The Impact of Dietary Fiber as a Prebiotic on Inflammation in Children with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Dietary Assessment
2.4. Anthropometry and Body Composition
2.5. Inflammatory Cytokines
2.6. Fecal Calprotectin
2.7. Metabolic Profiles
2.8. Statistical Analysis
3. Results
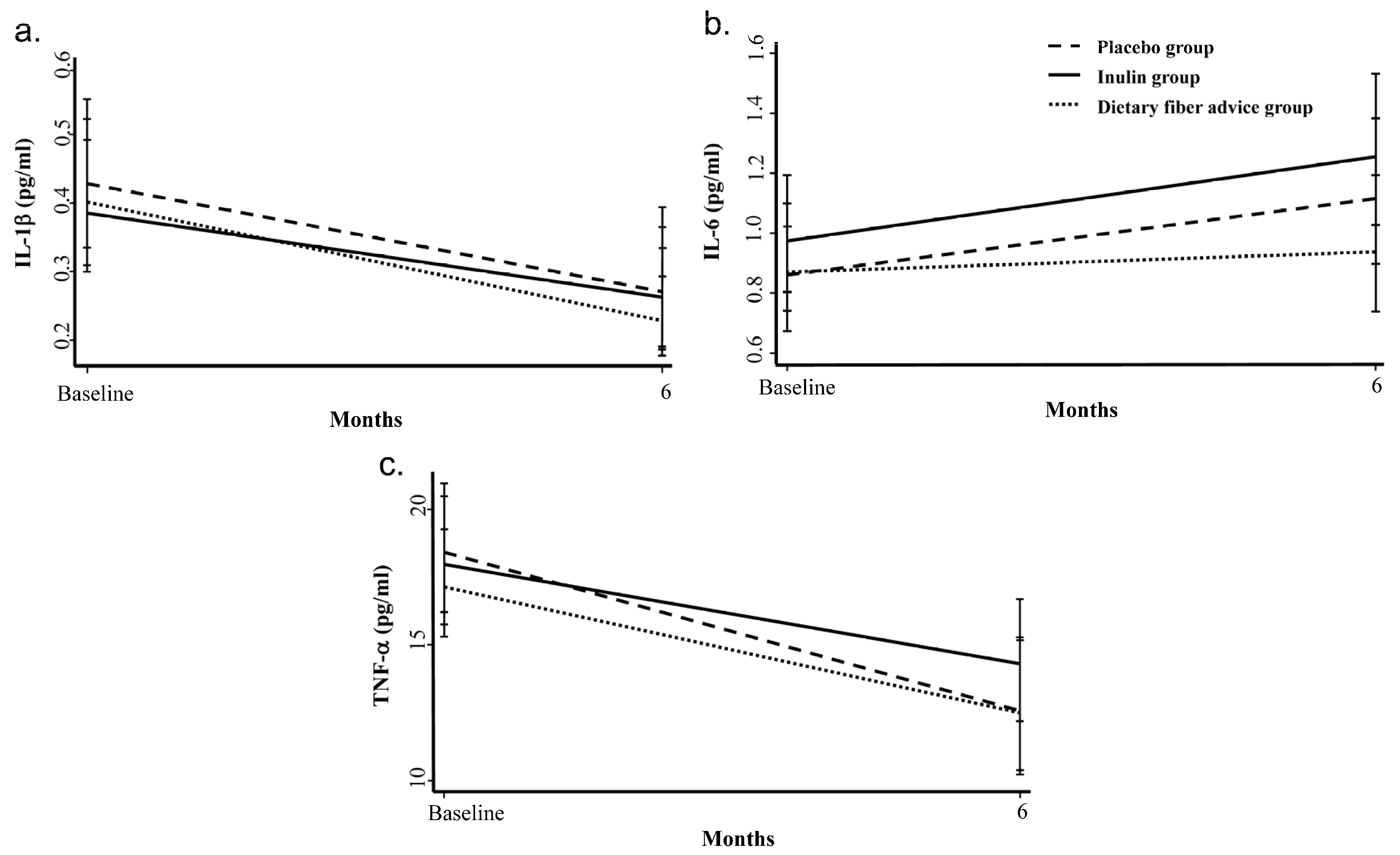
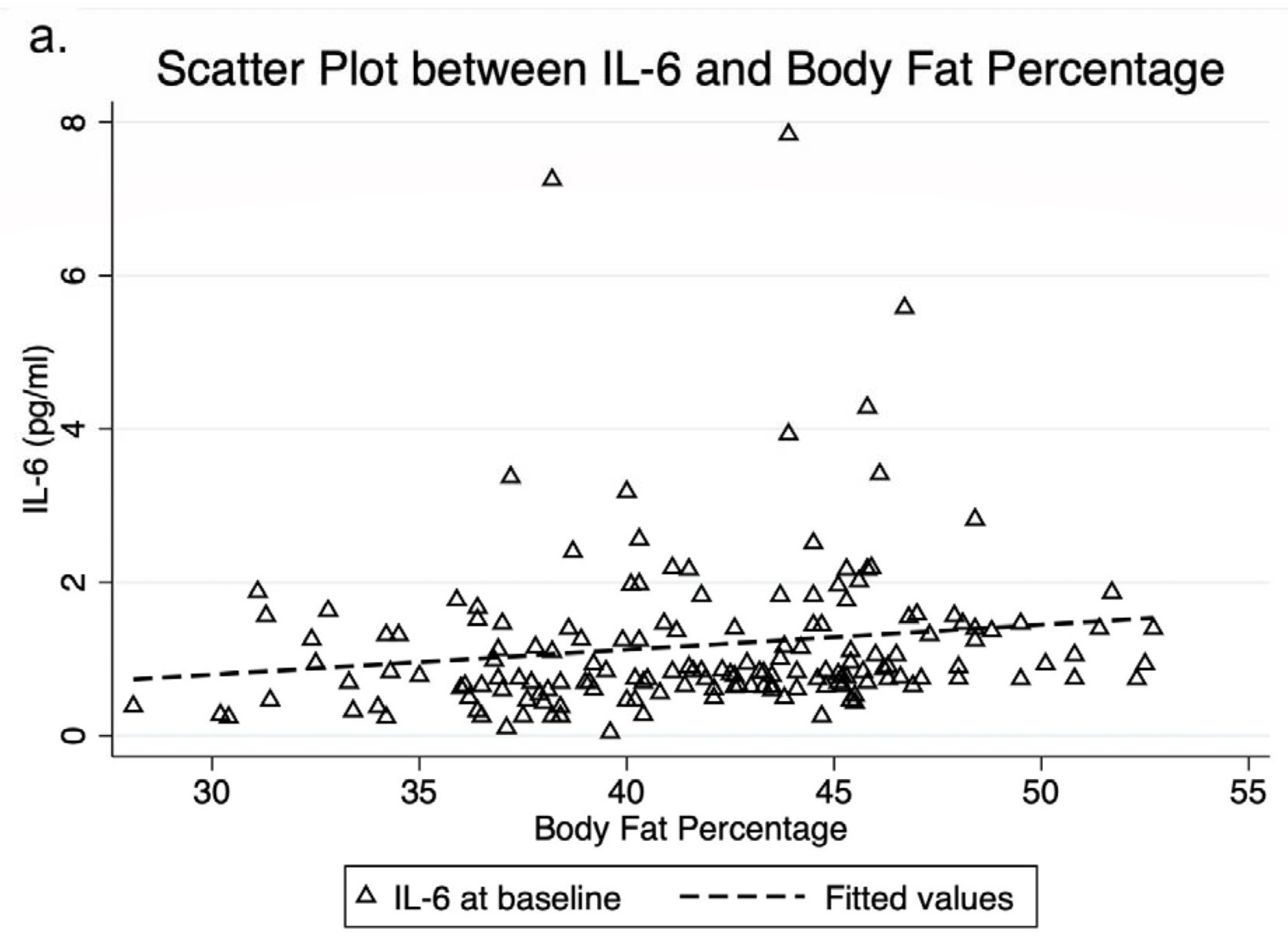
3.1. Inflammatory Cytokines
3.2. Fecal Calprotectin
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Overweight and Obesity. Available online: https://www.who.int/gho/ncd/risk_factors/overweight_adolescents_text/en/ (accessed on 6 July 2022).
- World Health Organization European Regional Obesity Report 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/353747/9789289057738-eng.pdf (accessed on 26 August 2022).
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with met-abolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Gut microbiota and obesity. Cell Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Galvão, J.G.F.M.; Silva, J.S.D.F.D.; De Sales-Neto, J.M.; Rodrigues-Mascarenhas, S. Obesity-Driven Gut Microbiota Inflammatory Pathways to Metabolic Syndrome. Front. Physiol. 2015, 6, 341. [Google Scholar] [CrossRef]
- Sun, L.; Ma, L.; Ma, Y.; Zhang, F.; Zhao, C.; Nie, Y. Insights into the role of gut microbiota in obesity: Pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 2018, 9, 397–403. [Google Scholar] [CrossRef]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef]
- Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.; Trindade, E.B. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: A systematic review. Clin. Nutr. 2017, 36, 1197–1206. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-enriched inulin improves some inflam-matory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical tri-al. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Gargari, B.P.; Jafarabadi, M.A.; Aliasgharzadeh, A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2013, 65, 117–123. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, W.J. A Study of Fecal Calprotectin in Obese Children and Adults. J. Obes. Metab. Syndr. 2018, 27, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Ayling, R.M.; Kok, K. Fecal Calprotectin. Adv. Clin. Chem. 2018, 87, 161–190. [Google Scholar]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2021).
