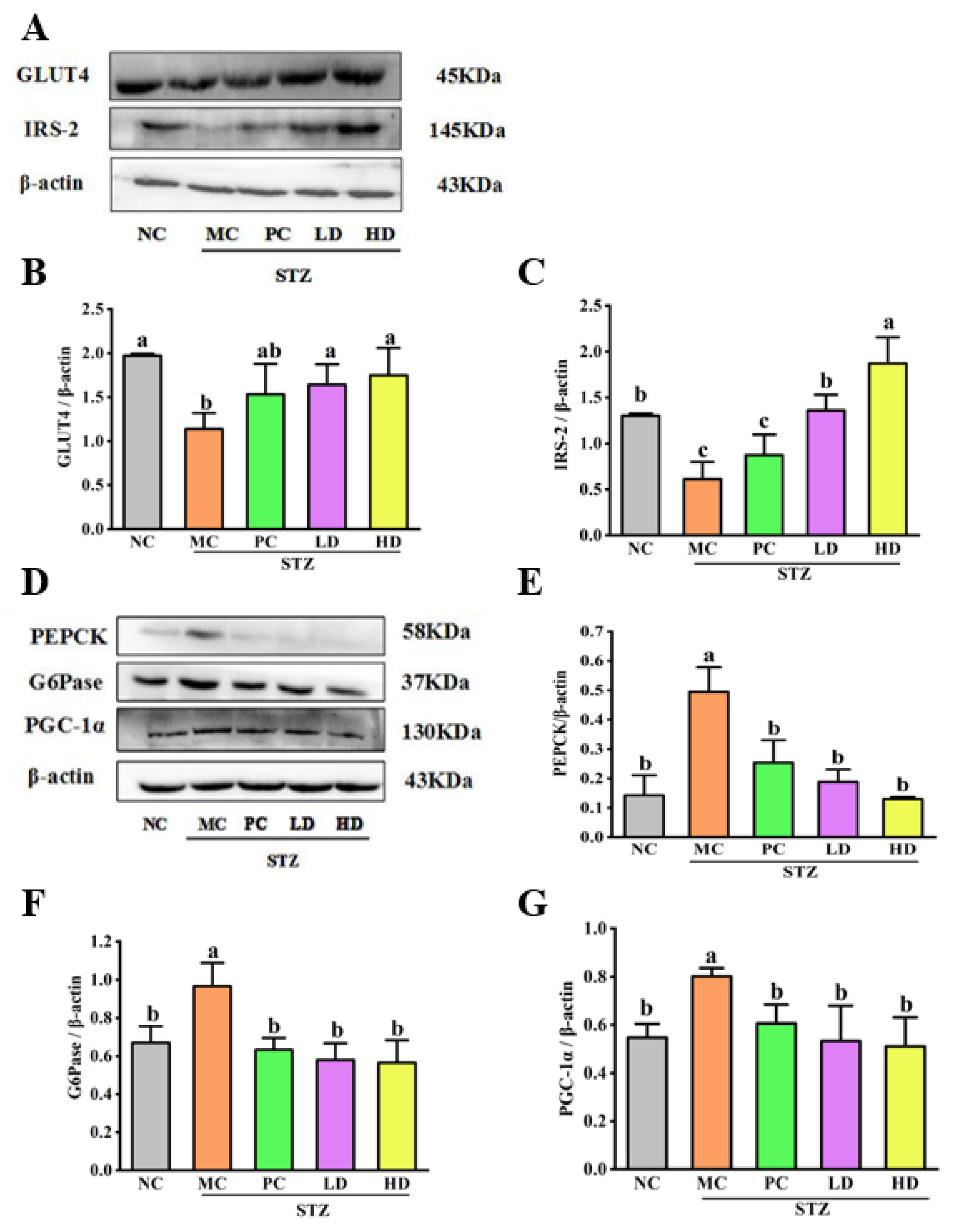
3.5. The Effect of EPS103 on Glucose Uptake
3.6. The Effect of EPS103 on Gluconeogenesis
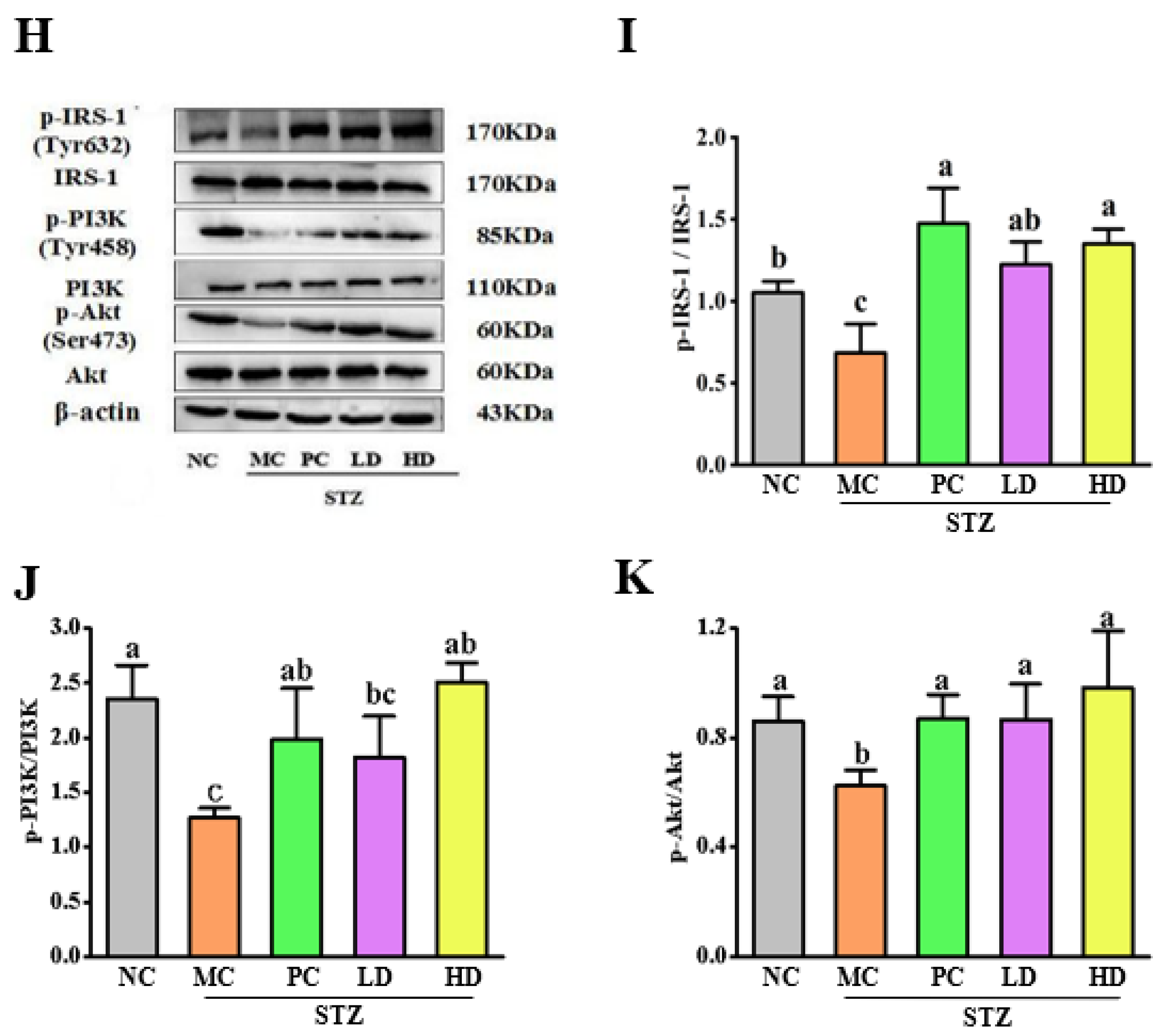
3.7. The Effect of EPS103 on the IRS-1/PI3K/Akt Pathway
3.8. EPS103 Alleviates Brain Nerve Damage of T2DM Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aettiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- la Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buij, R.M. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000, 871, 50–56. [Google Scholar] [CrossRef]
- Perseghin, G.; Regalia, E.; Battezzati, A.; Vergani, S.; Pulvirenti, A.; Terruzzi, I.; Baratti, D.; Bozzetti, F.; Mazzaferro, V.; Luzi, L. Regulation of glucose homeostasis in humans with denervated livers. J. Clin. Investig. 1997, 100, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Ferris, H.A.; Cai, W.K.; Kahn, C.R. Insulin Action in Brain Regulates Systemic Metabolism and Brain Function. Diabetes 2014, 63, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.T.; Tiganis, T. Insulin action in the brain: Roles in energy and glucose homeostasis. J. Neuroendocrinol. 2017, 29, e12513. [Google Scholar] [CrossRef]
- Ribeiro, I.; Ferreira-Neto, H.C.; Antunes, V. Subdiaphragmatic vagus nerve activity and hepatic venous glucose are differentially regulated by the central actions of insulin in Wistar and SHR. Physiol. Rep. 2015, 3, e12381. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.H.; Gao, Y.W.; Li, H.M.; Fang, L.; Liu, C.L.; Liu, X.T.; Min, W.H. Effects of Exopolysaccharides from Lactiplantibacillus plantarum JLAU103 on Intestinal Immune Response, Oxidative Stress, and Microbial Communities in Cyclophosphamide-Induced Immunosuppressed Mice. J. Agric. Food Chem. 2022, 70, 2197–2210. [Google Scholar] [CrossRef]
- Dilna, S.V.; Surya, H.; Aswathy, R.G.; Varsha, K.K.; Sakthikumar, D.N.; Pandeya, A.; Nampoothiria, K.M. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT-Food Sci. Technol. 2015, 64, 1179–1186. [Google Scholar] [CrossRef]
