Effect of Heat–Moisture Treatment on the Physicochemical Properties, Structure, Morphology, and Starch Digestibility of Highland Barley (Hordeum vulgare L. var. nudum Hook. f) Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Pasting Properties
2.4. Thermal Properties
2.5. Rheological Characteristics
2.6. Fourier-Transform Infrared Spectroscopy
2.7. X-ray Diffraction
2.8. Scanning Electron Microscopy
2.9. Confocal Laser Scanning Microscopy
2.10. In Vitro Digestibility of Starch
2.11. Statistical Analysis
3. Results and Discussion
3.1. Pasting Properties of Native and HMT HB Flour
3.2. Thermal Properties
3.3. Rheological Properties
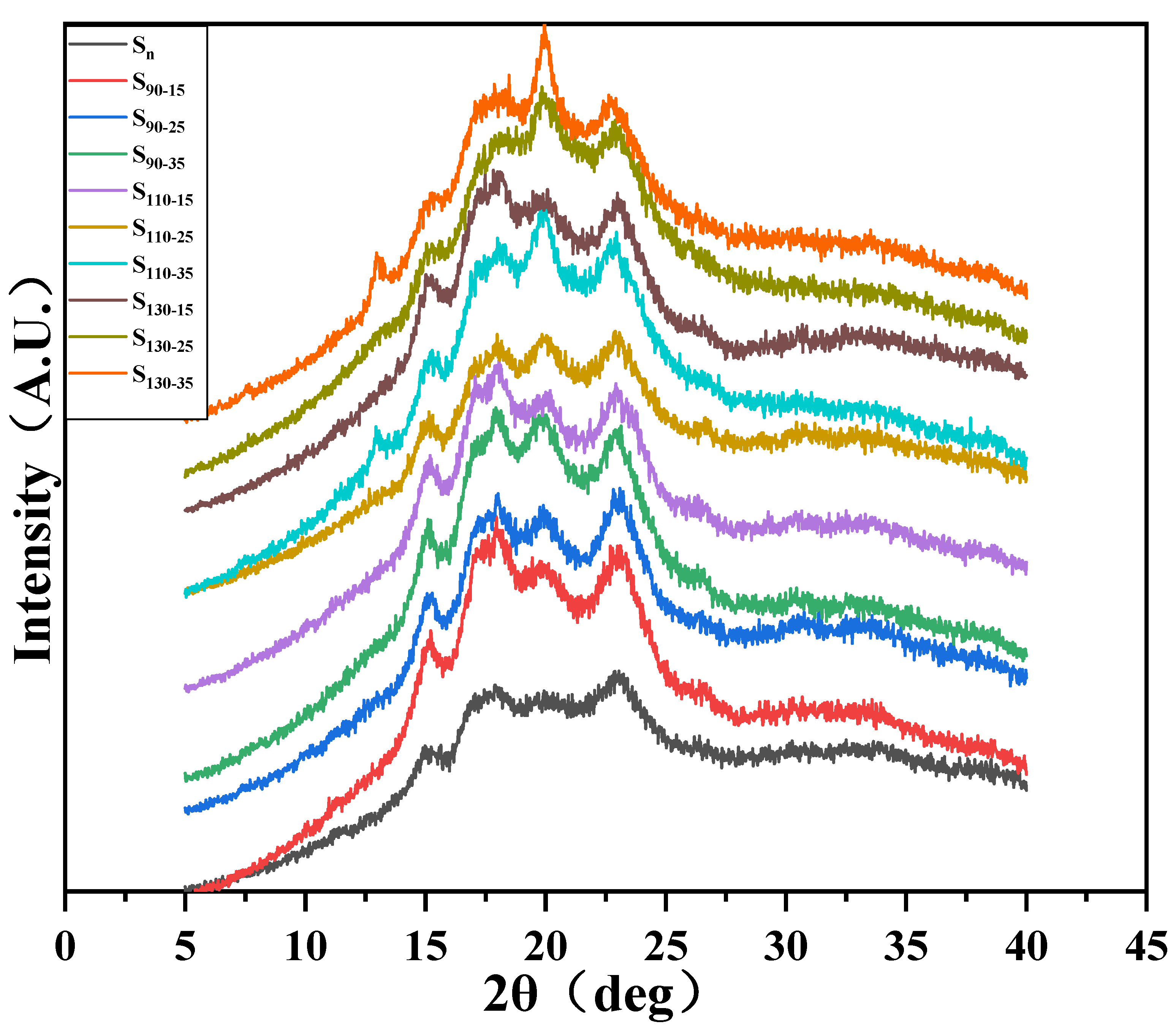
3.4. XRD Analysis
3.5. FTIR Spectrum Analysis
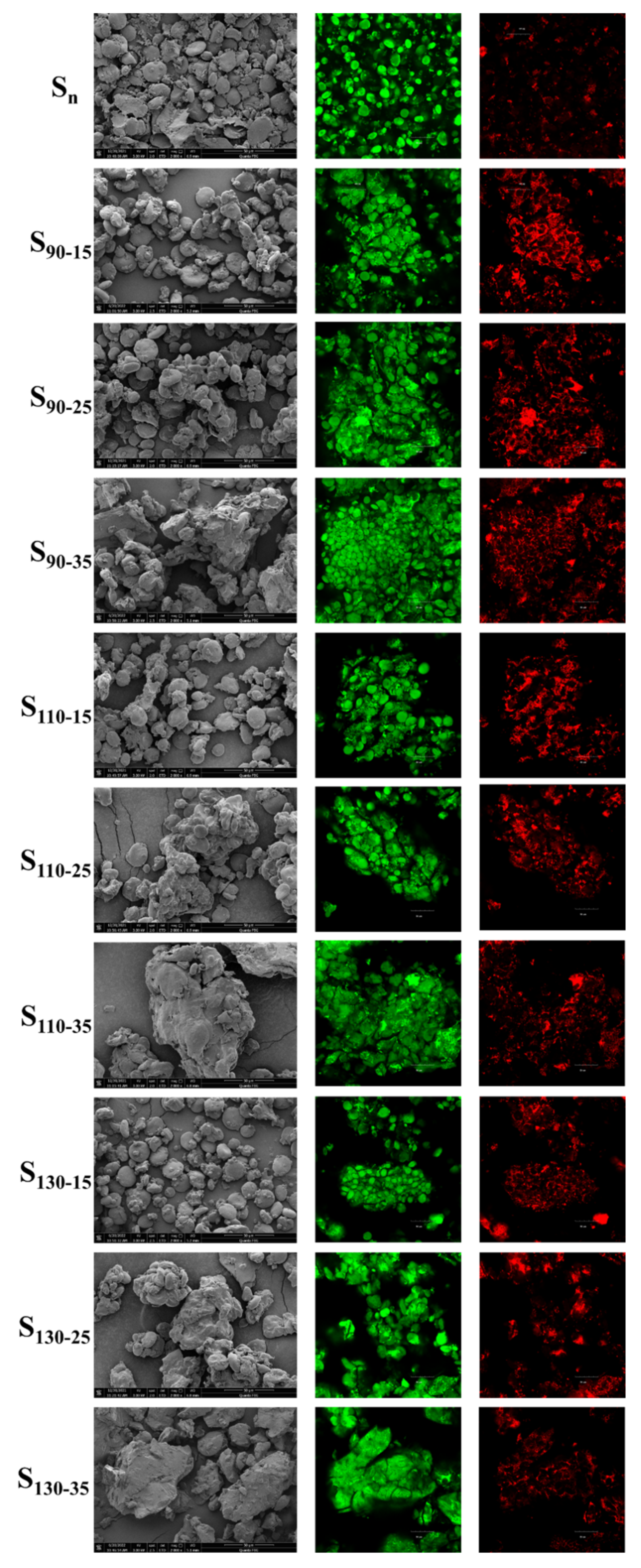
3.6. SEM and CLSM Analyses on HB Flour
3.7. Starch Digestibility Analysis of HB Flour
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Obadi, M.; Qi, Y.; Xu, B. Highland barley starch (Qingke): Structures, properties, modifications, and applications. Int. J. Biol. Macromol. 2021, 185, 725–738. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Influence of non-starchy polysaccharides on barley milling behavior and evaluating bioactive composition of milled fractions. Food Chem. 2017, 218, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, M.; Liu, H.; Fan, Z.; Zhang, Y.; Qin, X.; Liu, X. Effects of beta-glucan and various thermal processing methods on the in vitro digestion of hulless barley starch. Food Chem. 2021, 360, 129952. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N.; Huber, K.C. Physical modification of food starch functionalities. Annu. Rev. Food Sci. Technol. 2015, 6, 19–69. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Liu, C.; Law, C.L.; Mujumdar, A.S.; Xiao, H.W.; Zhang, C. Superheated steam processing: An emerging technology to improve food quality and safety. Crit. Rev. Food Sci. Nutr. 2022, 348, 129088. [Google Scholar] [CrossRef]
- Hoover, R. The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit. Rev. Food Sci. Nutr. 2010, 50, 835–847. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Liu, H.M.; Li, L.; Zheng, X.L.; Tian, X.L.; Sun, B.H.; Wang, X.X. Supplementation of wheat flour products with wheat bran dietary fiber: Purpose, mechanisms, and challenges. Trends Food Sci. Technol. 2022, 123, 281–289. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Collar, C.; Armero, E. Impact of Heat Moisture Treatment and Hydration Level of Flours on the Functional and Nutritional Value-Added Wheat-Barley Blended Breads. Food Bioprocess Technol. 2018, 11, 966–978. [Google Scholar] [CrossRef]
