Physicochemical Properties and Bacterial Community Profiling of Optimal Mahewu (A Fermented Food Product) Prepared Using White and Yellow Maize with Different Inocula
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sample Preparation
2.2. Processing of Maize into Mahewu
2.3. The Optimisation of Parameters to Produce Mahewu
2.4. pH, TTA and TSS
2.5. Multi-Response Numerical Optimisation and Processing of Optimal Mahewu Samples
2.6. Investigation of Parameters in Optimized Mahewu Samples
2.6.1. pH, TTA and TSS
2.6.2. Profiling of the Bacterial Community
The ZymoBIOMICSTM DNA Extraction
16s rRNA Amplicon Sequencing
Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. pH, TTA and TSS
3.2. Statistical Models and Validation
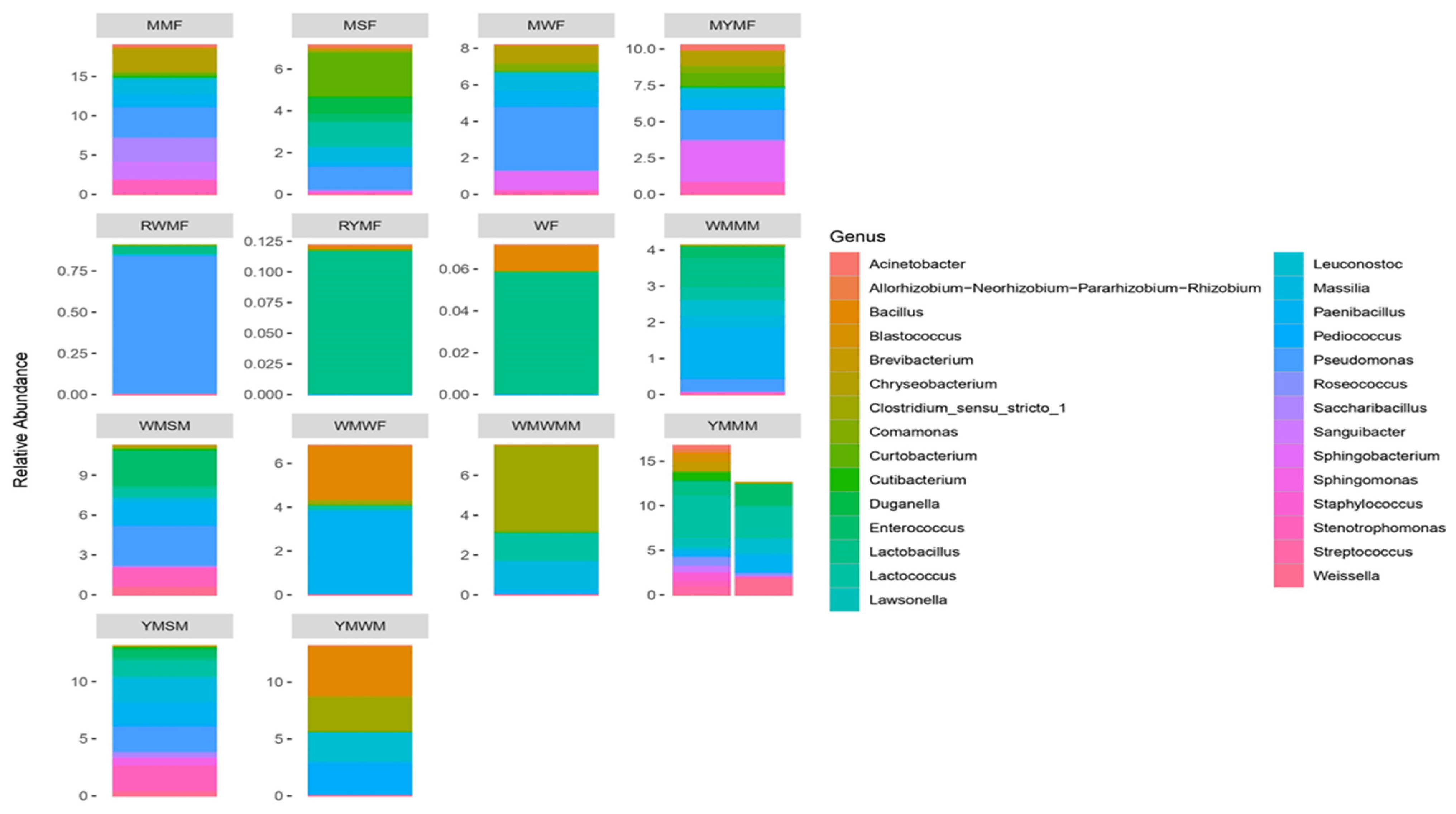
3.3. Profiling of Bacterial Community in Optimized Mahewu Samples
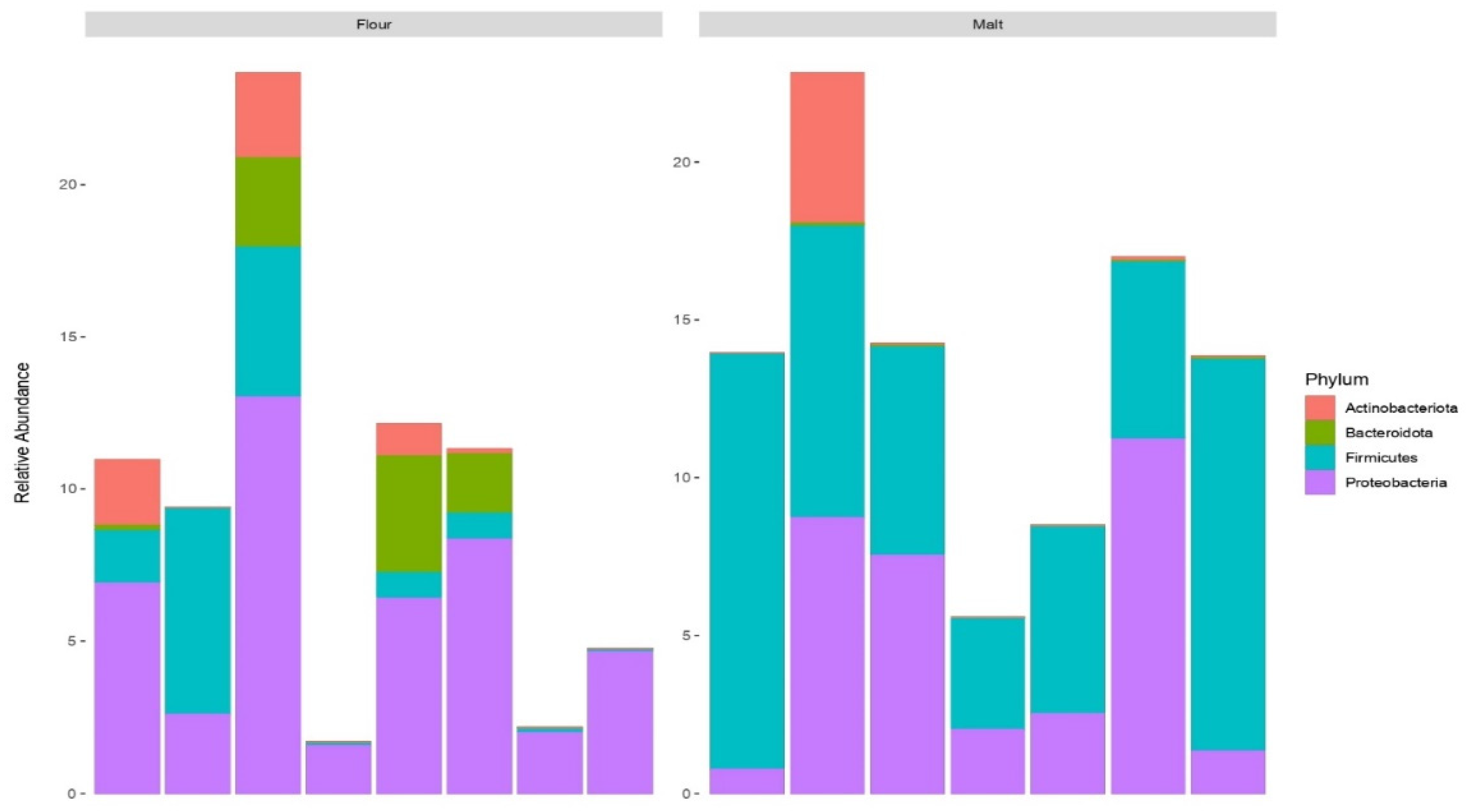
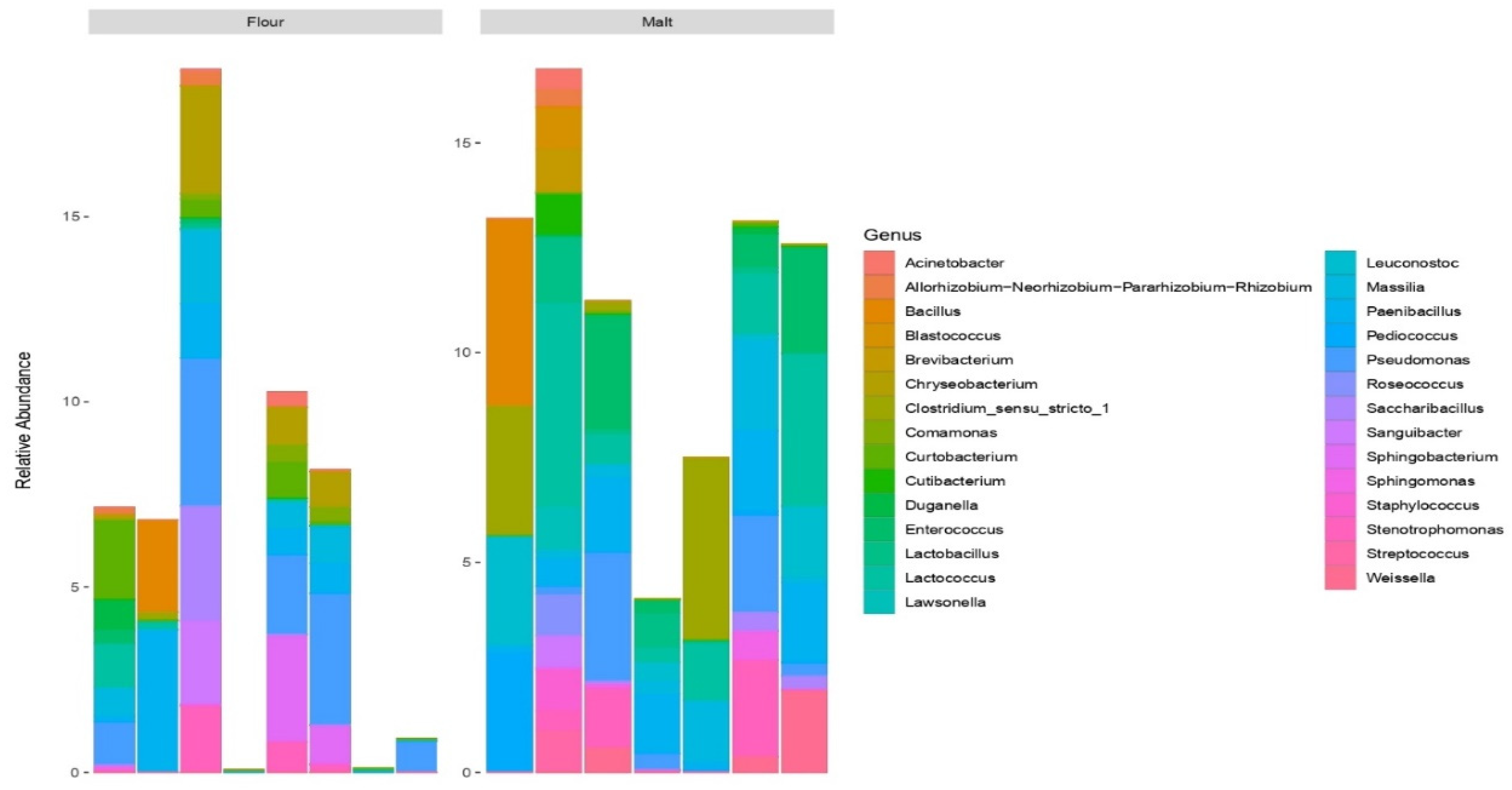
3.4. Profiling of Bacterial Community in Malt and Flour Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siyuan, S.; Tong, L.; Liu, R.H. Corn phytochemicals and their health benefits. Food Sci. Hum. Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1809–1834. [Google Scholar] [CrossRef] [PubMed]
- Cuvas-Limon, R.B.; Nobre, C.; Cruz, M.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Loredo-Trevino, A.; Texeira, J.A.; Belmares, R. Spontaneously fermented traditional beverage as a source of bioactive compounds: An overview. Cri. Rev. Food Sci. Nutr. 2020, 61, 2984–3006. [Google Scholar] [CrossRef] [PubMed]
- Ogunremi, O.R.; Banwo, K.; Sanni, A.L. Starter-culture to improve the quality of cereal-based fermented foods: Trends in selection and application. Curr. Opin. Food Sci. 2017, 13, 38–43. [Google Scholar] [CrossRef]
- Gotcheva, V.; Pandiella, S.S.; Angelov, A.; Roshkova, Z.G.; Webb, C. Microflora identification of the Bulgarian cereal-based fermented beverage boza. Process Biochem. 2020, 36, 127–130. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous years: Emerging trends and challenges in winemaking. Curr. Opin. Food Sci. 2020, 32, 133–147. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kenellaki, M.; Koutinas, A.A. Dairy and non-diary probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Kumar, R.S.; Varman, D.R.; Kanmani, P.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V. Isolation, Characterization and Identification of a Potential Probiont from South Indian Fermented Foods (Kallappam, Koozh and Mor Kuzhambu) and its use as Biopreservative. Probiotics Antimicrob. Proteins 2010, 2, 145–151. [Google Scholar] [CrossRef]