- Zheng, H.H.; Lin, F.X.; Zhu, X.Y.; Zhang, C. An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway. Int. J. Biol. Macromol. 2020, 143, 775–784. [Google Scholar]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food. Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef]
- Wang, Y.F.; Su, N.N.; Hou, G.H.; Li, J.L.; Ye, M. Hypoglycemic and hypolipidemic effects of a polysaccharide from Lachnum YM240 and its derivatives in mice, induced by a high fat diet and low dose STZ. MedChemComm 2017, 8, 964–974. [Google Scholar] [CrossRef]
- Wei, R.; Zhuge, X.; Yue, P.; Liu, M.; Zhu, L.; Liu, J.X.; Xia, C.B. Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression. Open Life Sci. 2020, 15, 311–317. [Google Scholar] [CrossRef]
- Sun, H.Q.; Yu, X.F.; Li, T.; Zhu, Z.Y. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 166, 496–508. [Google Scholar] [CrossRef]
- Liu, B.; Lu, H.; Li, Z.D.; Xiong, X.L.; Gao, L.; Wu, Z.X.; Lu, Y. Aberrant Expression of FBXO2 Disrupts Glucose Homeostasis Through Ubiquitin-Mediated Degradation of Insulin Receptor in Obese Mice. Diabetes 2017, 66, 689–698. [Google Scholar] [CrossRef]
- Ki-Chul, S.; Wild, S.H.; Byrne, C.D. Resolution of fatty liver and risk of incident. Diabetes 2013, 98, 3637–3643. [Google Scholar]
- Day, C.P.; Saksena, S. Non-alcoholic steatohepatitis: Definitions and pathogenesis. J. Gastroen. Hepatol. 2002, 17, S377–S384. [Google Scholar] [CrossRef]
- Knobler, H.; Schattner, A.; Zhornicki, T. Fatty Liver-an additional and treatable feature of the insulin resistance syndrome. QJM-Int. J. Med. 1999, 92, 73–79. [Google Scholar] [CrossRef]
- Mandrup-Poulsen, T.J. Beta-cell apoptosis: Stimuli and signaling. Diabetes 2001, 50, S58–S63. [Google Scholar] [CrossRef]
- Pan, L.H.; Li, X.F.; Wang, M.N.; Zha, X.Q.; Yang, X.F.; Liu, Z.J. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420–427. [Google Scholar] [CrossRef]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.X.; Kim, S.J.; Mehta, H.; Hevener, A.L.; Cabo, R.D.; et al. The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef]