- Visuthranukul, C.; Chamni, S.; Kwanbunbumpen, T.; Saengpanit, P.; Chongpison, Y.; Tepaamorndech, S.; Panichsillaphakit, E.; Uaariyapanichkul, J.; Nonpat, N.; Chomtho, S. Effects of inulin supplementation on body composition and metabolic outcomes in children with obesity. Sci. Rep. 2022, 12, 13014. [Google Scholar] [CrossRef] [PubMed]
- Willis, H.J.; Slavin, J.L. Dietary Fiber. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A., Catharine, Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012; pp. 58–64. [Google Scholar]
- Judprasong, K.; Puwastien, P.; Rojroongwasinkul, N.; Nitithamyong, A.; Sridonpai, P.; Somjai, A. Institute of Nutrition, Mahidol University 2015. Thai Food Composition Database, Online Version 2, September 2018, Thailand. Available online: http://www.inmu.mahidol.ac.th/thaifcd (accessed on 25 May 2022).
- Banjong, O.; Wanijjakul, C.; Peemanee, K. Application Manual: INMUCAL-Nutrients V.3. 2016. Available online: https://inmu2.mahidol.ac.th/inmucal/index.php (accessed on 25 May 2022).
- Blossner, M.; Siyam, A.; Borghi, E.; Onyango, A.; de Onis, M. WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World’s Children and Adolescents. Department of Nutrition for Health and Development, World Health Organization. Available online: https://www.who.int/growthref/tools/en/ (accessed on 25 May 2022).
- Wells, J.; ALSPAC Study Team; Cole, T. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int. J. Obes. 2002, 26, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Møller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2015, 17, 297–312. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Pipek, R.; Mandarino, L.J.; DeFronzo, R.A. Tumor necrosis factor α and insulin resistance in obese type 2 diabetic patients. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 88–94. [Google Scholar] [CrossRef]
- Vázquez-Carrera, M. Unraveling the Effects of PPARβ/δ on Insulin Resistance and Cardiovascular Disease. Trends Endocrinol. Metab. 2016, 27, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, M.; Albert, V.; Wölnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.; Claus, S.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.; Bindels, L.B.; De Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2012, 62, 1112–1121. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Lecerf, J.-M.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H.; et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of lep-tin in a population of Mexican Americans: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Hester, S.N.; Mastaloudis, A.; Gray, R.; Antony, J.M.; Evans, M.; Wood, S.M. Efficacy of an Anthocyanin and Prebiotic Blend on Intestinal Environment in Obese Male and Female Subjects. J. Nutr. Metab. 2018, 2018, 7497260. [Google Scholar] [CrossRef]
- Mendall, M.A.; Chan, D.; Patel, R.; Kumar, D. Faecal calprotectin: Factors affecting levels and its potential role as a surrogate marker for risk of de-velopment of Crohn’s Disease. BMC Gastroenterol. 2016, 16, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Placebo Group * (n = 55) | Inulin Group * (n = 55) | Dietary Fiber Advice Group * (n = 55) | |
|---|---|---|---|
| Age, years | 10.7 ± 2.4 | 10.3 ± 2.1 | 10.4 ± 2.0 |