- Setta, M.C.; Matemu, A.; Mbega, E.R. Potential of Potential of probiotics from fermented cereal-based beverages in improving health of poor people in Africa. J. Food. Sci. Technol. 2020, 57, 3935–3946. [Google Scholar] [CrossRef]
- Pswarayi, F. Composition and Origin of the Fermentation Microbiota of Mahewu, a Zimbabwean Fermented Cereal Beverage. Appl. Environ. Microbiol. 2019, 85, e03130-18. [Google Scholar] [CrossRef]
- Awobusuyi, T.D.; Siwela, M.; Kolanisi, U.; Amonsou, E.O. Provitamin A retention and sensory acceptability of amahewu, a non-alcoholic cereal-based beverage made with provitamin A-biofortified maize. J. Scie Food Agric. 2016, 96, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Fadahunsi, I.F.; Soremekun, O.O. Production, nutritional and microbiological evaluation of Mahewu a South African traditional fermented porridge. J. Adv. Biol. Biotechnol. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Kayitesi, E. Co-influence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- Li, H.; Driesche, S.; Van, D.; Bunge, F.; Yang, B.; Vellekoop, M.J. Optimization of on-chip bacterial culture conditions using the Box-Behnken design response surface methodology for faster drug susceptibility screening. Talanta 2019, 194, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Simango, C. Lactic acid fermentation of sour porridge and mahewu, a non-alcoholic fermented cereal beverage. JASSA 2000, 8, 89–98. [Google Scholar] [CrossRef]
- Gadaga, T.H.; Mutukumira, A.N.; Narvhus, J.A.; Feresu, S.B. A review of traditional fermented foods and beverages of Zimbabwe. Int. J. Food Microbiol. 1999, 53, 1–11. [Google Scholar] [CrossRef]
- Caro-quintero, A.; Konstantinidis, K.T. Minireview Bacterial species may exist, metagenomics reveal. Environ. microbiol. 2012, 14, 347–355. [Google Scholar] [CrossRef]
