Anti-Inflammatory and Antioxidant Effects Induced by Allium sativum L. Extracts on an Ex Vivo Experimental Model of Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Garlic Extracts
2.2. High Performance Liquid Chromatography (HPLC)–Diode Array (DAD)–Mass Spectrometry (MS) Analysis
2.3. Headspace Solid-Phase Microextraction-Gas Chromatography–Mass Spectrometry (HS–SPME–GC–MS) Analysis
2.4. Positive-Ion Direct Infusion–Electrospray Ionization–Mass Spectrometry (DI–ESI–MS) Analysis
2.5. Colorimetric Analysis
2.6. HPLC–DAD Analysis
2.7. Cell Lines
2.8. Cell Viability Assay
2.9. Apoptosis Assay
2.10. Ex Vivo Studies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Analyses
3.1.1. HPLC–DAD–MS Analysis
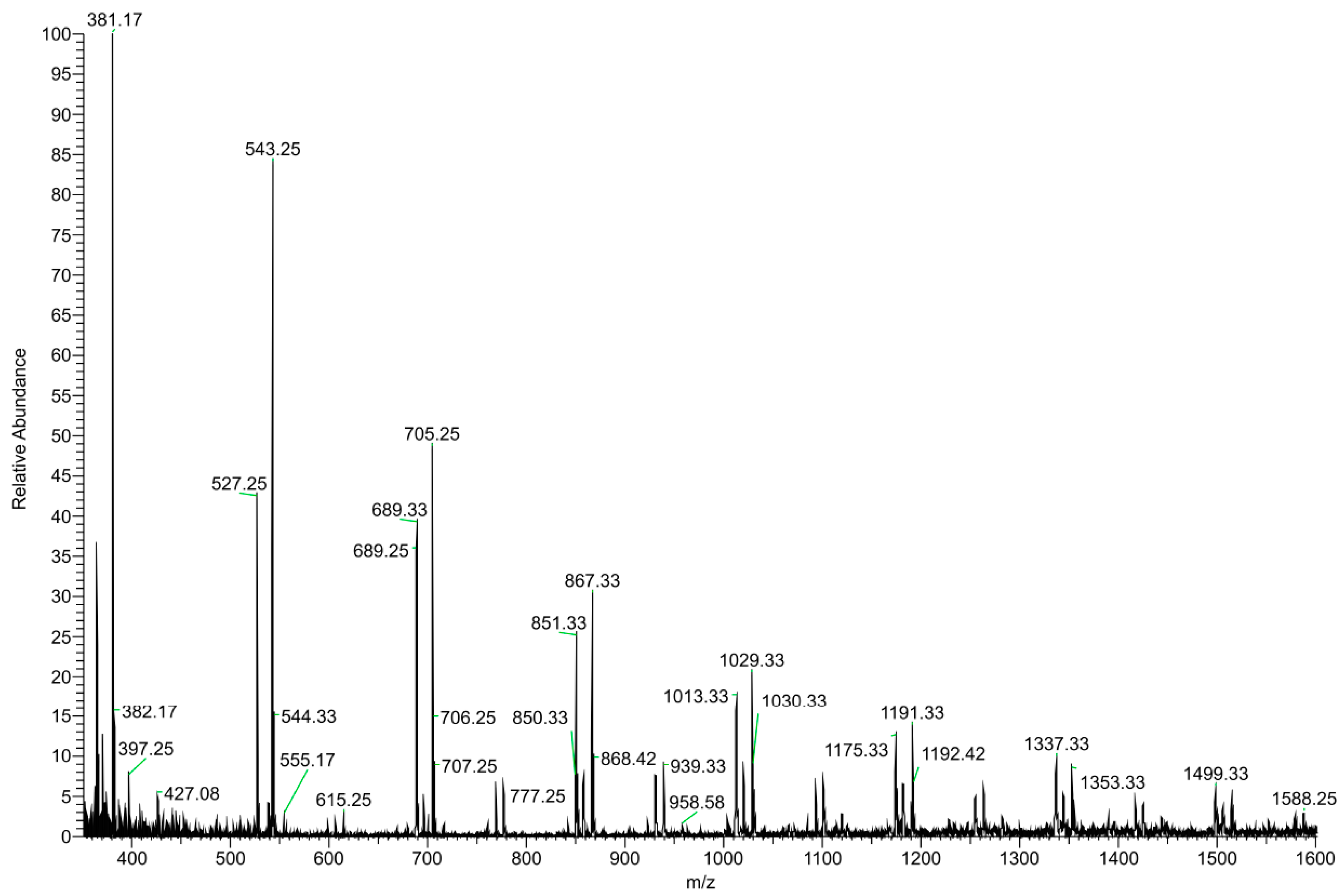
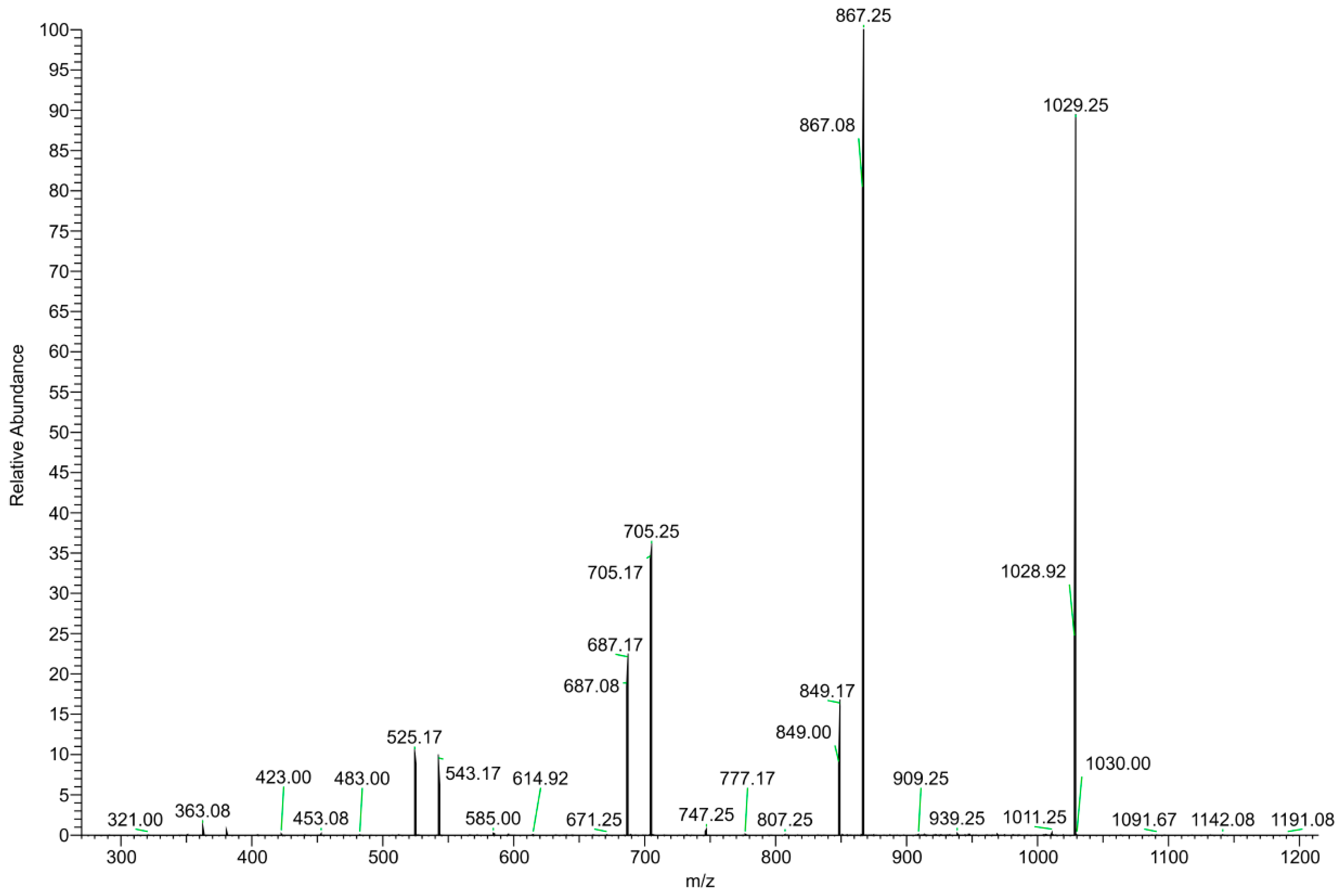
3.1.2. HS–SPME–GC–MS and DI–ESI–MS Analysis
3.1.3. Colorimetric Analysis
3.1.4. HPLC–DAD Analysis
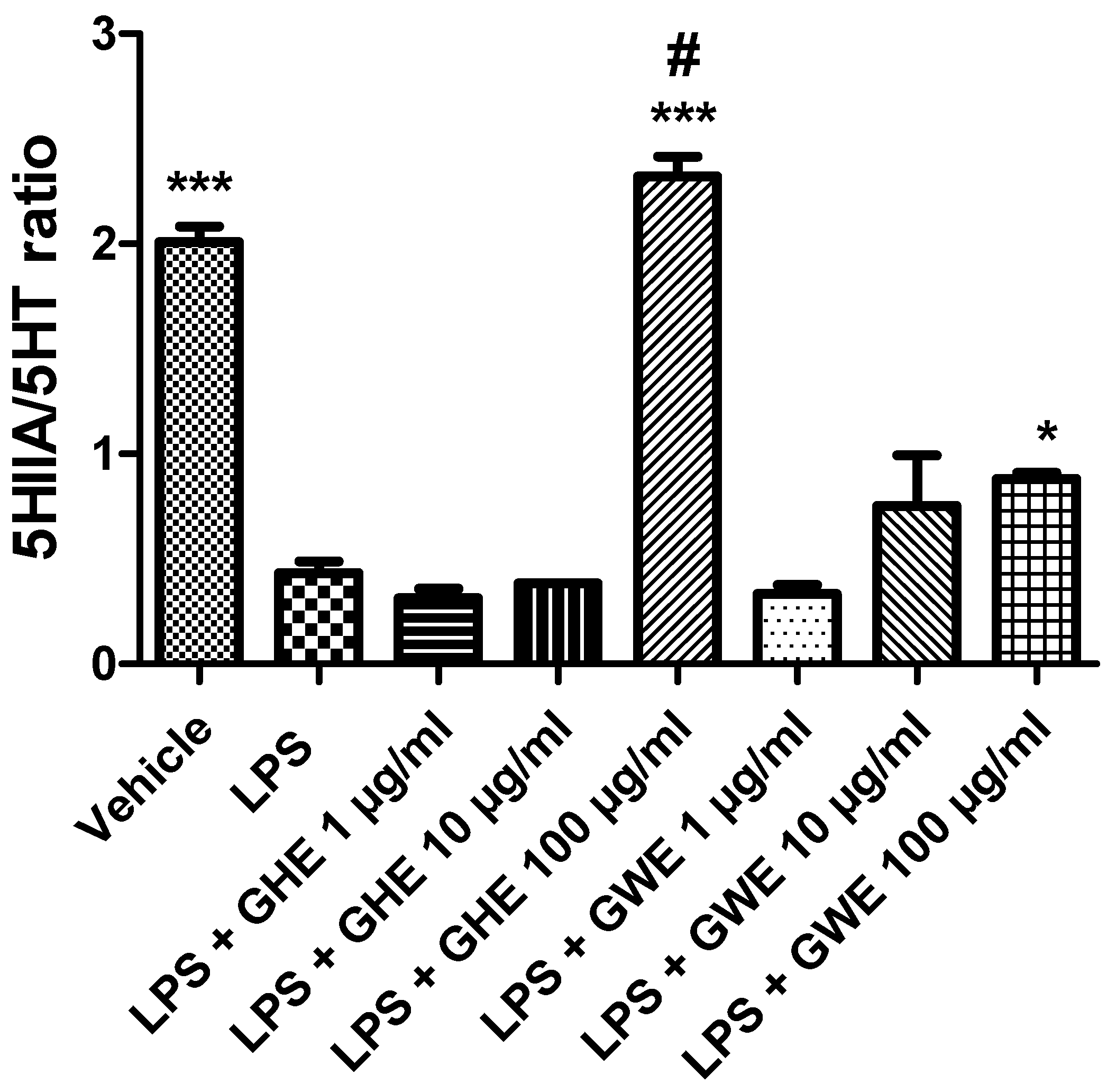
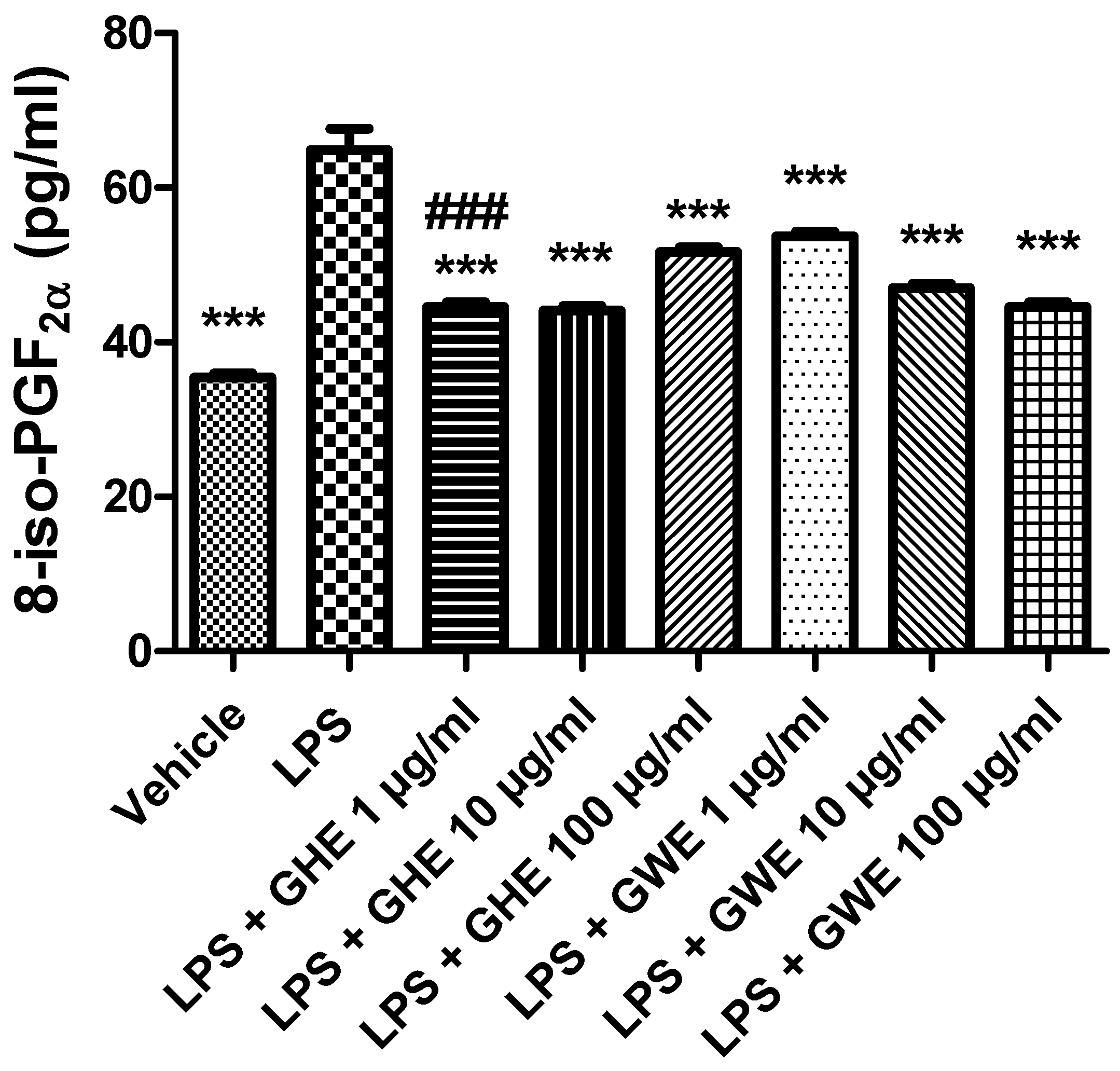
3.2. Toxicological and Pharmacological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gómez-Gómez, L.; Ahrazem, O. Tissue-specific accumulation of sulfur compounds and saponins in different parts of garlic cloves from purple and white ecotypes. Molecules 2017, 22, 1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, M.; Zhao, X.; Li, X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 2018, 56, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Waterer, D.; Schmitz, D. Influence of variety and cultural practices on garlic yields in Saskatchewan. Can. J. Plant Sci. 1994, 74, 611–614. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Nazareno, M.A.; Fusari, C.; Camargo, A. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017, 220, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; El-Hack, M.E.A.; Taha, A.E.; Abd-Elhakim, Y.M.; Devkota, H.P. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Malliaraki, N.; Dimoulios, P.D.; Karmiris, K.; Castanas, E.; Kouroumalis, E.A. Decreased Total and Corrected Antioxidant Capacity in Patients with Inflammatory Bowel Disease. Am. J. Dig. Dis. 2004, 49, 1433–1437. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Achitei, D.; Ciobica, A.; Balan, G.; Gologan, E.; Stanciu, C.; Stefanescu, G. Different Profile of Peripheral Antioxidant Enzymes and Lipid Peroxidation in Active and Non-active Inflammatory Bowel Disease Patients. Am. J. Dig. Dis. 2013, 58, 1244–1249. [Google Scholar] [CrossRef]