- Chung, H.-J.; Cho, A.; Lim, S.-T. Utilization of germinated and heat-moisture treated brown rices in sugar-snap cookies. LWT Food Sci. Technol. 2014, 57, 260–266. [Google Scholar] [CrossRef]
- Chandla, N.K.; Saxena, D.C.; Singh, S. Processing and evaluation of heat moisture treated (HMT) amaranth starch noodles; An inclusive comparison with corn starch noodles. J. Cereal Sci. 2017, 75, 306–313. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, S. Influence of heat-moisture treatment (HMT) on physicochemical and functional properties of starches from different Indian oat (Avena sativa L.) cultivars. Int. J. Biol. Macromol. 2019, 122, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ma, S.; Wang, F.; Wang, X. Effect of black rice flour with different particle sizes on frozen dough and steamed bread quality. Int. J. Food Sci. Technol. 2022, 57, 1748–1762. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Tian, X.; Sun, B.; Huang, J.; Yan, J.; Bao, Q.; Wang, X. Effect of synergistic fermentation of Lactobacillus plantarum and Saccharomyces cerevisiae on thermal properties of wheat bran dietary fiber-wheat starch system. Food Chem. 2022, 373, 131417. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Wang, L.; Cui, S.W.; Qiu, J. Different thermal treatments of highland barley kernel affect its flour physicochemical properties by structural modification of starch and protein. Food Chem. 2022, 387, 132835. [Google Scholar] [CrossRef]
- Gu, Y.; Qian, X.; Sun, B.; Tian, X.; Wang, X.; Ma, S. Effect of roasting treatment on the micromorphology, gelatinization, structure, and digestibility of whole oat flour. LWT Food Sci. Technol. 2022, 168, 113828. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Sudheesh, C.; Sunooj, K.V.; Alom, M.; Kumar, S.; Sajeevkumar, V.A.; George, J. Effect of dual modification with annealing, heat moisture treatment and cross-linking on the physico-chemical, rheological and in vitro digestibility of underutilised kithul (Caryota urens) starch. J. Food Meas. Charact. 2020, 14, 1557–1567. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Changes of starch during thermal processing of foods: Current status and future directions. Trends Food Sci. Technol. 2022, 119, 320–337. [Google Scholar] [CrossRef]