- Davies, H. A role for “omics” technologies in food safety assessment. Food Control 2010, 21, 1601–1610. [Google Scholar] [CrossRef]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of potentially probiotic lactic acid bacteria on the physicochemical composition and acceptance of fermented cereal beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Xiong, K.; Chen, Y.; Shen, S. Experimental optimization and mathematical modeling of supercritical carbon dioxide extraction of essential oil from Pogostemon cablin. Chin. J. Chem. Eng. 2019, 27, 2407–2417. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Mellem, J.J.; Ijabadeniyi, O.A.; Mellem, J.J.; Ijabadeniyi, O.A. Improvement of some quality attributes of soymilk through optimization of selected soybean sprouting parameters using response surface methodology. CyTA J. Food 2018, 16, 230–237. [Google Scholar] [CrossRef]
- Das, S.; Tamang, J.P. Changes in microbial communities and their predictive functionalities during fermentation of toddy, an alcoholic beverage of India. Microbiol. Res. 2021, 248, 126769. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P.; Gqaleni, N. Advantages of traditional lactic acid bacteria fermentation of food in Africa. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2016, 2, 1160–1167. [Google Scholar]
- Mashau, M.E.; Jideani, A.I.O.; Maliwichi, L.L. Evaluation of the shelf-life extension and sensory properties of mahewu–A non-alcoholic fermented beverage by adding Aloe vera (Aloe barbadensis) powder. Br. Food J. 2020, 122, 3419–3432. [Google Scholar] [CrossRef]
- Muyanja, C.; Namugumya, B.S. Traditional processing, microbiological phytochemical and sensory characteristics of Kwete, a Ugandan fermented maize based beverage. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1046–1059. [Google Scholar]
- Nanodoum, M. Sorghum Beer: Production, Nutritional Value and Impact upon Human Health. Beer Health Dis. Prev. 2010, 5, 53–60. [Google Scholar]
- Ibrahim, F.S.; Babiker, E.E.; Yousif, N.E.; El Tinay, A.H. Effect of fermentation on biochemical and sensory characteristics of sorghum flour supplemented with whey protein. Food Chem. 2005, 92, 285–292. [Google Scholar] [CrossRef]
- Kutyauripo, J.; Parawira, W.; Tinofa, S.; Kudita, I.; Ndengu, C. Investigation of shelf-life extension of sorghum beer (Chibuku) by removing the second conversion of malt. Int. J. Food Microbiol. 2009, 129, 271–276. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Milk fermentation, fermented and nonfermented dairy products. Food Microbiol. 2005, 149–173. [Google Scholar] [CrossRef]