- Meyer, F.; Heilmeyer, L.; Haschke, R.H.; Fischer, E. Control of phosphorylase activity in a muscle glycogen particle. 3. Regulation of phosphorylase phosphatase. J. Biol. 1970, 245, 6657–6663. [Google Scholar]
- Kido, Y.; Burks, D.J.; Withers, D.; Bruning, J.C.; Accili, D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J. Clin. Investig. 2000, 105, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Hatting, M.; Tavares, C.D.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Dey, D.K.; Vij, R.; Meena, S.; Kapila, R.; Kapila, S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats. Microb. Pathog. 2018, 125, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, X.; Huang, X.; Zhao, Y.; Ren, Q.; Hong, Z.; Xia, X. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J. Funct. Foods 2018, 48, 515–524. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Modlin, I.M.; Ballantyne, G. Changes in Insulin Resistance Following Bariatric Surgery: Role of Caloric Restriction and Weight Loss. Obes. Surg. 2005, 15, 462–473. [Google Scholar] [CrossRef]
- Logie, L.; Ruiz-Alcaraz, A.J.; Keane, M.; Woods, Y.L.; Bain, J.; Marquez, R.; Alessi, D.R.; Sutherland, C. Characterization of a Protein Kinase B Inhibitor In Vitro and in Insulin-Treated Liver Cells. Diabetes 2007, 56, 2218–2227. [Google Scholar] [CrossRef][Green Version]
- Jing, W.; Shi, Z.; Zou, T.; Zou, D.M.X.; Yang, H.X.; Zhang, C.P.; Xiang, D.B.; Lin, L.M.; Liu, H.Y.; Fang, D.Y.; et al. A novel recombinant peptide INSR-IgG4Fc (Yiminsu) restores insulin sensitivity in experimental insulin resistance models. Biomed. Pharmacother. 2019, 109, 1276–1286. [Google Scholar]
- Huang, H.Y.; Korivi, M.; Tsai, C.H.; Yang, J.H.; Tsai, Y.C. Supplementation of Lactobacillus plantarum K68 and Fruit-Vegetable Ferment along with High Fat-Fructose Diet Attenuates Metabolic Syndrome in Rats with Insulin Resistance. Evid. Based Complement. Altern. Med. 2013, 2013, 943020. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, F.; He, Y.; Zhang, Q.; Zhang, Y.; Zhou, G.; Yang, H. A novel PTP1B inhibitor extracted from Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food Funct. 