- Chung, H.-L.; Yue, G.G.-L.; To, K.-F.; Su, Y.-L.; Huang, Y.; Ko, W.-H. Effect of Scutellariae Radix extract on experimental dextran-sulfate sodium-induced colitis in rats. World J. Gastroenterol. 2007, 13, 5605. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, L.; Joubert-Zakeyh, J.; Texier, O.; Lamaison, J.-L.; Vasson, M.-P.; Felgines, C. Aloysia triphylla infusion protects rats against dextran sulfate sodium-induced colonic damage. J. Sci. Food Agric. 2011, 92, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.E.; Abo-Salem, O.M.; El-Sayed, E.-S.M.; Taha, E.I.; El-Halawany, N. L-arginine augments the antioxidant effect of garlic against acetic acid-induced ulcerative colitis in rats. Pak. J. Pharm. Sci. 2009, 22, 373–380. [Google Scholar] [PubMed]
- Kuo, C.H.; Lee, S.H.; Chen, K.M.; Lii, C.K.; Liu, C.T. Effect of garlic oil on neutrophil infiltration in the small intestine of endotoxin-injected rats and its association with levels of soluble and cellular adhesion molecules. J. Agric. Food Chem. 2011, 59, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Balaha, M.; Kandeel, S.; Elwan, W. Garlic oil inhibits dextran sodium sulfate-induced ulcerative colitis in rats. Life Sci. 2016, 146, 40–51. [Google Scholar] [CrossRef]
- Tanrıkulu, Y.; Şen Tanrıkulu, C.; Kılınç, F.; Can, M.; Köktürk, F. Effects of garlic oil (allium sativum) on acetic acid-induced colitis in rats: Garlic oil and experimental colitis. Turk. J. Trauma Emerg. Surg. 2020, 26, 503–508. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Ronci, M.; Menghini, L.; Brunetti, L.; Leone, S.; Tirillini, B.; Angelini, P.; Covino, S.; Venanzoni, R.; et al. Multidirectional Pharma-Toxicological Study on Harpagophytum procumbens DC. ex Meisn.: An IBD-Focused Investigation. Antioxidants 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, R.; Mottese, A.F.; Bua, G.D.; Salvo, A.; Mallamace, D.; Corsaro, C.; Vasi, S.; Giofrè, S.V.; Alfa, M.; Cicero, N.; et al. Statistical Analysis of Mineral Concentration for the Geographic Identification of Garlic Samples from Sicily (Italy), Tunisia and Spain. Foods 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of allicin determined by chemical and biological assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and chemical stability of garlic-derived allicin. J. Agric. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef] [PubMed]