- Abriouel, H.; Omar, N.B.; López, R.L.; Martínez-Cañamero, M.; Keleke, S.; Gálvez, A. Culture-independent analysis of the microbial composition of the African traditional fermented foods poto poto and dégué by using three different DNA extraction methods. Int. J. Food Microbiol. 2006, 111, 228–233. [Google Scholar] [CrossRef]
- Song, D.H.; Chun, B.H.; Lee, S.; Son, S.Y.; Reddy, C.K.; Mun, H.I. Comprehensive metabolite profiling and microbial communities of doenjang (Fermented soy paste) and ganjang (fermented soy sauce): A comparative study. Foods 2021, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wu, J.; An, F.; Yue, X.; Tao, D.; Wu, R.; Lee, Y. An integrated metagenomic/metaproteomic investigation of microbiota in dajiang-meju, a traditional fermented soybean product in Northeast China. Food Res. Int. 2019, 115, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Schoustra, S.E.; Kasase, C.; Toarta, C.; Kassen, R.; Poulain, A.J. Microbial Community Structure of Three Traditional Zambian Fermented Products: Mabisi, Chibwantu and Munkoyo. PLoS ONE 2013, 8, e63948. [Google Scholar]
- Teniola, O.D.; Odunfa, S.A. Microbial assessment and quality evaluation of ogi during spoilage. World J. Microbiol. Biotechnol. 2002, 18, 731–737. [Google Scholar] [CrossRef]
- Sohliya, I.; Joshi, S.R.; Bhagobaty, R.K.; Kumar, R. Tungrymbai—A traditional fermented soybean food of the ethnic tribes of Meghalaya. Indian J. Tradit. Knowl. 2009, 8, 559–561. [Google Scholar]
- Kouamé, A.K.; Djéni, T.N.; N’Guessan, F.K.; Dje, M.K. Postprocessing microflora of commercial attieke (a fermented cassava product) produced in the south of Côte d’Ivoire. Lett. Appl. Microbiol. 2013, 56, 44–50. [Google Scholar] [CrossRef]
- del Carmen Portillo, M.; Mas, A. Analysis of microbial diversity and dynamics during wine fermentation of Grenache grape variety by high-throughput barcoding sequencing. LWT Food Sci. Technol. 2016, 72, 317–321. [Google Scholar] [CrossRef]
- Ni, K.; Minh, T.T.; Tu, T.T.M.; Tsuruta, T.; Pang, H.; Nishino, N. Comparative microbiota assessment of wilted Italian ryegrass, whole crop corn and wilted alfalfa silage using denaturing gradient gel electrophoresis and next-generation sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 1385–1394. [Google Scholar] [CrossRef]
