Lettuce Contamination and Survival of Salmonella Typhimurium and Listeria monocytogenes in Hydroponic Nutrient Film Technique Systems
Abstract
1. Introduction
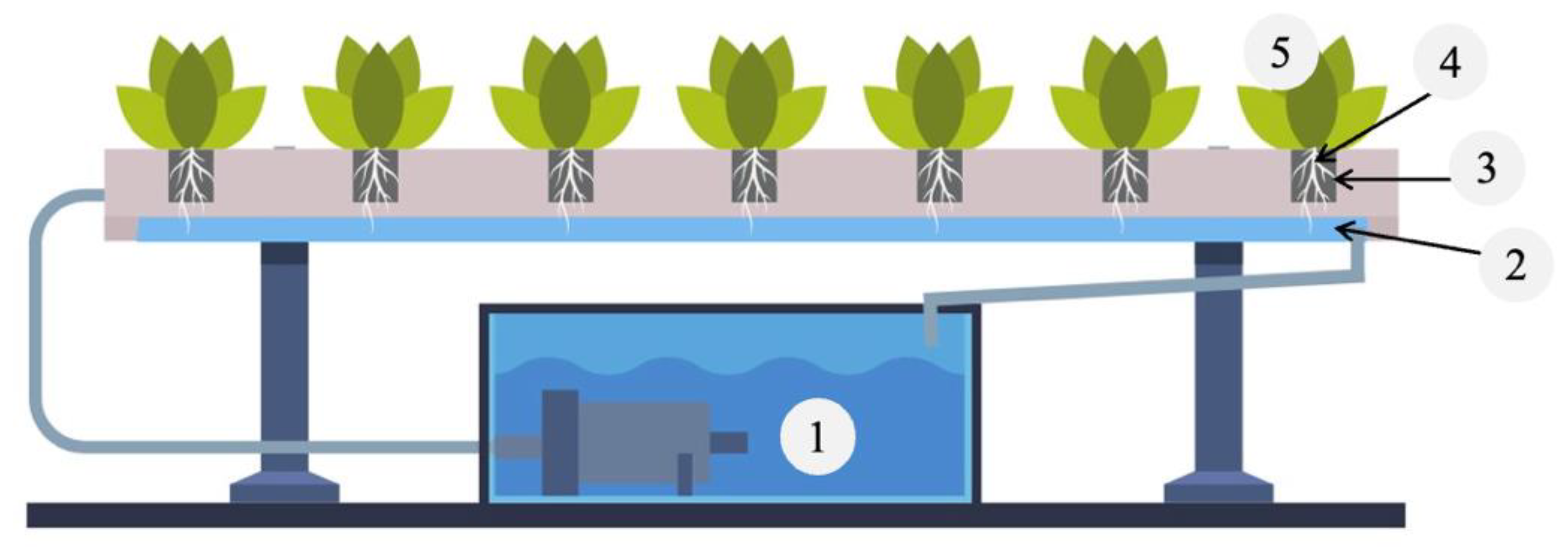
2. Materials and Methods
3. Results
3.1. Survival of Salmonella Typhimurium in NFT Hydroponic Systems under Extreme and Sporadic Contamination Events
3.2. Survival of Listeria monocytogenes in NFT Systems under Extreme and Sporadic Contamination Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bledsoe, M. The Greenhouse Industry in North America: Challenges, Regulatory Impacts between Borders and Phyto-Sanitation. Presented at The Ohio State University, Department of Horticulture and Crop Sciences Seminar Series, Wooster, OH, USA, 19 October 2020. [Google Scholar]
- Hydroponics Market Size & Share |Industry Analysis Report; 2025. Available online: https://www.grandviewresearch.com/industry-analysis/hydroponics-market (accessed on 12 January 2020).
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential Impacts of Climate Change on Vegetable Production and Product Quality—A Review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Leisner, C.P. Review: Climate Change Impacts on Food Security- Focus on Perennial Cropping Systems and Nutritional Value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Rao, T.C.; Solanki, S.; Suresh, M.V.; Menon, B.; Raghavendran, K. The Holy Basil Administration Diminishes the NF-KB Expression and Protects Alveolar Epithelial Cells from Pneumonia Infection through Interferon Gamma. Phytother. Res. PTR 2022, 36, 1822–1835. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, H. A Review on Relationship between Climate Change and Agriculture. J. Earth Sci. Amp Clim. Chang. 2016, 7, 335. [Google Scholar]
- Bartok, J.W., Jr. Hydroponic Systems. Available online: https://ag.umass.edu/greenhouse-floriculture/fact-sheets/hydroponic-systems (accessed on 10 October 2021).
