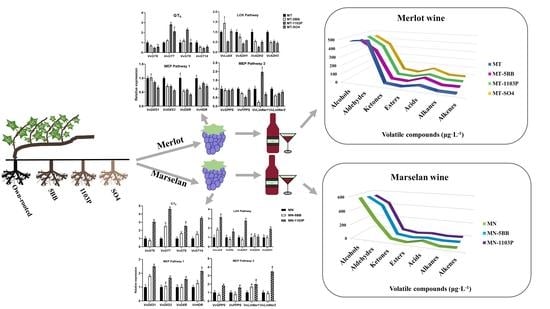
The Expression of Aroma Components and Related Genes in Merlot and Marselan Scion–Rootstock Grape and Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Material
2.2. Winemaking
2.3. Enological Parameter Analysis
2.4. Volatile Compounds SPME and GC-MS Analysis
2.5. Gene Expression Analysis by qRT-PCR
2.6. Data Analysis
3. Results
3.1. Effects of Rootstocks on Berry and Wine Physicochemical Parameters
3.2. Effects of Rootstocks on Merlot Berry and Wine Volatile Composition
3.3. Effects of Rootstocks on Marselan Berry and Wine Volatile Composition
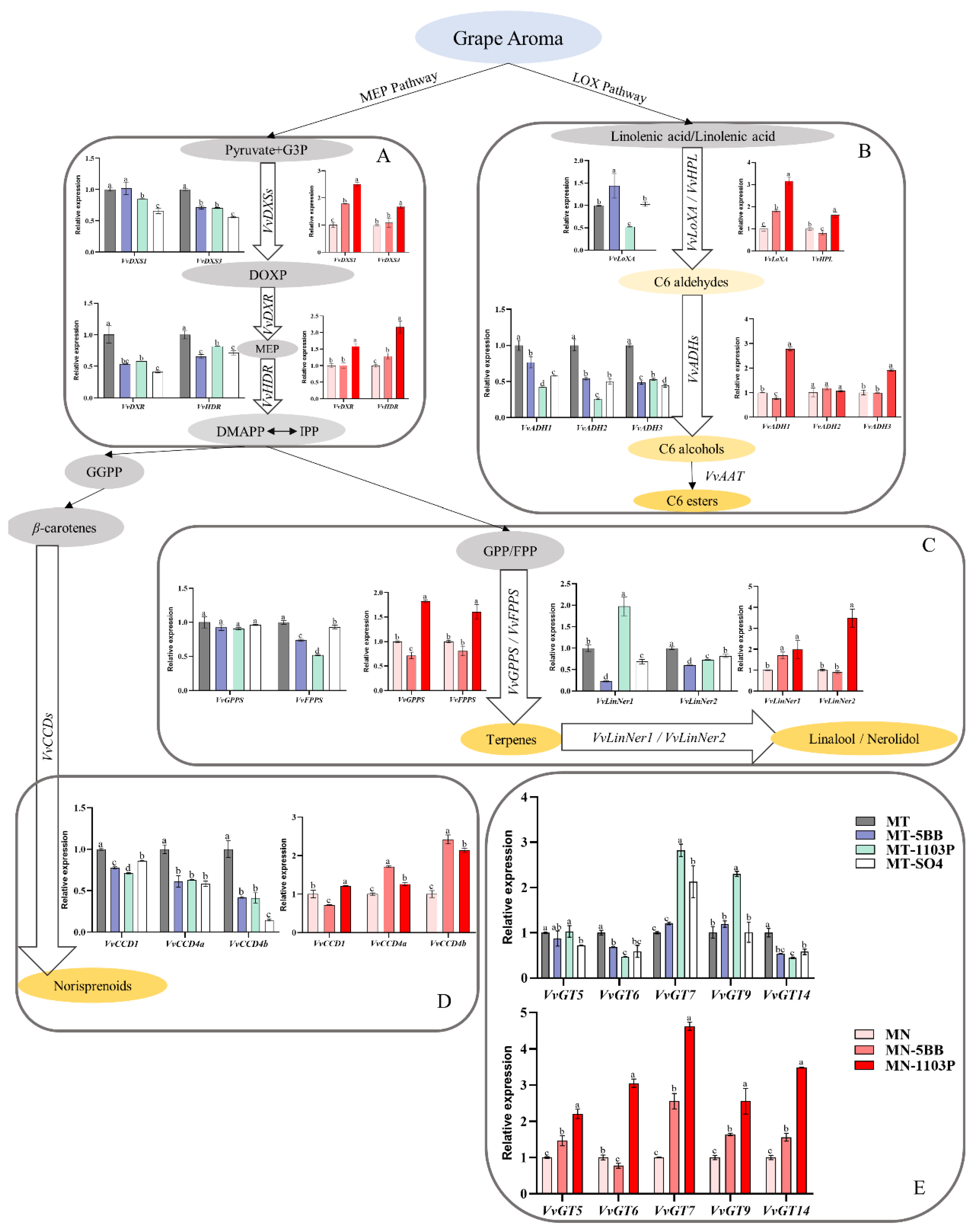
3.4. Rootstocks Effects on Volatile-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1103P | 1103 Paulsen |
| 5BB | Kober 5BB |
| AAT | Alcohol acyltransferases |
| ADH | Alcohol dehydrogenase |
| DMAPP | Dimethylallyl pyrophosphate |
| DXR | 1-Deoxy-D-xylulose 5-phosphate reductoisomerase |
| DXS | 1-Deoxy-D-xylulose-5-phosphate synthase |
| FPP | Farnesyldiphosphosphate |
| G3P | Glyceraldehyde 3-phosphate |
| GGPP | Geranylgeranylpyrophosphosphate |
| GPP | Geraniyl pyrophosphate |
| HDR | 1-Hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase |
| HPL | Hydroperoxides |
| IPP | Isopentenyl pyrophosphate |
| LOX | Lipoxygenase |
| MEP | Methylerythritol phosphate |
| MVA | Mevalonic acid |
| SO4 | Selection Oppenheim |
| TDN | 1,1,6-Trimethyl-1,2-dihydronaphthalene |
| TSS | Total soluble solids |
References
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. Review: The interaction between rootstocks and cultivars (vitis viniferal.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine R. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, H.; Sun, T.; Wang, Q.; Yao, Y. Modifications of ‘Gold Finger’ Grape Berry Quality as Affected by the Different Rootstocks. J. Agric. Food Chem. 2016, 64, 4189–4197. [Google Scholar] [CrossRef] [PubMed]
- Migicovsky, Z.; Harris, Z.N.; Klein, L.L.; Li, M.; McDermaid, A.; Chitwood, D.H.; Fennell, A.; Kovacs, L.G.; Kwasniewski, M.; Londo, J.P.; et al. Rootstock effects on scion phenotypes in a ‘Chambourcin’ experimental vineyard. Hort. Res. 2019, 6, 64. [Google Scholar] [CrossRef]