- Florio, R.; De Lellis, L.; Veschi, S.; Verginelli, F.; di Giacomo, V.; Gallorini, M.; Perconti, S.; Sanna, M.; Mariani-Costantini, R.; Natale, A.; et al. Effects of dichloroacetate as single agent or in combination with GW6471 and metformin in paraganglioma cells. Sci. Rep. 2018, 8, 13610. [Google Scholar] [CrossRef] [PubMed]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Menghini, L.; Ferrante, C.; Di Cesare Mannelli, L.; Ghelardini, C.; Brunetti, L.; Leone, S. Protective Effects Induced by Two Polyphenolic Liquid Complexes from Olive (Olea europaea, mainly Cultivar Coratina) Pressing Juice in Rat Isolated Tissues Challenged with LPS. Molecules 2019, 24, 3002. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Micheli, L.; Chiavaroli, A.; Libero, M.L.; Orlando, G.; Menghini, L.; Acquaviva, A.; Di Simone, S.; Ferrante, C.; Ghelardini, C.; et al. Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea europaea, Mainly Cultivar coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis. Nutrients 2022, 14, 1487. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Marconi, G.D.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; Schally, A.V.; et al. Antinflammatory, antioxidant, and behavioral effects induced by administration of growth hormone-releasing hormone analogs in mice. Sci. Rep. 2020, 10, 4850. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Di Valerio, V.; Veschi, S.; Orlando, G.; Ferrante, C.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; et al. Protective effects of growth hormone-releasing hormone analogs in DSS-induced colitis in mice. Sci. Rep. 2021, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Veschi, S.; Cama, A.; Marconi, G.D.; Diomede, F.; Gesmundo, I.; Granata, R.; et al. Effects of growth hormone-releasing hormone receptor antagonist MIA-602 in mice with emotional disorders: A potential treatment for PTSD. Mol. Psychiatry 2021, 26, 7465–7474. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Veschi, S.; Di Valerio, V.; Lattanzio, R.; Orlando, G.; Ferrante, C.; Gesmundo, I.; Granata, R.; Cai, R.; et al. Antagonist of growth hormone-releasing hormone MIA-690 attenuates the progression and inhibits growth of colorectal cancer in mice. Biomed. Pharmacother. 2022, 146, 112554. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Ferrante, C.; Menghini, L.; Orlando, G.; Brunetti, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Leporini, L.; et al. New pharmacological targets of three Asphodeline species using in vitro and ex vivo models of inflammation and oxidative stress. Int. J. Environ. Health Res. 2019, 29, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Sivam, G.P. Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr. 2001, 131, 1106S–1108S. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Altamirano, J.C.; Camargo, A.B. Multi-phytochemical determination of polar and non-polar garlic bioactive compounds in different food and nutraceutical preparations. Food Chem. 2021, 337, 127648. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Zhao, R.; Zhao, M.; Cui, X.; Xu, Y.; Qiao, X. In vitro Prebiotic Properties of Garlic Polysaccharides and Its Oligosaccharide Mixtures Obtained by Acid Hydrolysis. Front. Nutr. 2021, 8, 798450. [Google Scholar] [CrossRef]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kim, J.K.; Kim, H.H.; Lee, S.Y.; Park, N.I.; Park, S.U. Carotenoid accumulation and characterization of cDNAs encoding phytoene synthase and phytoene desaturase in garlic (Allium sativum). J. Agric. Food Chem. 2011, 59, 5412–5417. [Google Scholar] [CrossRef]
- Hong, S.I.; Kim, D.M. Storage quality of chopped garlic as influenced by organic acids and high-pressure treatment. J. Sci. Food Agric. 2001, 81, 397–403. [Google Scholar] [CrossRef]
- Liu, P.; Lu, X.; Li, N.; Zheng, Z.; Zhao, R.; Tang, X.; Qiao, X. Effects and mechanism of free amino acids on browning in the processing of black garlic. J. Sci. Food Agric. 2019, 99, 4670–4676. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ryu, K.; Yoshida, J.; Ide, N.; Kodera, Y.; Sasaoka, T.; Rosen, R.T. Identification of six phenylpropanoids from garlic skin as major antioxidants. J. Agric. Food Chem. 2003, 51, 7313–7317. [Google Scholar] [CrossRef]
- Eghdami, A.; Sohi, S.M.H.; Asli, D.E.; Houshmandfar, A. Antioxidant activity of methanolic and hydroalcohlic extracts of garlic plant. Adv. Environ. Biol. 2011, 5, 1575–1578. [Google Scholar]
- Dethier, B.; Laloux, M.; Hanon, E.; Nott, K.; Heuskin, S.; Wathelet, J.P. Analysis of the diastereoisomers of alliin by HPLC. Talanta 2012, 101, 447–452. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of nitrogen and sulfur fertilization on the alliin content of onions and garlic. J. Plant Nutr. 2005, 27, 1827–1839. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Bouguen, G.; Chevaux, J.B.; Peyrin-Biroulet, L. Recent advances in cytokines: Therapeutic implications for inflammatory bowel diseases. World J. Gastroenterol. 2011, 17, 547–556. [Google Scholar] [CrossRef]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-kB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hodge, G.; Hodge, S.; Han, P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry 2002, 48, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Ryu, J.H.; Kang, M.J.; Hwang, C.R.; Han, J.; Kang, D. Short-term heating reduces the anti-inflammatory effects of fresh raw garlic extracts on the LPS-induced production of NO and pro-inflammatory cytokines by downregulating allicin activity in RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 545–551. [Google Scholar] [CrossRef]