- Gaglio, R.; Cirlincione, F.; Di Miceli, G.; Franciosi, E.; Di Gerlando, R.; Francesca, N. Microbial dynamics in durum wheat kernels during aging. Int. J. Food Microbiol. 2020, 324, 108631. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, Y.; Huang, X.; Qiao, M.; He, R.K.; Song, L. Microbial diversity of representative traditional fermented sausages in different regions of China. J. Appl. Microbiol. 2021, 130, 133–141. [Google Scholar] [CrossRef]
- Nazari, M.; Mooraki, N.; Sedaghati, M. Chemical and microbial properties of a fermented fish sauce in the presence of Lactobacillus plantarum and Paenibacillus polymyxa. Iran J. Fish Sci. 2021, 20, 663–677. [Google Scholar]
- Zhou, J.; Luo, J.; Yang, S.; Xiao, Q.; Wang, X.; Zhou, Z. Different responses of microbiota across intestinal tract to Enterococcus faecium HDRsEf1 and their correlation with inflammation in weaned piglets. Microorganisms 2021, 9, 1767. [Google Scholar] [CrossRef]
- Tao, S.; Bai, Y.; Zhou, X.; Zhao, J.; Yang, H.; Zhang, S. In Vitro Fermentation Characteristics for Different Ratios of Soluble to Insoluble Dietary Fiber by Fresh Fecal Microbiota from Growing Pigs. ACS Omega 2019, 4, 15158–15167. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Kun-Young, P.; Ji-Kang, J.; Young-Eun, L.; James, W.D. Health Benefits of Kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar]
- Dharaneedharan, S.; Heo, M.S. Korean Traditional Fermented Foods—A Potential Resource of Beneficial Microorganisms and Their Applications. J. Life Sci. 2016, 26, 496–502. [Google Scholar] [CrossRef]
- Ashaolu, T.J. A review on selection of fermentative microorganisms for functional foods and beverages: The production and future perspectives. Int. J. Food Sci. Technol. 2019, 54, 2511–2519. [Google Scholar] [CrossRef]
- Sullivanh, T.F.O.; Mahonyi, A.O.; Fitzgeraldw, G.F.; Sinderen, D.V. A Comparative Study of Malthouse and Brewhouse Microflora. J. Inst Brew. 1999, 105, 55–61. [Google Scholar] [CrossRef]
- Justé, A.; Crauwels, S.; Willems, K.; Cooman, L.D.; Lievens, B.; Aerts, G. Assessing the xylanolytic bacterial diversity during the malting process. Food Microbiol. 2013, 36, 406–415. [Google Scholar]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Waters, D.M.; Mauch, A. Lactic Acid Bacteria as a Cell Factory for the Delivery of Functional Biomolecules and Ingredients in Cereal-Based Beverages: A review. Crit. Rev. Food. Sci. Nutr. 2015, 55, 503–520. [Google Scholar] [CrossRef]