- Ibacache, A.; Lbornoz, F.A.; Zurita-Silva, A. Yield responses in flame seedless, thompson seedless and red globe table grape cultivars are differentially modified by rootstocks under semi arid conditions. Sci. Hortic. 2016, 204, 25–32. [Google Scholar] [CrossRef]
- Cheng, J.; Wei, L.; Mei, J.; Wu, J. Effect of rootstock on phenolic compounds and antioxidant properties in berries of grape (vitis vinifera l.) cv. ‘red alexandria’. Sci. Hortic. 2017, 217, 137–144. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Wang, W.; Duan, C.; He, F. The influence of rootstocks on the scions’ aromatic profiles of vitis vinifera l. cv. chardonnay. Sci. Hortic. 2020, 272, 109517. [Google Scholar] [CrossRef]
- Lo’Ay, A.A.; El-Ezz, S. Performance of ‘flame seedless’ grapevines grown on different rootstocks in response to soil salinity stress. Sci. Hortic. 2021, 275, 109704. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Massera, A.; Assof, M.; Sari, S.; Ciklic, I.; Combina, M. Effect of low temperature fermentation on the yeast-derived volatile aroma composition and sensory profile in merlot wines. LWT 2021, 142, 111069. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. part 1. chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Martin, D.M.; Chiang, A.; Lund, S.T.; Bohlmann, J. Biosynthesis of wine aroma: Transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta 2012, 236, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. Cell Mol. Biol. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Dami, I.E. Characterization of free flavor compounds in traminette grape and their relationship to vineyard training system and location. J. Food Sci. 2008, 73, C262–C267. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.K.; Gao, X.T.; He, L.; Yang, X.H.; He, F.; Duan, C.Q.; Wang, J. Rootstock-Mediated Effects on Cabernet Sauvignon Performance: Vine Growth, Berry Ripening, Flavonoids, and Aromatic Profiles. Int. J. Mol. Sci. 2019, 20, 401. [Google Scholar] [CrossRef]
- Carrasco-Quiroz, M.; Martínez-Gil, A.M.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Effect of rootstocks on volatile composition of Merlot wines. J. Sci. Food Agric. 2020, 100, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Li, A.H.; Dizy, M.; Ullah, N.; Sun, W.X.; Tao, Y.S. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef]