- Han, L.; Wei, Q.; Cao, S.; Yu, Y.; Cao, X.; Chen, W. The assisting effects of ultrasound on the multiscale characteristics of heat-moisture treated starch from Agriophyllum squarrosum seeds. Int. J. Biol. Macromol. 2021, 187, 471–480. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kumar, M.; Singh, S.; Singh, P. Effect of Different Modifications (Physical and Chemical) on Morphological, Pasting, and Rheological Properties of Black Rice (Oryza sativa L. Indica) Starch: A Comparative Study. Starch Stärke 2020, 73, 2000098. [Google Scholar] [CrossRef]
- da RosaZavareze, E.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar]
- Chung, H.-J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Olayinka, O.; Adebowale, K.; Oluowolabi, B. Effect of heat-moisture treatment on physicochemical properties of white sorghum starch. Food Hydrocoll. 2008, 22, 225–230. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Xiao, N.; Liu, X.; Zhang, Y.; Zhang, H. Insights into the hierarchical structure and digestibility of starch in heat-moisture treated adlay seeds. Food Chem. 2020, 318, 126489. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Li, X.; Chen, L.; Zhang, B. Multi-scale structure, pasting and digestibility of heat moisture treated red adzuki bean starch. Int. J. Biol. Macromol. 2017, 102, 162–169. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch Stärke. 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Dong, J.; Huang, L.; Chen, W.; Zhu, Y.; Dun, B.; Shen, R. Effect of Heat-Moisture Treatments on Digestibility and Physicochemical Property of Whole Quinoa Flour. Foods 2021, 10, 3042. [Google Scholar] [CrossRef]
- Gou, M.; Wu, H.; Saleh, A.S.M.; Jing, L.; Liu, Y.; Zhao, K.; Su, C.; Zhang, B.; Jiang, H.; Li, W. Effects of repeated and continuous dry heat treatments on properties of sweet potato starch. Int. J. Biol. Macromol. 2019, 129, 869–877. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.-L. Gelatinization and rheological properties of starch. Starch-Stärke 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Xu, D.; Jin, Y.; Xu, X. Wheat flour superheated steam treatment induced changes in molecular rearrangement and polymerization behavior of gluten. Food Hydrocoll. 2021, 118, 106769. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Pan, Y.; Ali, B.; Xu, D.; Xu, X. Physicochemical, crystalline characterization and digestibility of wheat starch under superheated steam treatment. Food Hydrocoll. 2021, 118, 106720. [Google Scholar] [CrossRef]