- Dey, I.; Lejeune, M.; Chadee, K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br. J. Pharmacol. 2006, 149, 611–623. [Google Scholar] [CrossRef]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, D.Y.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, J.; Jiang, B.; Miao, M. Resveratrol and inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1403, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A review of the protective effects of chlorogenic acid against different chemicals. J. Food Biochem. 2022, 46, e14254. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef]
- Duan, L.; Cheng, S.; Li, L.; Liu, Y.; Wang, D.; Liu, G. Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 684486. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, A.; Zhang, Z.; Yu, Z.; Liu, Y.; Peng, S.; Wu, L.; Qin, H.; Wang, W. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-κB pathway. Pharmacol. Rep. 2016, 68, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Wang, J.; Le, T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-kappaB, attenuates bacterial peptidoglycan polysaccharide induced colitis in rats. J. Pharmacol. Exp. Ther. 2001, 299, 915–920. [Google Scholar]
- Liu, S.H.; Lu, T.H.; Su, C.C.; Lay, I.S.; Lin, H.Y.; Fang, K.M.; Ho, T.J.; Chen, K.L.; Su, Y.C.; Chiang, W.C.; et al. Lotus leaf (Nelumbo nucifera) and its active constituents prevent inflammatory responses in macrophages via JNK/NF-κB signaling pathway. Am. J. Chin. Med. 2014, 42, 869–889. [Google Scholar] [CrossRef]
- Sunil, M.A.; Sunitha, V.S.; Santhakumaran, P.; Mohan, M.C.; Jose, M.S.; Radhakrishnan, E.K.; Mathew, J. Protective effect of (+)-catechin against lipopolysaccharide-induced inflammatory response in RAW 264.7 cells through downregulation of NF-κB and p38 MAPK. Inflammopharmacology 2021, 29, 1139–1155. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. (Lond.) 2008, 5, 17. [Google Scholar] [CrossRef]
- Lim, R.; Barker, G.; Wall, C.A.; Lappas, M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol. Hum. Reprod. 2013, 19, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, S.C.; Senthil Kumar, K.J.; Yu, K.N.; Lee Chao, P.D.; Tsai, S.Y.; Hou, Y.C.; Hseu, Y.C. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J. Agric. Food Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.D.; Mahoney, C.R.; Linden, D.R.; Sampson, J.E.; Chen, J.; Blaszyk, H.; Crowell, M.D.; Sharkey, K.A.; Gershon, M.D.; Mawe, G.M.; et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004, 126, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Minderhoud, I.M.; Oldenburg, B.; Schipper, M.E.I.; ter Linde, J.J.M.; Samsom, M. Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin. Gastroenterol. Hepatol. 2007, 5, 714–720. [Google Scholar] [CrossRef]
