Vacuum Packaging Maintains Fresh Characteristics of Previously Frozen Beef Steaks during Simulated Retail Display
Abstract
:1. Introduction
2. Materials and Methods
2.1. Muscle Fabrication
2.2. Packaging Treatments
2.3. Simulated Storage Periods
2.4. Instrumental Color
2.5. Microbial Analysis: Aerobic Plate Count
2.6. Lipid Oxidation
2.7. Fresh pH
2.8. Purge Loss
2.9. Statistical Analysis
3. Results and Discussion
3.1. Instrumental Color
3.2. Aerobic Plate Count Changes
3.3. Lipid Oxidation
3.4. Fresh pH
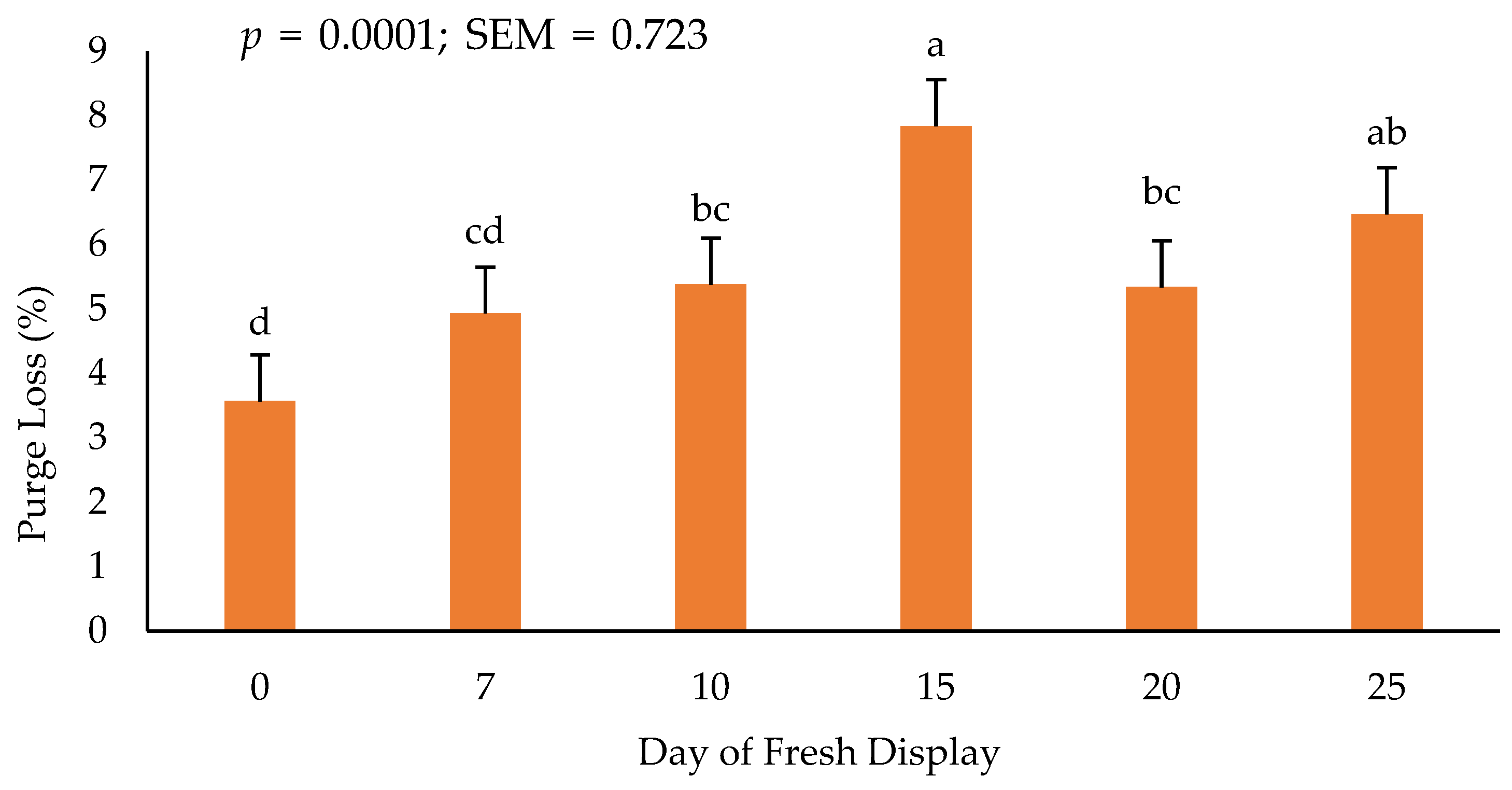
3.5. Purge Loss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muela, E.; Monge, P.; Sañudo, C.; Campo, M.M.; Beltrán, J.A. Meat quality of lamb frozen stored up to 21 months: Instrumental analyses on thawed meat during display. Meat Sci. 2015, 102, 35–40. [Google Scholar] [CrossRef]
- Lentz, C.P. Effect of light intensity and other factors on the color of frozen prepackaged beef. Can. Inst. Food Technol. J. 1979, 12, 47–50. [Google Scholar] [CrossRef]
- Zhan, X.; Sun, D.-W.; Zhu, Z.; Wang, Q.-J. Improving the quality and safety of frozen muscle foods by emerging freezing technologies: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2925–2938. [Google Scholar] [CrossRef]
- Okoro, C.; Umofia, E. Effects of freezing and thawing on the microbiological and physicochemical qualities of frozen pork. World J. Pharm. Med. Res. 2018, 4, 169–173. [Google Scholar]
- Löndahl, G.; Nilsson, T. Freezing|Storage of frozen foods. Encycl. Food Sci. Nutr. 2003, 2, 2732–2735. [Google Scholar] [CrossRef]
- Eastridge, J.S.; Bowker, B.C. Effect of rapid thawing on the meat quality attributes of USDA select beef strip loin steaks. J. Food Sci. 2011, 76, S156–S162. [Google Scholar] [CrossRef]
- Akhtar, S.; Issa Khan, M.; Faiz, F. Effect of thawing on frozen meat quality: A comprehensive review. Pak. J. Food Sci. 2013, 23, 198–211. [Google Scholar]
- Food Drug Administration. Available online: https://www.fda.gov/food/fda-food-code/food-code-2017 (accessed on 3 June 2022).