- Asranudin; Holilah; Syarifin, A.N.K.; Purnomo, A.S.; Ansharullah; Fudholi, A. The effect of heat moisture treatment on crystallinity and physicochemical-digestibility properties of purple yam flour. Food Hydrocoll. 2021, 120, 106889. [Google Scholar] [CrossRef]
- Cahyana, Y.; Wijaya, E.; Halimah, T.S.; Marta, H.; Suryadi, E.; Kurniati, D. The effect of different thermal modifications on slowly digestible starch and physicochemical properties of green banana flour (Musa acuminata colla). Food Chem. 2019, 274, 274–280. [Google Scholar] [CrossRef]
- Varatharajan, V.; Hoover, R.; Liu, Q.; Seetharaman, K. The impact of heat-moisture treatment on the molecular structure and physicochemical properties of normal and waxy potato starches. Carbohydr. Polym. 2010, 81, 466–475. [Google Scholar] [CrossRef]
- Zou, J.; Xu, M.; Tang, W.; Wen, L.; Yang, B. Modification of structural, physicochemical and digestive properties of normal maize starch by thermal treatment. Food Chem. 2020, 309, 125733. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, K.; Su, C.; Gong, B.; Ge, X.; Zhang, Q.; Li, W. Comparing the multi-scale structure, physicochemical properties and digestibility of wheat A- and B-starch with repeated versus continuous heat-moisture treatment. Int. J. Biol. Macromol. 2020, 163, 519–528. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, M.; Li, Y.; Xiong, L. Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch. Carbohydr. Polym. 2014, 110, 128–134. [Google Scholar] [CrossRef]
- Lu, H.; Ma, R.; Chang, R.; Tian, Y. Evaluation of starch retrogradation by infrared spectroscopy. Food Hydrocoll. 2021, 120, 106975. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.K.; Routray, W. Physicochemical characterization of modified lotus seed starch obtained through acid and heat moisture treatment. Food Chem. 2020, 319, 126513. [Google Scholar] [CrossRef]
- Lu, Z.H.; Donner, E.; Yada, R.Y.; Liu, Q. Physicochemical properties and in vitro starch digestibility of potato starch/protein blends. Carbohydr. Polym. 2016, 154, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Georget, D.M.; Belton, P.S. Effects of temperature and water content on the secondary structure of wheat gluten studied by FTIR spectroscopy. Biomacromolecules 2006, 7, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Li, T.; Zhang, Y.; Zhang, W.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. Interactions between gluten and water-unextractable arabinoxylan during the thermal treatment. Food Chem. 2021, 345, 128785. [Google Scholar] [CrossRef]