- Yue, X.; Shi, P.; Tang, Y.; Zhang, H.; Ma, X.; Ju, Y.; Zhang, Z. Effects of methyl jasmonate on the monoterpenes of Muscat Hamburg grapes and wine. J. Sci. Food Agric. 2021, 101, 3665–3675. [Google Scholar] [CrossRef]
- Wang, X.J.; Tao, Y.S.; Wu, Y.; An, R.Y.; Yue, Z.Y. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef]
- Zhang, E.; Chai, F.; Zhang, H.; Li, S.; Liang, Z.; Fan, P. Effects of sunlight exclusion on the profiles of monoterpene biosynthesis and accumulation in grape exocarp and mesocarp. Food Chem. 2017, 237, 379–389. [Google Scholar] [CrossRef]
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M. Light promotes expression of monoterpene and flavonol metabolic genes and enhances flavour of winegrape berries (Vitis vinifera L. cv. riesling). Aust. J. Grape Wine R. 2016, 22, 409–421. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Amor, F.D.; Gil-Muoz, R.; Flores, P.; Navarro, J.M. Interactive effects of the rootstock and the deficit irrigation technique on wine composition, nutraceutical potential, aromatic profile, and sensory attributes under semiarid and water limiting conditions. Agric. Water Manag. 2019, 225. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Comparison of major volatile compounds from riesling and cabernet sauvignon grapes (vitis vinifera l.) from fruitset to harvest. Aust. J. Grape Wine R. 2010, 16, 337–348. [Google Scholar] [CrossRef]
- Qian, X.; Xu, X.Q.; Yu, K.J.; Zhu, B.Q.; Lan, Y.B.; Duan, C.Q.; Pan, Q.H. Varietal Dependence of GLVs Accumulation and LOX-HPL Pathway Gene Expression in Four Vitis vinifera Wine Grapes. Int. J. Mol. Sci. 2016, 17, 1924. [Google Scholar] [CrossRef]
- Qian, X.; Sun, L.; Xu, X.Q.; Zhu, B.Q.; Xu, H.Y. Differential Expression of VvLOXA Diversifies C6 Volatile Profiles in Some Vitis vinifera Table Grape Cultivars. Int. J. Mol. Sci. 2017, 18, 2705. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Liang, Z.; Fan, P.; Li, S. Volatiles of grape berries evaluated at the germplasm level by headspace-spme with gc–ms. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Yang, C.X.; Wang, Y.; Wu, B.; Fang, J.; Li, S. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Jiang, W.; Li, J. Identification and quantification of impact aroma compounds in 4 nonfloral Vitis vinifera varieties grapes. J. Food Sci. 2010, 75, S81–S88. [Google Scholar] [CrossRef]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef] [PubMed]