- USDA, Food Safety and Inspection Service. The Big Thaw—Safe Defrosting Methods. Available online: http://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/big-thaw-safe-defrosting-methods (accessed on 13 June 2022).
- Aberle, E.; Forrest, J.; Gerrard, D.; Mills, E. Principles of Meat Science, 5th ed.; Kendall Hunt: Dubuque, IA, USA, 1989; ISBN 978-0-7575-9995-8. [Google Scholar]
- Kenawi, M.A. Evaluation of some packaging materials and treatments on some properties of beef during frozen storage. Food Chem. 1994, 51, 69–74. [Google Scholar] [CrossRef]
- Gray, J.I.; Gomaa, E.A.; Buckley, D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996, 43, 111–123. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Marchello, J.A.; Ahmad, H.A. Effect of freezing and microbial growth on myoglobin derivatives of beef. J. Agric. Food Chem. 1999, 47, 4093–4099. [Google Scholar] [CrossRef]
- Lagerstedt, Å.; Ahnström, M.L.; Lundström, K. Vacuum skin pack of beef—A consumer friendly alternative. Meat Sci. 2011, 88, 391–396. [Google Scholar] [CrossRef]
- Aidani, E.; Aghamohammadi, B.; Akbarian, M.; Morshedi, A.; Hadidi, M.; Ghasemkhani, N.; Akbarian, A. Effect of chilling, freezing and thawing on meat quality: A review. Int. J. Biosci. 2014, 5, 159–169. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Marriott, N.G.; Garcia, R.A.; Kurland, M.E.; Lee, D.R. Appearance and microbial quality of thawed retail cuts of beef, pork and lamb. J. Food Prot. 1980, 43, 185–189. [Google Scholar] [CrossRef]
- Lee, K.T. Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci. 2010, 86, 138–150. [Google Scholar] [CrossRef]
- American Meat Science Association. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Commission Internationale de l’ Eclairage. Recommendations on Uniform Color Spaces—Color Differences Equations, Psychometric Color Terms; C.I.E. Publication No. 15, Suppl. 2 (E-1.3.1), 1972/(TC-1-3); Bureau Central de la CIE: Paris, France, 1978. [Google Scholar]
- United States Food and Drug, Bacteriological Analytical Manual. Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam (accessed on 15 June 2022).