- Huang, T.T.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effect of debranching and heat-moisture treatments on structural characteristics and digestibility of sweet potato starch. Food Chem. 2015, 187, 218–224. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; George, J. Kithul palm (Caryota urens) as a new source of starch: Effect of single, dual chemical modifications and annealing on the physicochemical properties and in vitro digestibility. Int. J. Biol. Macromol. 2019, 125, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, F.; Ji, X.; Yan, Y.; Liu, Y. Effects of plasma-activated water and heat moisture treatment on the properties of wheat flour and dough. Int. J. Food Sci. Technol. 2021, 57, 1988–1994. [Google Scholar] [CrossRef]
- Gong, B.; Xu, M.; Li, B.; Wu, H.; Liu, Y.; Zhang, G.; Ouyang, S.; Li, W. Repeated heat-moisture treatment exhibits superiorities in modification of structural, physicochemical and digestibility properties of red adzuki bean starch compared to continuous heat-moisture way. Food Res. Int. 2017, 102, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, B.; Su, C.; Gong, B.; Zheng, J.; Jiang, H.; Zhang, G.; Li, W. Repeated Heat-Moisture Treatment: A more Effective Way for Structural and Physicochemical Modification of Mung Bean Starch Compared with Continuous Way. Food Bioprocess Technol. 2020, 13, 452–461. [Google Scholar] [CrossRef]
- Xie, X.; Qi, L.; Xu, C.; Shen, Y.; Wang, H.; Zhang, H. Understanding how the cooking methods affected structures and digestibility of native and heat-moisture treated rice starches. J. Cereal Sci. 2020, 95, 103085. [Google Scholar] [CrossRef]


| Sample | Peak Viscosity (cPa) | Trough Viscosity (cPa) | Breakdown (cPa) | Final Viscosity (cPa) | Setback (cPa) | Peak Time (s) | Pasting Temperature (°C) |
|---|---|---|---|---|---|---|---|
| Sn | 3626 ± 40 c | 2229 ± 7 c | 1397 ± 33 c | 3734 ± 26 d | 1505 ± 33 b | 6.32 ± 0.00 c | 66.93 ± 2.44 d |
| S90−15 | 4260 ± 184 b | 2649 ± 116 b | 1611 ± 68 b | 4180 ± 21 c | 1531 ± 136 b | 6.25 ± 0.10 c | 68.15 ± 0.64 d |
| S90−25 | 5147 ± 72 a | 3302 ± 1 a | 1845 ± 71 a | 5384 ± 122 a | 2082 ± 121 a | 6.35 ± 0.04 c | 69.68 ± 0.53 d |
| S90−35 | 2712 ± 8 e | 2177± 0 cd | 566 ± 36 e | 3299 ± 55 e | 1153 ± 83 c | 6.69 ± 0.05 b | 88.43 ± 1.66 ab |
| S110−15 | 4219 ± 111 b | 2566 ± 109 b | 1653 ± 2 b | 4515 ± 90 b | 1949 ± 199 a | 6.19 ± 0.09 cd | 70.13 ± 0.03 cd |