- Satisha, J.; Somkuwar, R.G.; Sharma, J.; Upadhyay, A.K.; Adsule, P.G. Influence of rootstocks on growth yield and fruit composition of thompson seedless grapes grown in the pune region of india. S. Afr. J Enol. Vitic. 2016, 31, 1–8. [Google Scholar] [CrossRef][Green Version]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Allen, M.S.; Jr, D.R.B.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 99, 273–286. [Google Scholar] [CrossRef]
- Bönisch, F.; Frotscher, J.; Stanitzek, S.; Rühl, E.; Wüst, M.; Bitz, O.; Schwab, W. Activity-based profiling of a physiologic aglycone library reveals sugar acceptor promiscuity of family 1 UDP-glucosyltransferases from grape. Plant Physiol. 2014, 166, 23–39. [Google Scholar] [CrossRef]
| Grapes | |||||||
|---|---|---|---|---|---|---|---|
| MT | MT-5BB | MT-1103P | MT-SO4 | MN | MN-5BB | MN-1103P | |
| Total sugar | 196.77 ± 0.42 b | 191.87 ± 0.26 c | 206.40 ± 0.64 a | 187.47 ± 0.62 d | 204.27 ± 0.53 A | 197.23 ± 0.45 B | 193.10 ± 0.43 C |
| Glucose | 96.40 ± 0.78 a | 86.70 ± 0.90 c | 93.17 ± 0.54 b | 84.60 ± 0.51 d | 93.06 ± 0.48 A | 90.03 ± 0.52 B | 87.43 ± 0.52 C |
| Fructose | 98.57 ± 1.09 c | 103.17 ± 0.90 b | 111.50 ± 0.78 a | 101.13 ± 0.86 b | 108.83 ± 0.59 A | 105.40 ± 0.50 B | 102.83 ± 0.52 C |
| Total soluble solids | 219.07 ± 0.21 d | 229.37 ± 0.53 b | 248.40 ± 0.70 a | 225.20 ± 0.99 c | 244.37 ± 0.54 A | 236.50 ± 0.64 B | 231.13 ± 0.33 C |
| Titratable acidity | 5.61 ± 0.06 b | 6.53 ± 0.08 a | 6.56 ± 0.03 a | 6.58 ± 0.02 a | 6.94 ± 0.04 C | 7.85 ± 0.05 A | 7.34 ± 0.10 B |
| TSS/TA | 39.04 | 35.13 | 37.86 | 34.22 | 35.21 | 30.13 | 31.49 |
| Wine | |||||||
|---|---|---|---|---|---|---|---|
| MT | MT-5BB | MT-1103P | MT-SO4 | MN | MN-5BB | MN-1103P | |
| Alcohol degree (%vol) | 12.06 ± 0.05 B | 11.78 ± 0.05 C | 12.56 ± 0.08 A | 12.23 ± 0.12 B | 12.32 ± 0.05 a | 9.65 ± 0.09 b | 9.33 ± 0.08 c |
| Titratable acidity (g/L) | 5.26 ± 0.04 B | 5.43 ± 0.08 A | 5.07 ± 0.05 C | 4.95 ± 0.04 C | 5.88 ± 0.04 a | 5.46 ± 0.06 b | 4.88 ± 0.05 c |
| Lactic acid (g/L) | 1.61 ± 0.04 A | 1.38 ± 0.04 B | 1.64 ± 0.04 A | 1.65 ± 0.03 A | 2.11 ± 0.04 a | 1.44 ± 0.02 b | 1.92 ± 0.03 c |