- Stoyanova, I.I.; Gulubova, M.V. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002, 104, 185–192. [Google Scholar] [CrossRef]
- Bearcroft, C.P.; Perrett, D.; Farthing, M.J.G. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: A pilot study. Gut 1998, 42, 42–46. [Google Scholar] [CrossRef]
- Spiller, R.C.; Jenkins, D.; Thornley, J.P.; Hebden, J.M.; Wright, T.; Skinner, M.; Neal, K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef]
- Chen, M.; Gao, L.; Chen, P.; Feng, D.; Jiang, Y.; Chang, Y.; Jin, J.; Chu, F.F.; Gao, Q. Serotonin-Exacerbated DSS-Induced Colitis Is Associated with Increase in MMP-3 and MMP-9 Expression in the Mouse Colon. Mediat. Inflamm. 2016, 2016, 5359768. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Orexigenic effects of omentin-1 related to decreased CART and CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides 2013, 44, 66–74. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides 2014, 51, 115–121. [Google Scholar] [CrossRef]
- Lee, J.; Chang, C.; Liu, I.; Chi, T.; Yu, H.; Cheng, J. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Ronci, M.; Orlando, G.; Di Simone, S.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Politi, M.; Tirillini, B.; et al. Protective effects induced by alcoholic Phlomis fruticosa and Phlomis herba-venti extracts in isolated rat colon: Focus on antioxidant, anti-inflammatory, and antimicrobial activities in vitro. Phytother. Res. 2019, 33, 2387–2400. [Google Scholar] [CrossRef] [PubMed]
- Batshaw, M.L.; Hyman, S.L.; Coyle, J.T.; Robinson, M.B.; Qureshi, I.A.; Mellits, E.D.; Quaskey, S. Effect of sodium benzoate and sodium phenylacetate on brain serotonin turnover in the ornithine transcarbamylase-deficient sparse-fur mouse. Pediatr. Res. 1988, 23, 368–374. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef]
- Praticò, D.; Lee, V.M.Y.; Trojanoswki, J.Q.; Rokach, J.; FitzGerald, G.A. Increased F2-isoprostanes in Alzheimer’s disease: Evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998, 12, 1777–1783. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef]



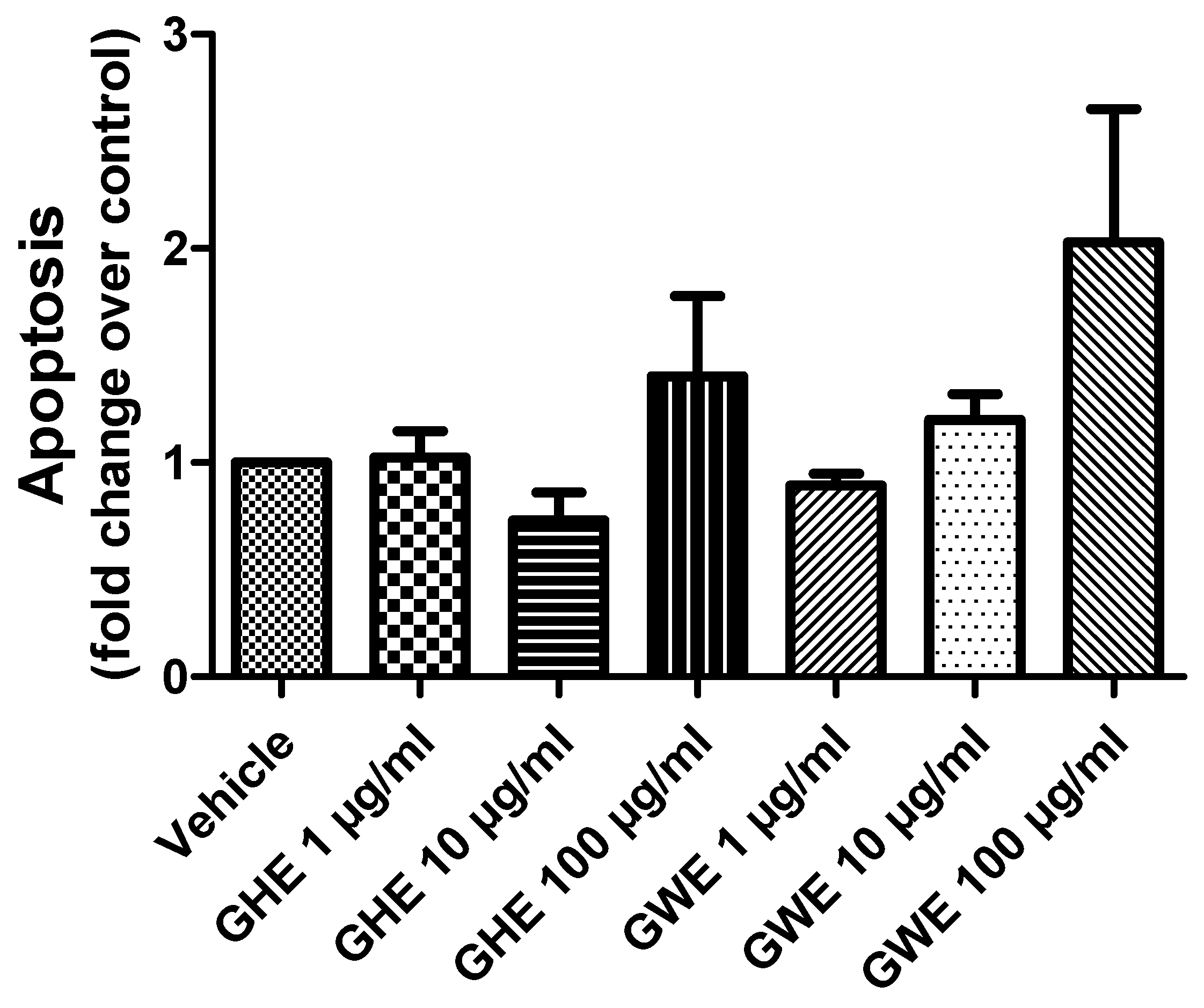

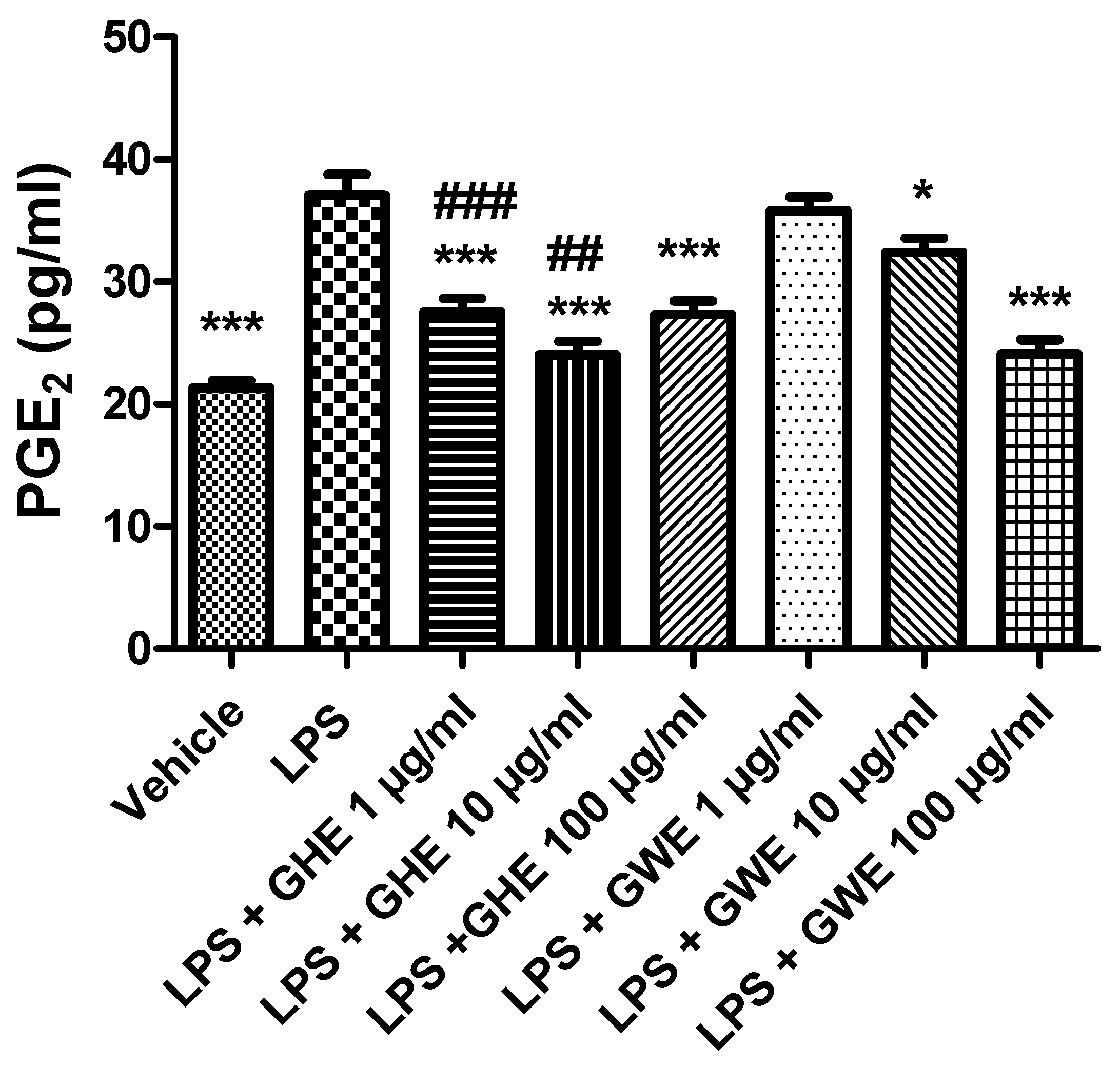
| Compound | GHE | GWE |
|---|---|---|
| Gallic acid | 17.21 ± 0.17 | 16.88 ± 0.12 |