| S110−25 | 3211 ± 3 d | 2228 ± 78 cd | 983 ± 81 d | 3717 ± 111 d | 1489 ± 189 b | 6.55 ± 0.14 b | 83.48 ± 0.53 ab |
| S110−35 | 1408 ± 32 g | 1374 ± 42 e | 272 ± 24 f | 2076 ± 38 g | 941 ± 30 c | 6.98 ± 0.00 a | 92.00 ± 0.07 a |
| S130−15 | 3662 ± 17 c | 2066 ±19 d | 1597 ± 2 b | 4062 ± 96 c | 1997 ± 115 a | 6.02 ± 0.05 d | 71.43 ± 0.53 cd |
| S130−25 | 1663 ± 21 f | 1419 ± 42 e | 244 ± 21 f | 2313 ± 1 f | 895 ± 43 c | 6.98 ± 0.00 a | 80.30 ± 13.08 bc |
| S130−35 | 471 ± 10 h | - | - | 912 ± 13 h | - | - | 93.58 ± 1.17 a |
| To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | |
|---|---|---|---|---|
| Sn | 55.77 ± 0.04 g | 60.47± 0.24 f | 66.16 ± 0.91 e | 6.41 ± 0.07 a |
| S90−15 | 56.11 ± 0.12 fg | 60.62 ± 0.01 f | 66.98 ± 0.30 e | 5.24 ± 0.57 ab |
| S90−25 | 62.54 ± 0.37 e | 66.66 ± 0.07 d | 73.47 ± 0.11 dc | 4.92 ± 0.05 bc |
| S90−35 | 67.88 ± 0.39 c | 71.64 ± 0.13 c | 75.28 ± 0.28 d | 3.40 ± 0.63 d |
| S110−15 | 56.80 ± 0.08 f | 61.98 ± 0.38 e | 67.42 ± 0.46 e | 5.58 ± 0.76 ab |
| S110−25 | 64.09 ± 0.99 d | 71.46 ± 0.76 c | 83.07 ± 0.79 c | 3.91 ± 0.15 cd |
| S110−35 | 68.02 ± 0.40 c | 72.67± 0.03 b | 85.14 ± 0.34 c | 1.31 ± 0.22 e |
| S130−15 | 57.04 ± 0.17 f | 62.17 ± 0.46 e | 70.96 ± 0.64 c | 5.98 ± 0.38 ab |
| S130−25 | 69.93 ± 0.11 b | 80.64 ± 0.80 a | 88.63 ± 0.12 b | 1.14 ± 0.40 e |
| S130−35 | 73.72 ± 0.01 a | 80.69 ± 0.00 a | 91.71 ± 0.16 a | 0.43 ± 0.01 e |
| Sample | Sn | S90−15 | S90−25 | S90−35 | S110−15 | S110−25 | S110−35 | S130−15 | S130−25 | S130−35 |
|---|---|---|---|---|---|---|---|---|---|---|
| RC (%) | 28.52 | 30.45 | 41.32 | 32.23 | 36.39 | 36.46 | 31.08 | 36.96 | 29.56 | 31.28 |
| Sample | Sn | S90−15 | S90−25 | S90−35 | S110−15 | S110−25 | S110−35 | S130−15 | S130−25 | S130−35 |
|---|---|---|---|---|---|---|---|---|---|---|
| R1047/1022 | 1.2584 ± 0.0032 c | 1.3245 ± 0.0062 abc | 1.48341 ± 0.0085 a | 1.5085 ± 0.0224 ab | 1.4599 ± 0.0159 abc | 1.3053 ± 0.0961 bc | 1.2974 ± 0.1192 c | 1.1997 ± 0.0929 c | 1.2139 ± 0.0850 c | 1.3579 ± 0.0289 abc |
| Sample | β-Sheet Structure | Random Coil Structure | α-Helix Structure | β-Turn Structure |
|---|---|---|---|---|
| Sn | 0.3084 ± 0.0221 b | 0.1610 ± 0.0053 a | 0.1679 ± 0.0199 bc | 0.3627 ± 0.0031 a |
| S90−15 | 0.3162 ± 0.0003 ab | 0.1657 ± 0.0032 a | 0.1929 ± 0.0034 ab | 0.3252 ± 0.0001 d |
| S90−25 | 0.3216 ± 0.0017 ab | 0.1622 ± 0.0002 a | 0.1872 ± 0.0001 ab | 0.3291 ± 0.0020 cd |
| S90−35 | 0.3352 ± 0.0272 ab | 0.1518 ± 0.0085 a | 0.1545 ± 0.0226 c | 0.3585 ± 0.0039 a |