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Fernández-López, J.; Sayas-Barberá, E.; Muñoz, T.; Sendra, E.; Navarro, C.; Pérez-Alvarez, J.A. Effect of packaging conditions on shelf-life of ostrich steaks. Meat Sci. 2008, 78, 143–152. [Google Scholar] [CrossRef]
- Coombs, C.E.O.; Holman, B.W.B.; Friend, M.A.; Hopkins, D.L. Long-term red meat preservation using chilled and frozen storage combinations: A review. Meat Sci. 2017, 125, 84–94. [Google Scholar] [CrossRef]
- Otremba, M.M.; Dikeman, M.E.; Boyle, E.A. Refrigerated shelf life of vacuum-packaged, previously frozen ostrich meat. Meat Sci. 1999, 52, 279–283. [Google Scholar] [CrossRef]
- Qian, S.; Li, X.; Wang, H.; Sun, Z.; Zhang, C.; Guan, W.; Blecker, C. Effect of sub-freezing storage (−6, −9 and −12 °C) on quality and shelf life of beef. Int. J. Food Sci. Technol. 2018, 53, 2129–2140. [Google Scholar] [CrossRef]
- Henriott, M.L.; Herrera, N.J.; Ribeiro, F.A.; Hart, K.B.; Bland, N.A.; Calkins, C.R. Impact of myoglobin oxygenation level on color stability of frozen beef steaks. J. Anim. Sci. 2020, 98, skaa193. [Google Scholar] [CrossRef]
- Shange, N.; Makasi, T.N.; Gouws, P.A.; Hoffman, L.C. The influence of normal and high ultimate muscle pH on the microbiology and colour stability of previously frozen black wildebeest (Connochaetes gnou) meat. Meat Sci. 2018, 135, 14–19. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Coombs, C.E.O.; Morris, S.; Kerr, M.J.; Hopkins, D.L. Effect of long term chilled (up to 5 weeks) then frozen (up to 12 months) storage at two different sub-zero holding temperatures on beef: Meat quality and microbial loads. Meat Sci. 2017, 133, 133–142. [Google Scholar] [CrossRef]