| 3-Hydroxytyrosol | 10.13 ± 0.15 *** | 12.86 ± 0.22 |
| Caftaric acid | 0.93 ± 0.06 | n.d. |
| Catechin | 37.40 ± 1.25 *** | 21.38 ± 0.72 |
| 4-Hydroxybenzoic acid | n.d. | n.d. |
| Loganic acid | n.d. | n.d. |
| Chlorogenic acid | 13.54 ± 0.72 | 12.14 ± 0.53 |
| Vanillic acid | n.d. | n.d. |
| Caffeic acid | 10.91 ± 0.95 | 10.19 ± 0.58 |
| Epicatechin | 8.94 ± 0.23 * | 7.23 ± 0.40 |
| Syringaldehyde | 9.53 ± 0.12 | 9.54 ± 0.23 |
| p-Coumaric acid | 13.33 ± 0.17 | 12.73 ± 0.15 |
| t-Ferulic acid | 15.82 ± 0.26 ** | 13.43 ± 0.21 |
| Benzoic acid | 22.66 ± 0.35 * | 21.51 ± 0.18 |
| Rutin | 13.68 ± 0.22 | 13.08 ± 0.23 |
| Resveratrol | 16.18 ± 0.27 ** | 13.97 ± 0.15 |
| t-Cinnamic acid | 11.83 ± 0.09 | 11.59 ± 0.12 |
| Quercetin | 15.86 ± 0.20 * | 14.76 ± 0.21 |
| Naringenin | 17.93 ± 0.21 * | 16.02 ± 0.37 |
| Hesperetin | 17.06 ± 0.58 ** | 12.72 ± 0.31 |
| Kaempferol | n.d. | n.d. |
| Carvacrol | n.d. | n.d. |
| Thymol | n.d. | n.d. |
| Flavone | 20.43 ± 0.15 | 19.83 ± 0.19 |
| 3-Hydroxyflavone | 10.27 ± 0.29 ** | 13.46 ± 0.25 |
| Emodin | n.d. | n.d. |
| Compound | Class | Area% | RI | RIL a |
|---|---|---|---|---|
| 2-Propenal b | Aldehyde | 1.89 | ||
| Heptane b | Other | 0.33 | ||
| Diallyl sulfide | SCC | 0.43 | 864 | 857 |
| Methyl allyl disulfide | SCC | 1.47 | 927 | 919 |
| Methoxy phenyl oxime b | Other | 5.72 | 943 | |
| p-cymene | Monoterpene | 0.47 | 1041 | 1025 |
| Allyl disulfide | SCC | 9.52 | 1088 | 1090 |
| 3-Allyl-thio-propionic acid | SCC | 1.70 | 1107 | 1093 |
| Linalyl anthranilate | Monoterpenoid | 0.95 | 1110 | 1104 |
| Nonanal | Aldehyde | 1.16 | 1113 | 1102 |
| Methyl 2-propenyl trisulfide | SCC | 2.51 | 1146 | 1142 |
| Isoborneol | Monoterpenoid | 0.44 | 1178 | 1165 |
| Terpinen-4-ol | Monoterpenoid | 0.55 | 1187 | 1184 |
| 3-Vinyl-1,2-dithiacyclohex-4-ene | SCC | 3.18 | 1195 | 1191 |
| Alpha-terpineol | Monoterpenoid | 0.27 | 1203 | 1207 |
| Decanal | Aldehyde | 0.63 | 1214 | 1208 |
| 3-Vinyl-1,2-dithiacyclohex-5-ene | SCC | 3.96 | 1222 | 1224 |
| Thymol | Monoterpenoid | 22.4 | 1310 | 1293 |
| Carvacrol | Monoterpenoid | 36.1 | 1319 | 1317 |
| 7 unknown compounds | 6.32 | |||
| Class | ||||
| Monoterpene and derivatives | 61.2 | |||
| SCC | 22.8 | |||
| Aldehyde | 3.68 | |||
| Other | 6.05 |
| m/z | Compound | Charge | |
|---|---|---|---|
| Positive mode | |||
| 163 | Allicin | H+ | |
| 175 | Arginine | H+ | |
| 214 | N-butylbenzene sulfonamide | H+ | |
| DP | |||
| 365 | Disaccharide | Na+ | |
| 381 | Disaccharide | K+ | |
| 527 | Trisaccharide | 3 | Na+ |
| 543 | Trisaccharide | 3 | K+ |
| 689 | Oligosaccharide | 4 | Na+ |