2018, 9, 397–406. [Google Scholar] [CrossRef]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Masanori, I.; Yukio, Y.; Yoshimitsu, N.; Osamu, N.; Makoto, M.; Morihiro, M.; Iichiro, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Liu, W.; Baker, S.S.; Baker, R.D.; Lixin, Z. Antioxidant Mechanisms in Nonalcoholic Fatty Liver Disease. Curr. Drug Targets 2015, 16, 1301–1314. [Google Scholar] [CrossRef]
- Yang, F.L.; Wei, Y.X.; Liao, B.Y.; Wei, G.J.; Qin, H.M.; Pang, X.X.; Wang, L.J. Effects of Lycium barbarum Polysaccharide on Endoplasmic Reticulum Stress and Oxidative Stress in Obese Mice. Front. Pharmacol. 2020, 11, 742. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.P.; Qiong, F.; Wei, G.J.; Qin, H.M.; Pang, X.X.; Wang, J.L. Cognitive deficits and Alzheimer-like neuropathological impairments during adolescence in a rat model of type 2 diabetes mellitus. Neural Regen. Res. 2018, 13, 1995–2004. [Google Scholar]
- Morshedi, M.; Valenlia, K.B.; Hosseinifard, E.S.; Shahabi, P.; Abbasi, M.M.; Ghorbani, M.; Barzegari, A.; Sadigh-Eteghad, S.; Saghafi-Asl, M. Beneficial psychological effects of novel psychobiotics in diabetic rats: The interaction among the gut, blood, and amygdala. J. Nutr. Biochem. 2018, 57, 145–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Widmayer, M.A.; Zhang, B.X.; Cui, J.K.; Baskin, D.S. Suppression of post-ischemic-induced fos protein expression by an antisense oligonucleotide to c-fos mRNA leads to increased tissue damage. Brain Res. 1999, 832, 1–2. [Google Scholar] [CrossRef]
- Heijer, T.D.; Vermeer, S.E.; Dijk, E.J.V.; Prins, N.D.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M.B. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003, 46, 1604–1610. [Google Scholar] [CrossRef]


| Groups | Fasting Blood Glucose Level (mmol/L) | ||||||
|---|---|---|---|---|---|---|---|
| Week 2 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | |
| NC | 4.6 ± 0.6 b | 5.3 ± 0.4 c | 5.4 ± 0.8 b | 5.7 ± 0.7 b | 5.4 ± 0.9 c | 5 ± 1 c | 5.8 ± 0.6 c |
| MC | 7.7 ± 0.6 a | 8.7 ± 0.5 b | 12.2 ± 0.7 a | 12.5 ± 0.9 a | 12.8 ± 0.6 a | 13.6 ± 1.1 a | 12 ± 1 a |