- MacDougall, D.B. Changes in the colour and opacity of meat. Food Chem. 1982, 9, 75–88. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Hanna, M.A.; Mandigo, R.W. Lipid oxidation in ground beef patties as affected by time-temperature and product packaging parameters. J. Food Sci. 1988, 53, 714–717. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Li, C.; Xu, B. Effect of frozen storage on the lipid oxidation, protein oxidation, and flavor profile of marinated raw beef meat. Food Chem. 2022, 376, 131881. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Kubiak, D.; Panfil, A. The effect of long-term frozen storage on the quality of meat (Longissimus Thoracis et Lumborum) from female roe deer (Capreolus capreolus L.). J. Food Qual. 2018, 2018, 4691542. [Google Scholar] [CrossRef]
- Jenkins, W.A.; Harrington, J.P. Packaging Foods with Plastics; Technomic Publishing Company: Lancaster, PA, USA, 1991; ISBN 08-776-27908. [Google Scholar]
- Limbo, S.; Torri, L.; Sinelli, N.; Franzetti, L.; Casiraghi, E. Evaluation and predictive modeling of shelf life of minced beef stored in high-oxygen modified atmosphere packaging at different temperatures. Meat Sci. 2010, 84, 129–136. [Google Scholar] [CrossRef]
- Owen, J.E.; Lawrie, R.A. The effect of an artificially induced high pH on the susceptibility of minced porcine muscle to undergo oxidative rancidity under frozen storage. Int. J. Food Sci. Technol. 1975, 10, 169–180. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: A review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Dainty, R.H.; Mackey, B.M. The Relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. Soc. Appl. Bacteriol. Symp. Ser. 1992, 21, 103S–114S. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O. Extending the storage life of raw chilled meats. Meat Sci. 1996, 43, 99–109. [Google Scholar] [CrossRef]