- Mohammed, S.S.D.; Orukotan, A.A.; Musa, J. Effect of fermentation and malting on some cereal weaning foods enriched with African locust beans. J. Appl. Sci. Environ. Manag. 2017, 21, 911–921. [Google Scholar] [CrossRef][Green Version]
- Krasauskas, A. Fungi in malting barley grain and malt production. Biologija 2017, 63, 283–288. [Google Scholar] [CrossRef][Green Version]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha tea fermentation: A review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Justé, A.; Malfliet, S.; Lenaerts, M.; De Cooman, L.; Aerts, G.; Willems, K.A. Microflora during malting of barley: Overview and impact on malt quality. Brew. Sci. 2011, 64, 22–31. [Google Scholar]
- Hejazi, S.N.; Orsat, V.; Azadi, B.; Kubow, S. Improvement of the in vitro protein digestibility of amaranth grain through optimization of the malting process. J. Cereal Sci. 2016, 68, 59–65. [Google Scholar] [CrossRef]
| Experimental Runs | Btm (min) | Ftp (°C) | Ftm (h) |
|---|---|---|---|
| 1 | 15 | 25 | 16 |
| 2 | 15 | 25 | 72 |
| 3 | 10 | 25 | 44 |
| 4 | 20 | 25 | 44 |
| 5 | 10 | 35 | 72 |
| 6 | 15 | 35 | 44 |
| 7 | 15 | 35 | 44 |
| 8 | 20 | 35 | 16 |
| 9 | 15 | 35 | 44 |
| 10 | 15 | 35 | 44 |
| 11 | 10 | 35 | 16 |
| 12 | 20 | 35 | 72 |
| 13 | 15 | 35 | 44 |
| 14 | 10 | 45 | 44 |
| 15 | 15 | 45 | 16 |
| 16 | 20 | 45 | 44 |
| 17 | 15 | 45 | 72 |
| Ftp (°C) | Ftm (h) | Btm (min) | pH | TTA (%) | TSS (°Brix) |
|---|---|---|---|---|---|
| White Maize | |||||
| 25 | 16 | 15 | 3.98 j ± 0.01 | 0.25 b ± 0 | 4.60 abc ± 0.17 |
| 25 | 72 | 15 | 3.50 a ± 0.01 | 0.50 e ± 0 | 5.03 e ± 0.15 |
| 25 | 44 | 10 | 3.56 b ± 0.01 | 0.50 e ± 0 | 4.60 abc ± 0 |
| 25 | 44 | 20 | 3.59 cd ± 0.01 | 0.50 e ± 0 | 4.43 a ± 0.29 |
| 35 | 16 | 10 | 3.98 j ± 0 | 0.25 b ± 0 | 4.60 abc ± 0.10 |
| 35 | 72 | 10 | 3.73 g ± 0.01 | 0.48 de ± 0.03 | 4.57 ab ± 0.15 |
| 35 | 16 | 20 | 4.03 k ± 0 | 0.20 a ± 0 | 5.90 g ± 0.10 |
| 35 | 72 | 20 | 3.71 f ± 0.01 | 0.48 de ± 0.03 | 4.97 de ± 0.06 |
| 35 | 44 | 15 | 3.76 h ± 0.02 | 0.48 de ± 0.03 | 5.43 f ± 0.06 |
| 35 | 44 | 15 | 3.62 e ± 0.01 | 0.48 de ± 0.03 | 4.73 bd ± 0.12 |
| 35 | 44 | 15 | 3.62 e ± 0.01 | 0.50 e ± 0 | 4.63 ab ± 0.15 |
| 35 | 44 | 15 | 3.60 d ± 0.01 | 0.47 d ± 0.03 | 4.93 de ± 0.06 |