| Tartaric acid (g/L) | 1.29 ± 0.05 B | 1.50 ± 0.00 A | 1.14 ± 0.02 C | 1.29 ± 0.02 B | 1.34 ± 0.02 c | 2.13 ± 0.02 a | 1.77 ± 0.03 b |
| pH | 3.62 ± 0.02 B | 3.60 ± 0.01 B | 3.73 ± 0.01 A | 3.70 ± 0.01 A | 3.73 ± 0.00 a | 3.50 ± 0.01 c | 3.64 ± 0.00 b |
| Glycerol (g/L) | 8.00 ± 0.08 C | 8.23 ± 0.09B C | 8.77 ± 0.12 A | 8.37 ± 0.12 B | 7.90 ± 0.24 a | 6.90 ± 0.14 b | 6.83 ± 0.12 b |
| Grape | Wine | |||||||
|---|---|---|---|---|---|---|---|---|
| MT | MT-5BB | MT-1103P | MT-SO4 | MT | MT-5BB | MT-1103P | MT-SO4 | |
| C6 compounds | ||||||||
| Hexanal | 123.97 ± 0.57 A | 125.42 ± 6.03 A | ND | ND | 262.88 ± 11.28 a | 174.84 ± 2.21 c | 188.42 ± 2.82 b | 181.73 ± 1.22 b |
| (E)-2-hexenal | 91.11 ± 0.34 A | 79.90 ± 1.90 B | ND | ND | 199.41 ± 7.10 a | 134.31 ± 2.29 b | 142.32 ± 2.14 b | 140.79 ± 1.47 b |
| (Z)/(E)-3-hexenol | 1.14 ± 0.00 B | 1.27 ± 0.51 A | ND | ND | 0.90 ± 0.09 d | 3.60 ± 0.04 a | 1.12 ± 0.04 c | 1.38 ± 0.06 b |
| (E)-2-hexenol | 14.15 ± 0.72 B | 17.85 ± 1.55 A | ND | ND | 32.49 ± 0.24 c | 41.12 ± 0.63 a | 31.42 ± 0.02 d | 35.16 ± 0.16 b |
| Hexanol | 16.20 ± 0.02 B | 22.27 ± 2.22 A | 20.29 ± 0.69 A | 21.46 ± 0.06 A | 53.47 ± 0.72 c | 82.34 ± 1.11 a | 62.77 ± 0.19 b | 54.77 ± 0.42 c |
| Hexanoic acid | 2.08 ± 0.72 B | 2.95 ± 0.13 A | ND | ND | 32.43 ± 7.30 b | 56.22 ± 1.37 a | 39.95 ± 2.37 b | 60.72 ± 5.69 a |
| Isobutyl acetate | ND | ND | 2.87 ± 0.12 A | 2.81 ± 0.02 A | ND | ND | ND | ND |
| Butanoic acid, ethyl ester | ND | ND | 5.73 ± 0.27 A | 5.25 ± 0.00 A | ND | ND | ND | ND |
| Monoterpenes | ||||||||
| Linalool | 0.75 ± 0.01 | ND | ND | ND | 1.58 ± 0.035 c | ND | 1.90 ± 0.02 b | 2.18 ± 0.03 a |
| Geraniol | 1.81 ± 0.12 A | 1.22 ± 0.08 B | ND | ND | 6.41 ± 0.20 a | 1.79 ± 0.00 b | 6.50 ± 0.12 a | ND |
| Citronellol | 0.44 ± 0.00 B | ND | 7.29 ± 0.17 A | 7.23 ± 0.05 A | ND | ND | ND | ND |
| Alcohols | 141.97 ± 0.49 B | 154.38 ± 5.21 B | 1088.93 ± 42.95 A | 1065.72 ± 11.24 A | 478.45 ± 16.29 a | 458.80 ± 0.90 ab | 470.14 ± 3.11 ab | 451.02 ± 5.38 b |
| Aldehydes | 230.08 ± 0.77 A | 217.78 ± 6.90 A | 4.67 ± 0.10 B | 3.64 ± 0.10 B | 494.27 ± 18.21 a | 340.68 ± 4.0 1b | 357.51 ± 6.13 b | 347.98 ± 1.14 b |