| S110−15 | 0.3385 ± 0.0076 ab | 0.1620 ± 0.0001 a | 0.1723 ± 0.0006 abc | 0.3330 ± 0.0013 c |
| S110−25 | 0.3119 ± 0.0026 ab | 0.1590 ± 0.0040 a | 0.1863 ± 0.0009 ab | 0.3429 ± 0.0004 b |
| S110−35 | 0.3196 ± 0.0055 ab | 0.1613 ± 0.0060 a | 0.1901 ± 0.0020 ab | 0.3290 ± 0.0015 cd |
| S130−15 | 0.3423 ± 0.0283 ab | 0.1335 ± 0.0441 a | 0.1965 ± 0.0097 a | 0.3276 ± 0.0061 cd |
| S130−25 | 0.3251 ± 0.000 ab | 0.1639 ± 0.0001 a | 0.1863 ± 0.0002 ab | 0.3246 ± 0.0011 d |
| S130−35 | 0.3530 ± 0.0153 a | 0.1485 ± 0.0070 a | 0.1827 ± 0.0087 ab | 0.3157 ± 0.0004 e |
| Sample | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| Sn | 60.35 ± 1.28 e | 14.87 ± 0.22 d | 24.77 ± 1.50 bc |
| S90−15 | 61.57 ± 0.25 e | 10.96 ± 0.00 e | 27.47 ± 0.25 b |
| S90−25 | 60.33 ± 0.44 e | 15.89 ± 0.78 d | 23.78 ± 1.21 c |
| S90−35 | 62.27 ± 0.14 e | 35.19 ± 0.64 a | 2.55 ± 0.78 ef |
| S110−15 | 61.98 ± 0.14 e | 4.62 ± 0.55 f | 33.40 ± 0.70 a |
| S110−25 | 65.96 ± 0.57 d | 15.89 ± 1.00 d | 18.15 ± 0.43 d |
| S110−35 | 68.74 ± 1.09 c | 29.64 ± 0.52 b | 1.62 ± 1.61 ef |
| S130−15 | 59.96 ± 1.52 e | 13.53 ± 2.13 de | 26.51 ± 0.60 bc |
| S130−25 | 71.50 ± 0.70 b | 24.90 ± 0.66 c | 3.61 ± 1.36 e |
| S130−35 | 85.30 ± 0.85 a | 14.12 ± 0.88 d | 0.58 ± 0.03 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Ma, S.; Yan, J.; Sun, B.; Wang, X. Effect of Heat–Moisture Treatment on the Physicochemical Properties, Structure, Morphology, and Starch Digestibility of Highland Barley (Hordeum vulgare L. var. nudum Hook. f) Flour. Foods 2022, 11, 3511. https://doi.org/10.3390/foods11213511
Lv Y, Ma S, Yan J, Sun B, Wang X. Effect of Heat–Moisture Treatment on the Physicochemical Properties, Structure, Morphology, and Starch Digestibility of Highland Barley (Hordeum vulgare L. var. nudum Hook. f) Flour. Foods. 2022; 11(21):3511. https://doi.org/10.3390/foods11213511
Chicago/Turabian StyleLv, Yiming, Sen Ma, Jingyao Yan, Binghua Sun, and Xiaoxi Wang. 2022. "Effect of Heat–Moisture Treatment on the Physicochemical Properties, Structure, Morphology, and Starch Digestibility of Highland Barley (Hordeum vulgare L. var. nudum Hook. f) Flour" Foods 11, no. 21: 3511. https://doi.org/10.3390/foods11213511
APA StyleLv, Y., Ma, S., Yan, J., Sun, B., & Wang, X. (2022). Effect of Heat–Moisture Treatment on the Physicochemical Properties, Structure, Morphology, and Starch Digestibility of Highland Barley (Hordeum vulgare L. var. nudum Hook. f) Flour. Foods, 11(21), 3511. https://doi.org/10.3390/foods11213511