| PC | 8.1 ± 0.5 a | 11 ± 1 a | 13.1 ± 0.7 a | 12 ± 1 a | 11 ± 1 b | 8.8 ± 0.8 b | 8 ± 1 b |
| LD | 7 ± 1 a | 8.9 ± 0.8 ab | 12.9 ± 0.7 a | 12 ± 1 a | 11.1 ± 0.9 ab | 9.5 ± 0.7 b | 8 ± 1 b |
| HD | 8 ± 1 a | 10.2 ± 1.1 ab | 13.5 ± 0.9 a | 12.1 ± 0.9 a | 10.6 ± 0.4 b | 8.1 ± 0.8 b | 6.2 ± 0.7 bc |
| Groups | TG (mmol/L) | TC (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) |
|---|---|---|---|---|
| NC | 0.7 ± 0.1 c | 3.7 ± 0.2 c | 0.6 ± 0.1 c | 2.84 ± 0.06 a |
| MC | 1.45 ± 0.07 a | 5.7 ± 0.1 a | 2.0 ± 0.2 a | 1.59 ± 0.09 d |
| PC | 0.76 ± 0.04 bc | 5.0 ± 0.2 b | 1.77 ± 0.05 a | 2.28 ± 0.09 bc |
| LD | 0.9 ± 0.1 b | 5.1 ± 0.1 b | 1.4 ± 0.1 b | 2.12 ± 0.07 c |
| HD | 0.81 ± 0.08 bc | 5.1 ± 0.1 b | 1.36 ± 0.08 b | 2.35 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wang, D.; Fang, L.; Liu, X.; Liu, C.; Zhao, F.; Wu, D.; Wang, X.; Wang, J.; Min, W. Hypoglycemic Effect of Exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on Streptozotocin and High-Fat Diet-Induced Type 2 Diabetic Mice. Foods 2022, 11, 3571. https://doi.org/10.3390/foods11223571
Qi Y, Wang D, Fang L, Liu X, Liu C, Zhao F, Wu D, Wang X, Wang J, Min W. Hypoglycemic Effect of Exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on Streptozotocin and High-Fat Diet-Induced Type 2 Diabetic Mice. Foods. 2022; 11(22):3571. https://doi.org/10.3390/foods11223571
Chicago/Turabian StyleQi, Yuan, Danyang Wang, Li Fang, Xiaoting Liu, Chunlei Liu, Fanrui Zhao, Dan Wu, Xiyan Wang, Ji Wang, and Weihong Min. 2022. "Hypoglycemic Effect of Exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on Streptozotocin and High-Fat Diet-Induced Type 2 Diabetic Mice" Foods 11, no. 22: 3571. https://doi.org/10.3390/foods11223571
APA StyleQi, Y., Wang, D., Fang, L., Liu, X., Liu, C., Zhao, F., Wu, D., Wang, X., Wang, J., & Min, W. (2022). Hypoglycemic Effect of Exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on Streptozotocin and High-Fat Diet-Induced Type 2 Diabetic Mice. Foods, 11(22), 3571. https://doi.org/10.3390/foods11223571