| Ketones | 10.82 ± 0.04 B | 10.52 ± 0.75 B | 309.71 ± 9.97 A | 4.94 ± 0.04 B | 20.83 ± 6.67 ab | 11.71 ± 0.15 bc | 8.79 ± 0.63 c | 22.60 ± 4.79 a |
| Esters | 4.93 ± 0.00 B | 1.64 ± 0.08 C | 11.89 ± 0.47 A | 5.89 ± 0.54 B | 4.28 ± 0.97 a | 1.51 ± 0.21 b | 1.45 ± 0.02 b | 1.61 ± 0.05 b |
| Acids | 6.18 ± 0.12 C | 6.27 ± 0.99 C | 427.88 ± 16.41 B | 684.63 ± 2.86 A | 41.96 ± 8.06 b | 65.08 ± 2.21 a | 45.26 ± 3.87 b | 65.66 ± 5.32 a |
| Alkanes | 0.68 ± 0.08 A | 0.52 ± 0.04 A | 0.00 B | 0.45 ± 0.01 A | 2.62 ± 0.30 a | 1.33 ± 0.03 bc | 1.14 ± 0.01 c | 2.15 ± 0.66 ab |
| Alkenes | 0.85 ± 0.22 B | 0.83 ± 0.03 B | 1.78 ± 0.05 A | 1.19 ± 0.09 B | 1.95 ± 0.13 b | 5.01 ± 1.40 a | 1.98 ± 0.22b | 1.92 ± 0.29 b |
| Total | 395.51 ± 1.63 | 391.93 ± 17.16 | 1844.87 ± 85.67 | 1766.45 ± 17.85 | 1044.36 ± 41.65 | 884.12 ± 0.64 | 886.29 ± 16.6 | 892.95 ± 9.15 |
| Grape | Wine | |||||
|---|---|---|---|---|---|---|
| MN | MN-5BB | MN-1103P | MN | MN-5BB | MN-1103P | |
| C6 compounds | ||||||
| Hexanal | ND | ND | ND | 124.28 ± 3.92 b | 196.12 ± 0.16 a | 204.66 ± 17.47 a |
| (E) (Z)-2-hexenol | ND | ND | ND | 31.35 ± 0.97 b | 25.65 ± 0.12 c | 34.67 ± 1.56 a |
| Hexanoic acid | ND | ND | ND | 26.36 ± 1.12 a | 10.59 ± 0.22 c | 13.39 ± 0.65 b |
| (E)-2-hexenal | ND | ND | ND | 117.51 ± 5.43 b | 210.41 ± 1.13 a | 201.29 ± 14.27 a |
| (E)-3-hexenol | ND | ND | ND | 5.95 ± 0.19 a | 4.73 ± 0.00 b | 4.66 ± 0.15 b |
| 2-Ethyl-furan | ND | ND | ND | ND | 0.66 ± 0.02 a | 0.54 ± 0.05 a |
| Hexanol | 22.86 ± 0.14 A | 23.46 ± 0.57 A | 18.70 ± 0.31 B | 52.59 ± 1.44 b | 45.40 ± 0.24 c | 64.74 ± 2.28 a |
| Isobutyl acetate | 3.34 ± 0.06 B | 3.80 ± 0.09 A | 2.31 ± 0.01 C | ND | ND | ND |
| Ethyl ester-butanoic acid | 6.44 ± 0.15 A | 6.49 ± 0.13 A | 6.20 ± 0.11 A | ND | ND | ND |
| 3-Methyl-4-oxo-pentanoic acid | 395.60 ± 3.80 B | 426.47 ± 10.10 A | 372.25 ± 6.89 C | ND | ND | ND |
| 3-Methyl-1-pentanol | 0.53 ± 0.00 A | 0.37 ± 0.01 C | 0.48 ± 0.00 B | ND | ND | ND |
| Terpenes | ||||||
| Linalool | ND | ND | ND | 9.28 ± 0.51 a | 9.69 ± 0.15 a | 9.62 ± 0.18 a |
| α-Terpineol | 0.53 ± 0.01 C | 0.67 ± 0.03 B | 1.35 ± 0.02 A | 1.05 ± 0.05 a | 0.86 ± 0.02 b | 0.93 ± 0.00 b |
| Geraniol | ND | ND | ND | 28.92 ± 1.68 b | 31.97 ± 0.39 a | 26.87 ± 0.27 b |
| Citronellol | 8.14 ± 0.18 C | 8.79 ± 0.24 B | 9.67 ± 0.18 A | ND | 3.96 ± 0.05 a | 3.85 ± 0.03 a |