| 705 | Oligosaccharide | 4 | K+ |
| 851 | Oligosaccharide | 5 | Na+ |
| 867 | Oligosaccharide | 5 | K+ |
| 1013 | Oligosaccharide | 6 | Na+ |
| 1029 | Oligosaccharide | 6 | K+ |
| 1175 | Oligosaccharide | 7 | Na+ |
| 1191 | Oligosaccharide | 7 | K+ |
| 1337 | Oligosaccharide | 8 | Na+ |
| 1353 | Oligosaccharide | 8 | K+ |
| 1449 | Oligosaccharide | 9 | Na+ |
| 1515 | Oligosaccharide | 9 | K+ |
| 1661 | Oligosaccharide | 10 | Na+ |
| 1677 | Oligosaccharide | 10 | K+ |
| 1823 | Oligosaccharide | 11 | Na+ |
| 1839 | Oligosaccharide | 11 | K+ |
| Negative mode | |||
| 191 | Citric acid | [M-H]− | |
| 289 | Catechin | [M-H]− | |
| L* | a* | b* | c* | h° | DE° | |
|---|---|---|---|---|---|---|
| GP t° | 90.15 | 0.47 | 16.02 | 16.03 | 88.32 | - |
| GP t12m | 78.55 | 1.11 | 20.17 | 20.20 | 86.85 | 12.34 a |
| GWE t° | 76.41 | −1.10 | 16.83 | 16.87 | 93.74 | 13.85 a |
| GWE t12m | 65.00 | −2.87 | 28.00 | 27.95 | 95.90 | 28.06 a–16.06 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recinella, L.; Gorica, E.; Chiavaroli, A.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Martelli, A.; Calderone, V.; Veschi, S.; et al. Anti-Inflammatory and Antioxidant Effects Induced by Allium sativum L. Extracts on an Ex Vivo Experimental Model of Ulcerative Colitis. Foods 2022, 11, 3559. https://doi.org/10.3390/foods11223559
Recinella L, Gorica E, Chiavaroli A, Fraschetti C, Filippi A, Cesa S, Cairone F, Martelli A, Calderone V, Veschi S, et al. Anti-Inflammatory and Antioxidant Effects Induced by Allium sativum L. Extracts on an Ex Vivo Experimental Model of Ulcerative Colitis. Foods. 2022; 11(22):3559. https://doi.org/10.3390/foods11223559
Chicago/Turabian StyleRecinella, Lucia, Era Gorica, Annalisa Chiavaroli, Caterina Fraschetti, Antonello Filippi, Stefania Cesa, Francesco Cairone, Alma Martelli, Vincenzo Calderone, Serena Veschi, and et al. 2022. "Anti-Inflammatory and Antioxidant Effects Induced by Allium sativum L. Extracts on an Ex Vivo Experimental Model of Ulcerative Colitis" Foods 11, no. 22: 3559. https://doi.org/10.3390/foods11223559
APA StyleRecinella, L., Gorica, E., Chiavaroli, A., Fraschetti, C., Filippi, A., Cesa, S., Cairone, F., Martelli, A., Calderone, V., Veschi, S., Lanuti, P., Cama, A., Orlando, G., Ferrante, C., Menghini, L., Di Simone, S. C., Acquaviva, A., Libero, M. L., Nilofar, ... Leone, S. (2022). Anti-Inflammatory and Antioxidant Effects Induced by Allium sativum L. Extracts on an Ex Vivo Experimental Model of Ulcerative Colitis. Foods, 11(22), 3559. https://doi.org/10.3390/foods11223559


