| 35 | 44 | 15 | 3.57 c ± 0.01 | 0.47 d ± 0.03 | 4.83 de ± 0.06 |
| 45 | 16 | 15 | 4.20 n ± 0.01 | 0.20 a ± 0 | 4.83 de ± 0.06 |
| 45 | 72 | 15 | 3.90 i ± 0.01 | 0.40 c ± 0 | 5.30 f ± 0.20 |
| 45 | 44 | 10 | 4.08 m ± 0.01 | 0.20 a ± 0 | 4.60 abc ± 0 |
| 45 | 44 | 20 | 4.07 l ± 0.02 | 0.20 a ± 0 | 5.40 f ± 0.10 |
| Yellow Maize | |||||
| 25 | 16 | 15 | 4.01 j ± 0.01 | 0.25 b ± 0 | 4.43 ab ± 0.31 |
| 25 | 72 | 15 | 3.48 a ± 0.01 | 0.50 d ± 0 | 5.00 def ± 0 |
| 25 | 44 | 10 | 3.51 b ± 0.01 | 0.48 d ± 0.03 | 4.60 bc ± 0 |
| 25 | 44 | 20 | 3.56 c ± 0.01 | 0.50 d ± 0 | 5.43 gh ± 0.15 |
| 35 | 72 | 10 | 3.92 i ± 0.01 | 0.40 c ± 0 | 4.77 bcde ± 0.06 |
| 35 | 44 | 15 | 3.69 g ± 0.01 | 0.48 d ± 0 | 4.73 bcd ± 0.15 |
| 35 | 44 | 15 | 3.92 i ± 0.01 | 0.40 c ± 0 | 5.20 fgh ± 0.10 |
| 35 | 16 | 20 | 3.73 h ± 0.01 | 0.48 d ± 0.03 | 5.23 fgh ± 0.15 |
| 35 | 44 | 15 | 3.73 h ± 0.02 | 0.48 d ± 0.03 | 5.87 i ± 0.15 |
| 35 | 44 | 15 | 3.60 d ± 0 | 0.48 d ± 0.03 | 4.80 bcde ± 0.10 |
| 35 | 16 | 10 | 3.61 e ± 0.01 | 0.48 d ± 0.03 | 4.90 cdef ± 0.17 |
| 35 | 72 | 20 | 3.61 e ± 0.01 | 0.48 d ± 0.03 | 4.57 bc ± 0.12 |
| 35 | 44 | 15 | 3.63 f ± 0.01 | 0.48 d ± 0.03 | 4.10 a ± 0.36 |
| 45 | 44 | 10 | 4.87 l ± 0 | 0.20 a ± 0 | 5.47 bcd ± 0.05 |
| 45 | 16 | 15 | 5.28 n ± 0.01 | 0.20 a ± 0 | 4.63 efg ± 0.12 |
| 45 | 44 | 20 | 5.00 m ± 0.01 | 0.20 a ± 0 | 5.57 gh ± 0.15 |
| 45 | 72 | 15 | 4.34 k ± 0 | 0.20 a ± 0 | 5.13 hi ± 0.21 |
| Coefficient | pH | TTA | TSS |
|---|---|---|---|
| White Maize | |||
| β0 | 5.70414 | −0.950000 | 3.56018 |
| β1 | −0.06275 | 0.043339 | 0.02193 |
| β2 | −0.02664 * | 0.012366 | 0.00673 |
| β3 | −0.08255 | 0.063821 | 0.00871 |
| β11 | 0.00113 * | −0.000725 | −0.00111 |
| β22 | 0.00019 * | −0.000089 * | 0.00018 |
| β33 | 0.00321 | −0.002300 * | −0.00165 |
| β12 | 0.00016 | −0.000045 | 0.00004 |
| β13 | −0.00020 | 0.000000 | 0.00485 |
| β23 | −0.00013 | 0.000089 | −0.00161 |
| R2 (%) | 94.31 | 90.37 | 60.58 |
| Yellow Maize | |||
| β0 | 7.47591 | −1.12260 | 3.90414 |
| β1 | −0.30732 * | 0.10172 * | −0.01424 |
| β2 | 0.01592 | 0.00988 | 0.04825 |
| β3 | 0.04174 | −0.03239 | −0.03851 |
| β11 | 0.00542 * | −0.00146 * | 0.00126 |
| β22 | 0.00003 | −0.00004 | −0.00035 |
| β33 | −0.00074 | 0.00107 | 0.00781 |
| β12 | −0.00037 | −0.00022 | −0.00006 |
| β13 | 0.00040 | −0.00010 | −0.00365 |
| β23 | −0.00077 | 0.00014 | −0.00095 |