| Trans-β-ocimene | ND | ND | ND | 0.89 ± 0.07 a | 0.76 ± 0.18 a | 0.72 ± 0.16 a |
| Neral | ND | ND | ND | 0.29 ± 0.01 a | 0.25 ± 0.00 b | 0.25 ± 0.00 b |
| β-Myrcene | ND | ND | ND | 5.05 ± 0.45 a | 5.70 ± 0.42 a | 4.95 ± 0.19 a |
| Farnesene | ND | 1.38 ± 0.02 A | 0.58 ± 0.01 B | ND | ND | ND |
| Nerolidol | ND | 3.39 ± 0.06 B | 5.25 ± 0.11 A | ND | ND | ND |
| Alcohols | 1030.39 ± 15.44 A | 951.14 ± 23.12 B | 839.52 ± 13.05 C | 580.09 ± 11.28 a | 561.15 ± 5.66 a | 546.38 ± 20.56 a |
| Aldehydes | 7.93 ± 0.27 A | 2.53 ± 0.31 B | 0.42 ± 0.34 C | 274.43 ± 11.42 b | 439.18 ± 0.26 a | 433.46 ± 28.19 a |
| Ketones | 4.98 ± 0.07 B | 5.74 ± 0.13 B | 15.18 ± 0.72 A | 29.76 ± 4.46 a | 27.96 ± 0.53 a | 32.62 ± 0.72 a |
| Esters | 6.60 ± 0.61 B | 10.47 ± 0.29 A | 5.13 ± 0.02 C | 2.13 ± 0.17 a | 0.71 ± 0.51 b | 2.05 ± 0.29 a |
| Acids | 799.80 ± 30.18 A | 798.29 ± 24.26 A | 733.09 ± 11.80 B | 51.77 ± 17.74 a | 18.82 ± 1.51 b | 18.28 ± 0.57 b |
| Alkanes | 0 | 0.66 ± 0.54 B | 0.96 ± 0.00 A | 2.69 ± 0.50 ab | 4.05 ± 1.12 a | 1.99 ± 0.25 b |
| Alkenes | 1.28 ± 0.06 C | 2.05 ± 0.06 B | 2.95 ± 0.34 A | 11.24 ± 1.31 a | 12.58 ± 0.02 a | 10.98 ± 0.62 a |
| Total | 1850.99 ± 56.45 | 1770.88 ± 57.43 | 1597.25 ± 32.18 | 952.10 ± 57.42 | 1064.46 ± 4.68 | 1045.77 ± 62.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, H.; Li, Y.; Du, T.; Jia, J.; Xi, Z. The Expression of Aroma Components and Related Genes in Merlot and Marselan Scion–Rootstock Grape and Wine. Foods 2022, 11, 2777. https://doi.org/10.3390/foods11182777
Li C, Chen H, Li Y, Du T, Jia J, Xi Z. The Expression of Aroma Components and Related Genes in Merlot and Marselan Scion–Rootstock Grape and Wine. Foods. 2022; 11(18):2777. https://doi.org/10.3390/foods11182777
Chicago/Turabian StyleLi, Chan, Hao Chen, Yiran Li, Tiantian Du, Jia Jia, and Zhumei Xi. 2022. "The Expression of Aroma Components and Related Genes in Merlot and Marselan Scion–Rootstock Grape and Wine" Foods 11, no. 18: 2777. https://doi.org/10.3390/foods11182777
APA StyleLi, C., Chen, H., Li, Y., Du, T., Jia, J., & Xi, Z. (2022). The Expression of Aroma Components and Related Genes in Merlot and Marselan Scion–Rootstock Grape and Wine. Foods, 11(18), 2777. https://doi.org/10.3390/foods11182777