- Honikel, K.O.; Fischer, A.; Hamm, R. Influence of postmortem changes in bovine muscle on the water-holding capacity of beef. Postmortem storage of muscle at 20 °C. J. Food Sci. 1981, 46, 1–6. [Google Scholar] [CrossRef]
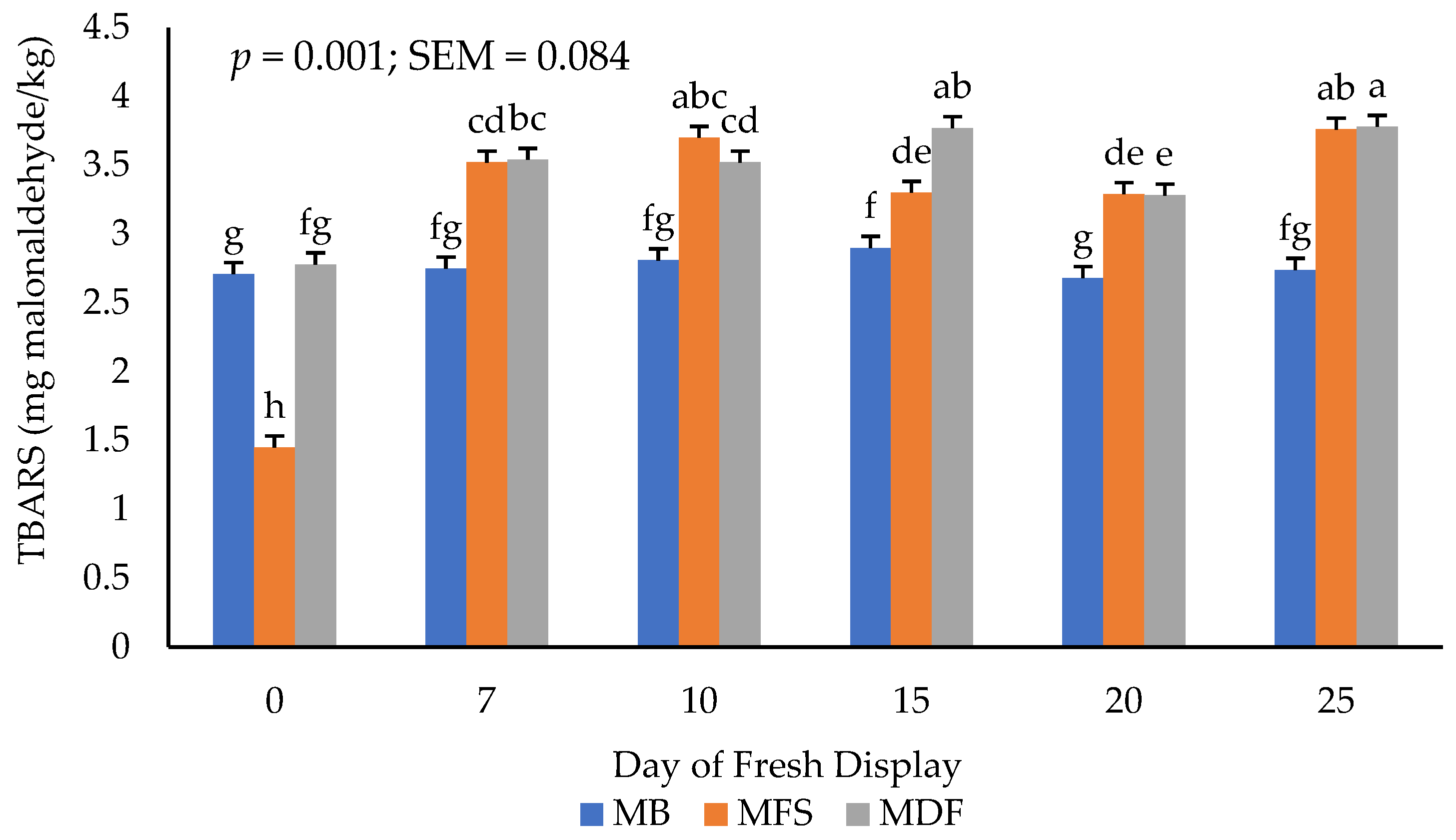
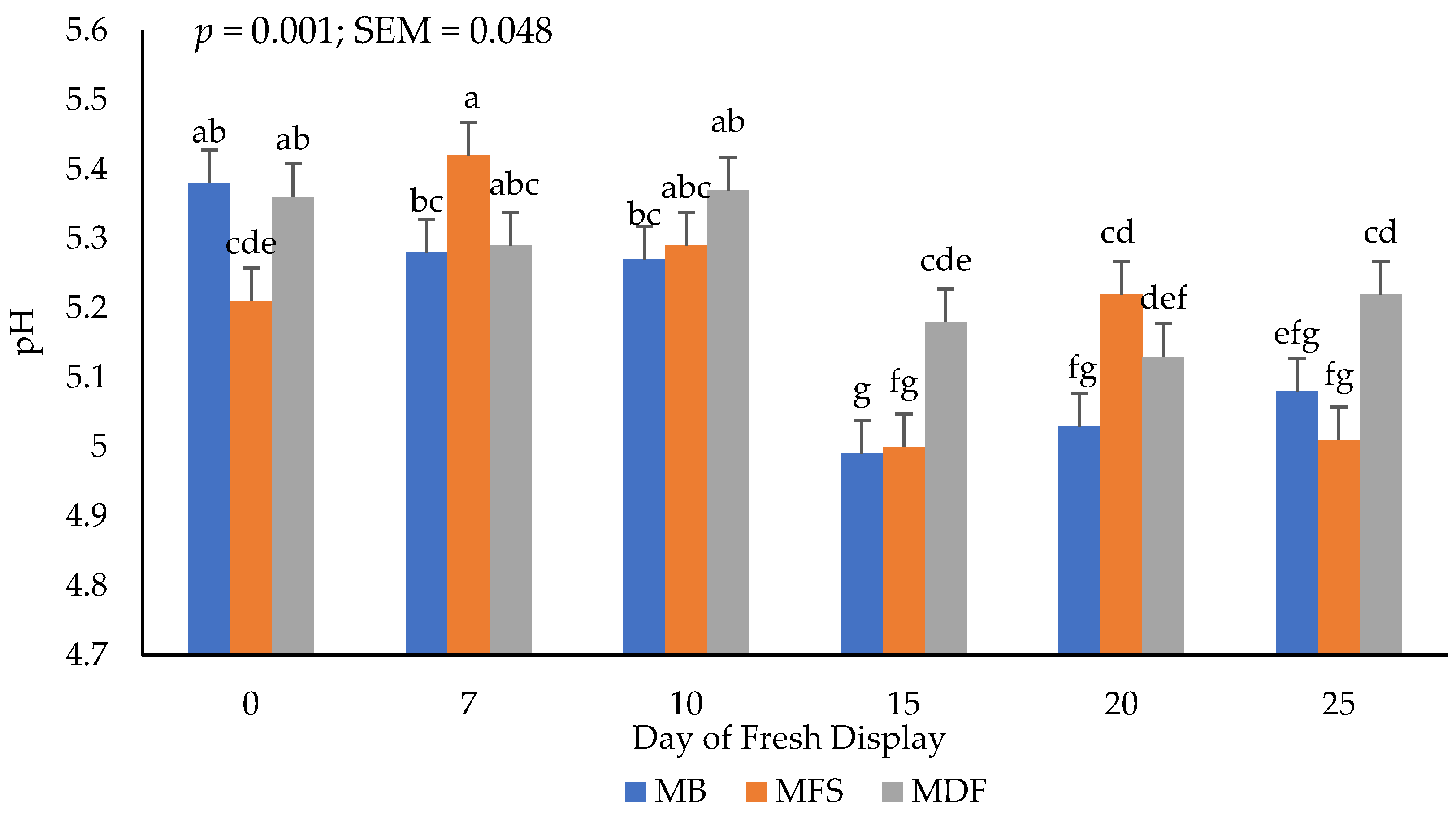
| Day | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 10 | 15 | 20 | 25 | SEM * | |
| MB 1 | |||||||
| a* 2 | 13.61 e | 22.11 a | 20.45 b | 20.15 b | 17.52 c | 15.76 d | 0.330 |
| b* 2 | 13.94 fg | 13.17 hi | 13.03 hi | 13.77 gh | 13.59 ghi | 12.88 hi | 0.251 |
| C* 3 | 19.69 e | 25.79 a | 24.27 b | 24.45 b | 22.25 d | 20.40 e | 0.326 |
| Hue (°) 4 | 45.87 d | 30.85 i | 32.52 h | 34.41 g | 38.03 f | 39.51 f | 0.716 |
| MFS 1 | |||||||
| a* 2 | 17.21 c | 10.46 gh | 10.46 gh | 10.78 fgh | 10.03 hi | 10.17 ghi | 0.345 |
| b* 2 | 16.28 a | 16.25 ab | 15.33 cd | 14.66 ef | 13.94 fg | 13.92 fgh | 0.251 |
| C* 3 | 23.76 bc | 19.94 e | 18.61 f | 18.32 fg | 17.25 h | 17.29 gh | 0.344 |
| Hue (°) 4 | 43.63 e | 54.83 b | 55.85 b | 53.90 b | 54.36 b | 53.69 b | 0.716 |
| MDF 1 | |||||||
| a* 2 | 17.04 c | 11.25 fg | 9.19 i | 10.29 gh | 10.69 fgh | 11.98 f | 0.345 |
| b* 2 | 15.79 bc | 16.05 ab | 14.99 de | 14.34 fg | 12.99 hi | 12.75 i | 0.251 |
| C* 3 | 23.31 c | 19.65 e | 17.62 fgh | 17.72 fgh | 16.94 h | 17.56 fgh | 0.344 |
| Hue (°) 4 | 43.06 e | 55.24 b | 58.58 a | 54.34 b | 50.45 c | 47.06 d | 0.716 |
| Day | SEM * | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 15 | 20 | 25 | ||