| R2 (%) | 90.45 | 89.80 | 31.99 |
| WM | pH | TTA | TSS | |||
|---|---|---|---|---|---|---|
| BF | AF | BF | AF | BF | AF | |
| MS | 5.96 b ± 0.01 | 3.54 b ± 0.01 | 0.20 b ± 0 | 0.60 c ± 0 | 5.20 a ± 0.21 | 4.67 a ± 0.21 |
| W | 6.39 d ± 0.01 | 4.51 d ± 0.01 | 0.15 a ± 0.03 | 0.47 a ± 0.06 | 5.90 d ± 0.31 | 5.17 b ± 0.31 |
| MM | 5.90 a ± 0.02 | 3.41 a ± 0.02 | 0.20 b ± 0 | 0.68 d ± 0.03 | 5.61 c ± 0.15 | 5.03 ab ± 0.15 |
| MWM | 5.95 c ± 0.01 | 3.63 c ± 0.01 | 0.20 b ± 0 | 0.57 b ± 0.03 | 5.50 b ± 0.17 | 4.70 a ± 0.17 |
| YM | ||||||
| MS | 5.97 b ± 0.02 | 3.47 b ± 0.02 | 0.20 b ± 0 | 0.60 bc ± 0 | 5.90 d ± 0.12 | 5.17 bc ± 0.15 |
| W | 6.41 c ± 0.01 | 4.65 c ± 0.01 | 0.15 a ± 0.03 | 0.38 a ± 0.03 | 5.50 b ± 0.12 | 4.93 a ± 0.12 |
| MM | 5.96 b ± 0.01 | 3.49 b ± 0.01 | 0.20 a ± 0 | 0.57 b ± 0.03 | 5.61 c ± 0.01 | 5.53 c ± 0.12 |
| MWM | 5.91 a ± 0.01 | 3.44 a ± 0.01 | 0.20 b ± 0 | 0.62 c ± 0.03 | 5.20 a ± 0.15 | 4.80 a ± 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daji, G.A.; Green, E.; Abrahams, A.; Oyedeji, A.B.; Masenya, K.; Kondiah, K.; Adebo, O.A. Physicochemical Properties and Bacterial Community Profiling of Optimal Mahewu (A Fermented Food Product) Prepared Using White and Yellow Maize with Different Inocula. Foods 2022, 11, 3171. https://doi.org/10.3390/foods11203171
Daji GA, Green E, Abrahams A, Oyedeji AB, Masenya K, Kondiah K, Adebo OA. Physicochemical Properties and Bacterial Community Profiling of Optimal Mahewu (A Fermented Food Product) Prepared Using White and Yellow Maize with Different Inocula. Foods. 2022; 11(20):3171. https://doi.org/10.3390/foods11203171
Chicago/Turabian StyleDaji, Grace Abosede, Ezekiel Green, Adrian Abrahams, Ajibola Bamikole Oyedeji, Kedibone Masenya, Kulsum Kondiah, and Oluwafemi Ayodeji Adebo. 2022. "Physicochemical Properties and Bacterial Community Profiling of Optimal Mahewu (A Fermented Food Product) Prepared Using White and Yellow Maize with Different Inocula" Foods 11, no. 20: 3171. https://doi.org/10.3390/foods11203171
APA StyleDaji, G. A., Green, E., Abrahams, A., Oyedeji, A. B., Masenya, K., Kondiah, K., & Adebo, O. A. (2022). Physicochemical Properties and Bacterial Community Profiling of Optimal Mahewu (A Fermented Food Product) Prepared Using White and Yellow Maize with Different Inocula. Foods, 11(20), 3171. https://doi.org/10.3390/foods11203171