- Shrestha, A.; Dunn, B. Hydroponics. Available online: https://extension.okstate.edu/fact-sheets/hydroponics.html (accessed on 10 October 2022).
- Brauther, C. Greenhouse Produce: Challenges & Opportunities. Prod. Bus. 2010, 960, 277–280. [Google Scholar]
- Buchholz, U.; Bernard, H.; Werber, D.; Böhmer, M.M.; Remschmidt, C.; Wilking, H.; Deleré, Y.; an der Heiden, M.; Adlhoch, C.; Dreesman, J.; et al. German Outbreak of Escherichia coli O104:H4 Associated with Sprouts. N. Engl. J. Med. 2011, 365, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- CDC—MMWR—MMWR Publications—MMWR Weekly: Past Volume. 2013. Available online: https://www.cdc.gov/mmwr/index2013.html (accessed on 10 October 2022).
- Verhaelen, K.; Bouwknegt, M.; Carratalà, A.; Lodder-Verschoor, F.; Diez-Valcarce, M.; Rodríguez-Lázaro, D.; de Roda Husman, A.M.; Rutjes, S.A. Virus Transfer Proportions between Gloved Fingertips, Soft Berries, and Lettuce, and Associated Health Risks. Int. J. Food Microbiol. 2013, 166, 419–425. [Google Scholar] [CrossRef]
- Orozco, L.; Rico-Romero, L.; Escartín, E.F. Microbiological Profile of Greenhouses in a Farm Producing Hydroponic Tomatoes. J. Food Prot. 2008, 71, 60–65. [Google Scholar] [CrossRef]
- Orozco, R.L.; Iturriaga, M.H.; Tamplin, M.L.; Fratamico, P.M.; Call, J.E.; Luchansky, J.B.; Escartin, E.F. Animal and Environmental Impact on the Presence and Distribution of Salmonella and Escherichia coli in Hydroponic Tomato Greenhouses. J. Food Prot. 2008, 71, 676–683. [Google Scholar] [CrossRef]
- Ilic, S.; Ivey, M.; Ilic, S. Food Safety of Hydroponic Fruits and Vegetables—What We Do and Don’t Know. In Proceedings of the International Association of Food Protection Annual Meeting, Salt Lake City, UT, USA, 8–11 July 2018; S48. [Google Scholar]
- CDC. CDC: Escherichia coli O157 Infections Linked to Alfalfa Sprouts Produced by Jack & The Green Sprouts. Available online: https://www.cdc.gov/ecoli/2016/o157-02-16/index.html (accessed on 5 August 2021).
- CDC. CDC: Salmonella Outbreak Linked to BrightFarms Packaged Salad Greens. Available online: https://www.cdc.gov/salmonella/typhimurium-07-21/details.html (accessed on 10 October 2022).
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical Review: Vegetables and Fruit in the Prevention of Chronic Diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Kirezieva, K.; Luning, P.; Jacxsens, L.; Allende, A.; Johannessen, G.; Tondo, E.; Rajković, A.; Uyttendaele, M.; Boekel, M.V. Factors Affecting the Status of Food Safety Management Systems in the Global Fresh Produce Chain. Food Control. 2015, 52, 85–97. [Google Scholar] [CrossRef]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by Using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA: Full Text of the Food Safety Modernization Act (FSMA). Available online: https://www.fda.gov/food/food-safety-modernization-act-fsma/full-text-food-safety-modernization-act-fsma (accessed on 10 October 2022).