| Sex, male, % | 56.4 | 54.6 | 67.3 |
| BMI, kg/m2 | 28.5 ± 4.6 | 28.3 ± 4.5 | 27.4 ± 3.4 |
| BMI for age z-score | 3.2 ± 1.1 | 3.3 ± 1.0 | 3.2 ± 0.95 |
| Waist circumference, cm | 90.6 ± 10.8 | 89.9 ± 11.3 | 88.6 ± 10.0 |
| SBP, mmHg | 115.3 ± 10.0 | 114.1 ± 8.3 | 117.4 ± 11.1 |
| Acanthosis nigricans, % | 83.6 | 76.4 | 80.0 |
| Tanner stage | |||
| Stage 1, % | 56.36 | 65.45 | 69.09 |
| Stage 2, % | 10.91 | 20 | 12.73 |
| Stage 3, % | 20 | 12.73 | 10.91 |
| Stage 4, % | 10.91 | 1.82 | 7.27 |
| Stage 5, % | 1.82 | 0 | 0 |
| BIA | |||
| FMI, kg/m2 | 12.0 ± 2.9 | 11.9 ± 3.1 | 11.4 ± 2.7 |
| FFMI, kg/m2 | 16.3 ± 2.5 | 16.2 ± 1.9 | 16.0 ± 1.7 |
| Percent body fat, % | 42.0 ± 5.5 | 41.9 ± 4.8 | 41.3 ± 4.9 |
| Trunk FMI, kg/m2 | 5.7 ± 1.4 | 5.7 ± 1.5 | 5.5 ± 1.3 |
| VFA, cm2 | 133.9 ± 39.3 | 128.8 ± 42.0 | 125.3 ± 39.7 |
| Total nutrient intake | |||
| Caloric intake, kcal/day | 1419 ± 537 | 1470 ± 571 | 1463 ± 513 |
| Protein intake, g/kg/day | 1.53 ± 0.70 | 1.54 ± 0.57 | 1.67 ± 0.66 |
| Dietary fiber, g/1000 kcal | 2.8 ± 1.9 | 2.9 ± 2.3 | 2.6 ± 1.9 |
| Fat intake, g/day | 56.9 ± 28.0 | 60.9 ± 31.1 | 58.3 ± 27.4 |
| Cholesterol intake, mg/day | 300 ± 198 | 316 ± 250 | 330 ± 236 |
| Caloric distribution (%C:P:F) | 48:16:36 | 48:16:36 | 47:17:36 |
| Metabolic profiles | |||
| Total cholesterol, mg/dL | 189.8 ± 29.0 | 189.6 ± 33.7 | 189.3 ± 32.8 |
| LDL-C, mg/dL | 129.5 ± 27.2 | 130.4 ± 36.7 | 126.9 ± 30.5 |
| HDL-C, mg/dL | 50.1 ± 9.2 | 50.4 ± 10.2 | 53.2 ± 9.1 |
| Triglyceride, mg/dL | 103.8 ± 52.6 | 99.3 ± 36.5 | 101.6 ± 53.2 |
| ALT, U/L | 32.8 ± 32.5 | 31.3 ± 22.8 | 27.1 ± 17.2 |
| FPG, mg/dL | 82.6 ± 5.9 | 83.7 ± 5.5 | 83.2 ± 7.4 |
| Inflammatory cytokines † | |||
| IL-1β, pg/mL | 0.33 (110.6) | 0.31 (108.2) | 0.29 (118.8) |
| IL-6, pg/mL | 0.99 (108.2) | 1.10 (80.0) | 0.89 (63.0) |
| TNF-α, pg/mL | 15.20 (48.8) | 15.70 (49.2) | 14.40 (43.0) |
| Fecal calprotectin, µg/g † | 81.00 (124.26) | 93.55 (104.88) | 76.78 (125.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visuthranukul, C.; Kwanbunbumpen, T.; Chongpison, Y.; Chamni, S.; Panichsillaphakit, E.; Uaariyapanichkul, J.; Maholarnkij, S.; Chomtho, S. The Impact of Dietary Fiber as a Prebiotic on Inflammation in Children with Obesity. Foods 2022, 11, 2856. https://doi.org/10.3390/foods11182856
Visuthranukul C, Kwanbunbumpen T, Chongpison Y, Chamni S, Panichsillaphakit E, Uaariyapanichkul J, Maholarnkij S, Chomtho S. The Impact of Dietary Fiber as a Prebiotic on Inflammation in Children with Obesity. Foods. 2022; 11(18):2856. https://doi.org/10.3390/foods11182856
Chicago/Turabian StyleVisuthranukul, Chonnikant, Tanisa Kwanbunbumpen, Yuda Chongpison, Supakarn Chamni, Ekkarit Panichsillaphakit, Jaraspong Uaariyapanichkul, Settachote Maholarnkij, and Sirinuch Chomtho. 2022. "The Impact of Dietary Fiber as a Prebiotic on Inflammation in Children with Obesity" Foods 11, no. 18: 2856. https://doi.org/10.3390/foods11182856
APA StyleVisuthranukul, C., Kwanbunbumpen, T., Chongpison, Y., Chamni, S., Panichsillaphakit, E., Uaariyapanichkul, J., Maholarnkij, S., & Chomtho, S. (2022). The Impact of Dietary Fiber as a Prebiotic on Inflammation in Children with Obesity. Foods, 11(18), 2856. https://doi.org/10.3390/foods11182856