| MB 1 | >0.001 e | 0.57 d | 1.25 ab | 0.85 bcd | 0.57 d | 0.142 |
| MFS 1 | 0.04 e | 0.56 d | 0.86 bcd | 1.46 a | 1.03 bc | 0.142 |
| MDF 1 | >0.001 e | 0.83 cd | 0.85 cd | 1.47 a | 1.44 a | 0.142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagoner, M.P.; Reyes, T.M.; Zorn, V.E.; Coursen, M.M.; Corbitt, K.E.; Wilborn, B.S.; Starkey, C.W.; Brandebourg, T.D.; Belk, A.D.; Bonner, T.; et al. Vacuum Packaging Maintains Fresh Characteristics of Previously Frozen Beef Steaks during Simulated Retail Display. Foods 2022, 11, 3012. https://doi.org/10.3390/foods11193012
Wagoner MP, Reyes TM, Zorn VE, Coursen MM, Corbitt KE, Wilborn BS, Starkey CW, Brandebourg TD, Belk AD, Bonner T, et al. Vacuum Packaging Maintains Fresh Characteristics of Previously Frozen Beef Steaks during Simulated Retail Display. Foods. 2022; 11(19):3012. https://doi.org/10.3390/foods11193012
Chicago/Turabian StyleWagoner, Madison P., Tristan M. Reyes, Virgina E. Zorn, Madison M. Coursen, Katie E. Corbitt, Barney S. Wilborn, Charles W. Starkey, Terry D. Brandebourg, Aeriel D. Belk, Tom Bonner, and et al. 2022. "Vacuum Packaging Maintains Fresh Characteristics of Previously Frozen Beef Steaks during Simulated Retail Display" Foods 11, no. 19: 3012. https://doi.org/10.3390/foods11193012
APA StyleWagoner, M. P., Reyes, T. M., Zorn, V. E., Coursen, M. M., Corbitt, K. E., Wilborn, B. S., Starkey, C. W., Brandebourg, T. D., Belk, A. D., Bonner, T., & Sawyer, J. T. (2022). Vacuum Packaging Maintains Fresh Characteristics of Previously Frozen Beef Steaks during Simulated Retail Display. Foods, 11(19), 3012. https://doi.org/10.3390/foods11193012