- Patchanee, P.; Molla, B.; White, N.; Line, D.E.; Gebreyes, W.A. Tracking Salmonella Contamination in Various Watersheds and Phenotypic and Genotypic Diversity. Foodborne Pathog. Dis. 2010, 7, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, F.; Valero, A.; Carrasco, E.; García, R.M.; Zurera, G. Understanding and Modelling Bacterial Transfer to Foods: A Review. Trends Food Sci. Technol. 2008, 19, 131–144. [Google Scholar] [CrossRef]
- Shaw, A.; Helterbran, K.; Evans, M.R.; Currey, C. Growth of Escherichia coli O157:H7, Non-O157 Shiga Toxin-Producing Escherichia coli, and Salmonella in Water and Hydroponic Fertilizer Solutions. J. Food Prot. 2016, 79, 2179–2183. [Google Scholar] [CrossRef]
- Ilic, S.; LeJeune, J.; Lewis Ivey, M.L.; Miller, S. Delphi Expert Elicitation to Prioritize Food Safety Management Practices in Greenhouse Production of Tomatoes in the United States. Food Control. 2017, 78, 108–115. [Google Scholar] [CrossRef]
- Deblais, L.; Miller, S.A.; Rajashekara, G. Impact of plant pathogen infection on Salmonella enterica subsp. enterica Serotype Typhimurium on Tomato Plants. J. Food Prot. 2021, 84, 563–571. [Google Scholar] [CrossRef]
- Stroup, W.W.; Milliken, G.A.; Claassen, E.A.; Wolfinger, R.D. SAS® for Mixed Models: Introduction and Basic Applications; SAS Institute Inc.: Cary, NC, USA, 2018; ISBN 978-1-63526-152-3. [Google Scholar]
- Xylia, P.; Chrysargyris, A.; Botsaris, G.; Skandamis, P.; Tzortzakis, N. Salmonella Enteritidis Survival in Different Temperatures and Nutrient Solution PH Levels in Hydroponically Grown Lettuce. Food Microbiol. 2022, 102, 103898. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated With Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Jagannathan, B.V.; Vijayakumar, P.P. The Need for Prevention-Based Food Safety Programs for Fresh Produce. Anim. Food Sci. 2019, 39, 572–579. [Google Scholar]
- Jung, J.; Friedrich, L.M.; Danyluk, M.D.; Schaffner, D.W. Quantification of Transfer of Salmonella from Citrus Fruits to Peel, Edible Portion, and Gloved Hands during Hand Peeling. J. Food Prot. 2017, 80, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; Monaghan, J. Irrigation Water Quality for Leafy Crops: A Perspective of Risks and Potential Solutions. Int. J. Environ. Res. Public. Health 2015, 12, 7457–7477. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R. Vectors and Conditions for Preharvest Contamination of Fruits and Vegetables with Pathogens Capable of Causing Enteric Diseases. Br. Food J. 2006, 108, 38–53. [Google Scholar] [CrossRef]
- Dankwa, A.S.; Machado, R.M.; Perry, J.J. Sanitizer Efficacy in Reducing Microbial Load on Commercially Grown Hydroponic Lettuce. J. Sci. Food Agric. 2021, 101, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Uyttendaele, M.; Jaykus, L.-A.; Amoah, P.; Chiodini, A.; Cunliffe, D.; Jacxsens, L.; Holvoet, K.; Korsten, L.; Lau, M.; McClure, P.; et al. Microbial Hazards in Irrigation Water: Standards, Norms, and Testing to Manage Use of Water in Fresh Produce Primary Production. Compr. Rev. Food Sci. Food Saf. 2015, 14, 336–356. [Google Scholar] [CrossRef]
- Riggio, G.M.; Jones, S.L.; Gibson, K.E. Risk of Human Pathogen Internalization in Leafy Vegetables During Lab-Scale Hydroponic Cultivation. Horticulturae 2019, 5, 25. [Google Scholar] [CrossRef]
- Warriner, K.; Spaniolas, S.; Dickinson, M.; Wright, C.; Waites, W.M. Internalization of Bioluminescent Escherichia coli and Salmonella Montevideo in Growing Bean Sprouts. J. Appl. Microbiol. 2003, 95, 719–727. [Google Scholar] [CrossRef]
- Yang, J.; Kharbanda, P.D.; Mirza, M. Evaluation of Paenibacillus polymyxa PKB1 for Biocontrol of Pythium disease of cucumber in a hydroponic system. Acta Hortic. 2004, 635, 59–66. [Google Scholar] [CrossRef]

| Time Post-Inoculation | Reservoir Nutrient Solution 1 | Channel Nutrient Solution | Root | Rockwool | Edible Leaf Tissue |
|---|---|---|---|---|---|
| 1 h | 4.29 a 2 | 3.91 a | 6.21 a | 3.97 a | 0.97 |
| 12 h | 3.07 b | 2.67 b | 5.34 b | 3.66 ab | 0.08 |
| 24 h | 2.99 b | 2.96 b | 3.95 c | 3.30 abc | 1.02 |
| 7 days | 1.18 c | 1.19 c | 3.70 cd | 3.16 bc | 0.39 |
| 14 days | 0.56 c | 0.79 c | 2.96 de | 2.19 d | 0.55 |
| 21 days | 0.52 c | 0.65 c | 3.03 de | 2.76 cd | 0.30 |
| 28 days | 0.60 c | 0.80 c | 2.90 e | 2.22 d | 0.00 3 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.2151 |
| Time Post-Inoculation | Reservoir Nutrient Solution 1 | Channel Nutrient Solution | Root | Rockwool | Edible Leaf Tissue |
|---|---|---|---|---|---|
| 1 h | 2.21 a 2 | 2.19 ab | 5.72 a | 3.70 ab | 0.00 3 |
| 12 h | 1.54 b | 1.99 b | 6.24 a | 3.78 ab | 0.36 |
| 24 h | 2.22 a | 2.30 a | 5.55 a | 4.26 a | 0.00 3 |
| 7 days | 0.93 c | 0.54 c | 4.34 b | 3.28 b | 0.43 |
| 14 days | 0.44 d | 0.65 c | 3.35 c | 2.52 c | 0.14 |
| 21 days | 0.38 d | 0.53 c | 2.10 d | 2.18 cd | 0.11 |
| 28 days | 0.30 d | 0.28 d | 2.11 d | 1.81 d | 0.23 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.5171 |
| Time Post-Inoculation | Reservoir Nutrient Solution 1 | Channel Nutrient Solution | Root | Rockwool | Edible Leaf Tissue |
|---|---|---|---|---|---|
| 1 h | 2.49 a 2 | 2.57 a | 5.33 ab | 4.35 | 0.00 c 3 |
| 12 h | 1.64 ab | 1.85 ab | 5.65 ab | 4.21 | 0.19 bc |
| 24 h | 1.18 bc | 1.33 bc | 5.27 ab | 3.46 | 1.12 ab |
| 7 days | 0.90 bc | 0.79 bc | 3.79 c | 3.01 | 1.99 a |
| 14 days | 0.84 bc | 1.31 bc | 5.52 ab | 4.05 | 1.47 a |
| 21 days | 0.57 c | 0.59 c | 4.82 bc | 4.28 | 1.29 a |
| 28 days | 1.24 bc | 1.52 abc | 6.07 a | 3.91 | 1.74 a |
| p value | 0.0169 | 0.0312 | 0.0047 | 0.0826 | 0.0004 |
| Time Post-Inoculation | Reservoir Nutrient Solution 1 | Channel Nutrient Solution | Root | Rockwool | Edible Leaf Tissue |
|---|---|---|---|---|---|
| 1 h | - 2 | - 2 | 5.43 | 3.49 abc | 1.93 ab |
| 12 h | 1.35 b 3 | 1.22 c | 5.53 | 2.73 d | 0.84 c |
| 24 h | 1.13 b | 1.35 c | 5.76 | 3.13 cd | 2.21 ab |
| 7 days | 1.87 a | 2.22 a | 5.73 | 3.72 ab | 2.39 a |
| 14 days | 1.90 a | 1.89 ab | 5.30 | 3.47 bc | 1.85 ab |
| 21 days | 1.34 b | 1.60 bc | 5.06 | 3.91 ab | 1.53 bc |
| 28 days | 1.85 a | 2.12 a | 5.38 | 3.97 a | 1.82 ab |
| p value | <0.0001 | <0.0001 | 0.2913 | <0.0001 | 0.0135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilic, S.; Moodispaw, M.R.; Madden, L.V.; Lewis Ivey, M.L. Lettuce Contamination and Survival of Salmonella Typhimurium and Listeria monocytogenes in Hydroponic Nutrient Film Technique Systems. Foods 2022, 11, 3508. https://doi.org/10.3390/foods11213508
Ilic S, Moodispaw MR, Madden LV, Lewis Ivey ML. Lettuce Contamination and Survival of Salmonella Typhimurium and Listeria monocytogenes in Hydroponic Nutrient Film Technique Systems. Foods. 2022; 11(21):3508. https://doi.org/10.3390/foods11213508
Chicago/Turabian StyleIlic, Sanja, Margaret R. Moodispaw, Lawrence V. Madden, and Melanie L. Lewis Ivey. 2022. "Lettuce Contamination and Survival of Salmonella Typhimurium and Listeria monocytogenes in Hydroponic Nutrient Film Technique Systems" Foods 11, no. 21: 3508. https://doi.org/10.3390/foods11213508
APA StyleIlic, S., Moodispaw, M. R., Madden, L. V., & Lewis Ivey, M. L. (2022). Lettuce Contamination and Survival of Salmonella Typhimurium and Listeria monocytogenes in Hydroponic Nutrient Film Technique Systems. Foods, 11(21), 3508. https://doi.org/10.3390/foods11213508






