Succinylation Modified Ovalbumin: Structural, Interfacial, and Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
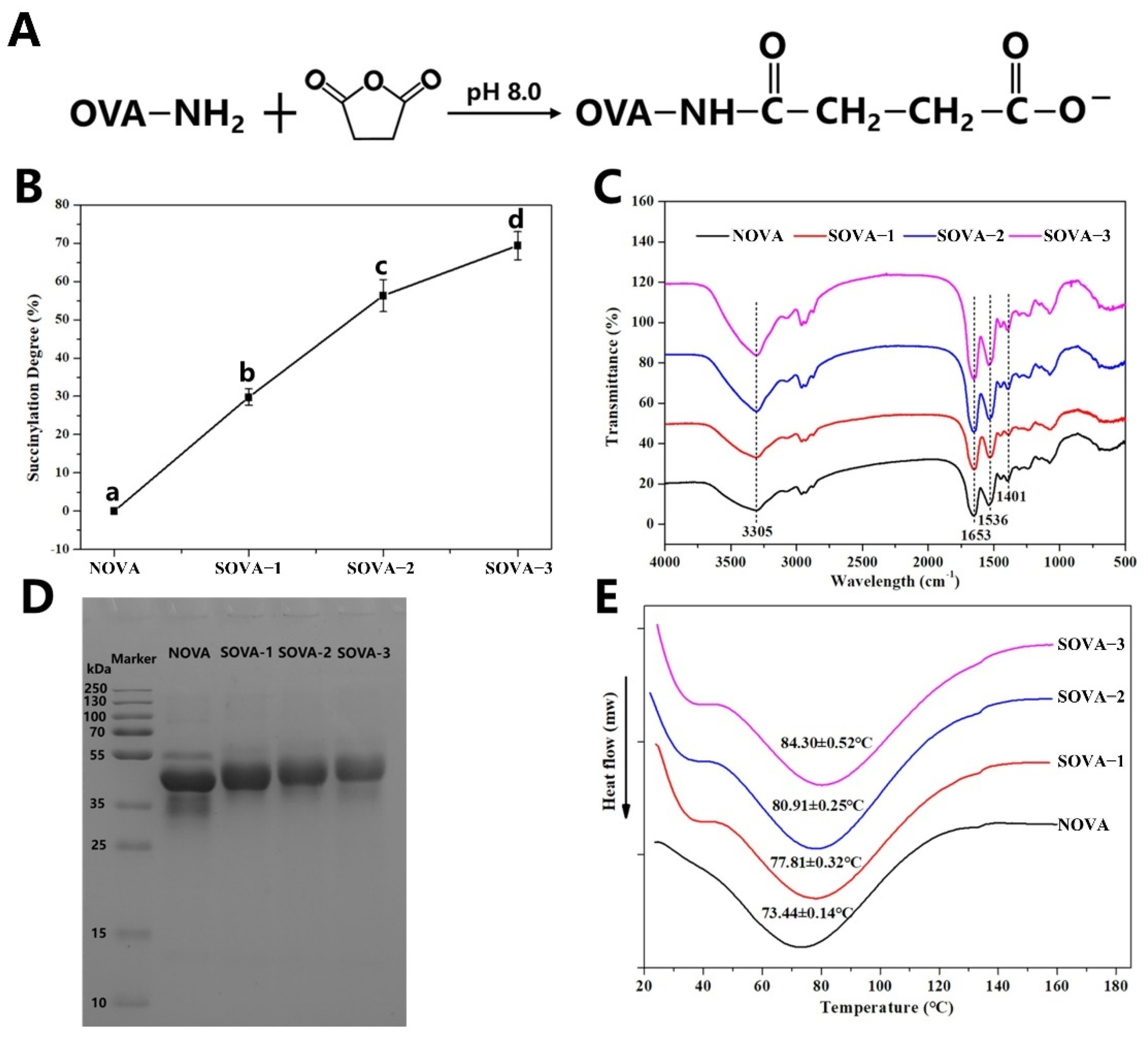
2.2. Synthesis of Succinylated Ovalbumin (SOVA)
2.3. Measurement of the Degree of Succinylation
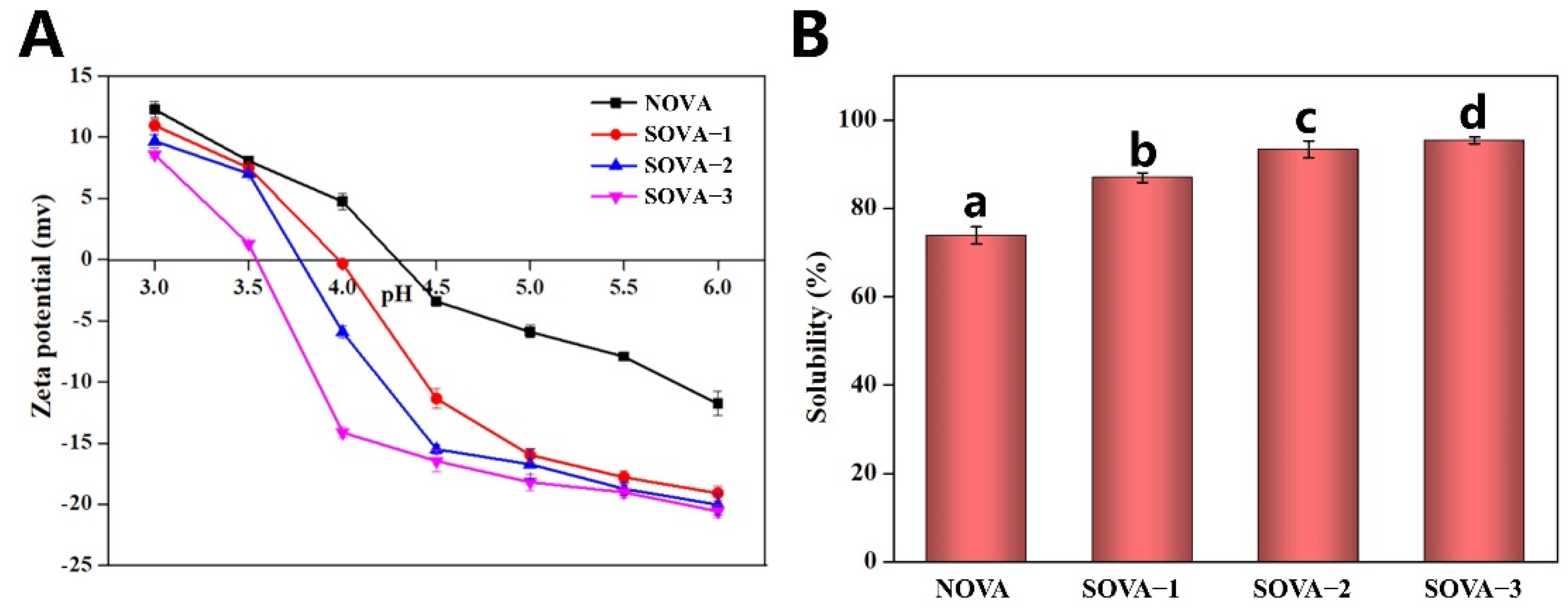
2.4. Zeta-Potential Analysis
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
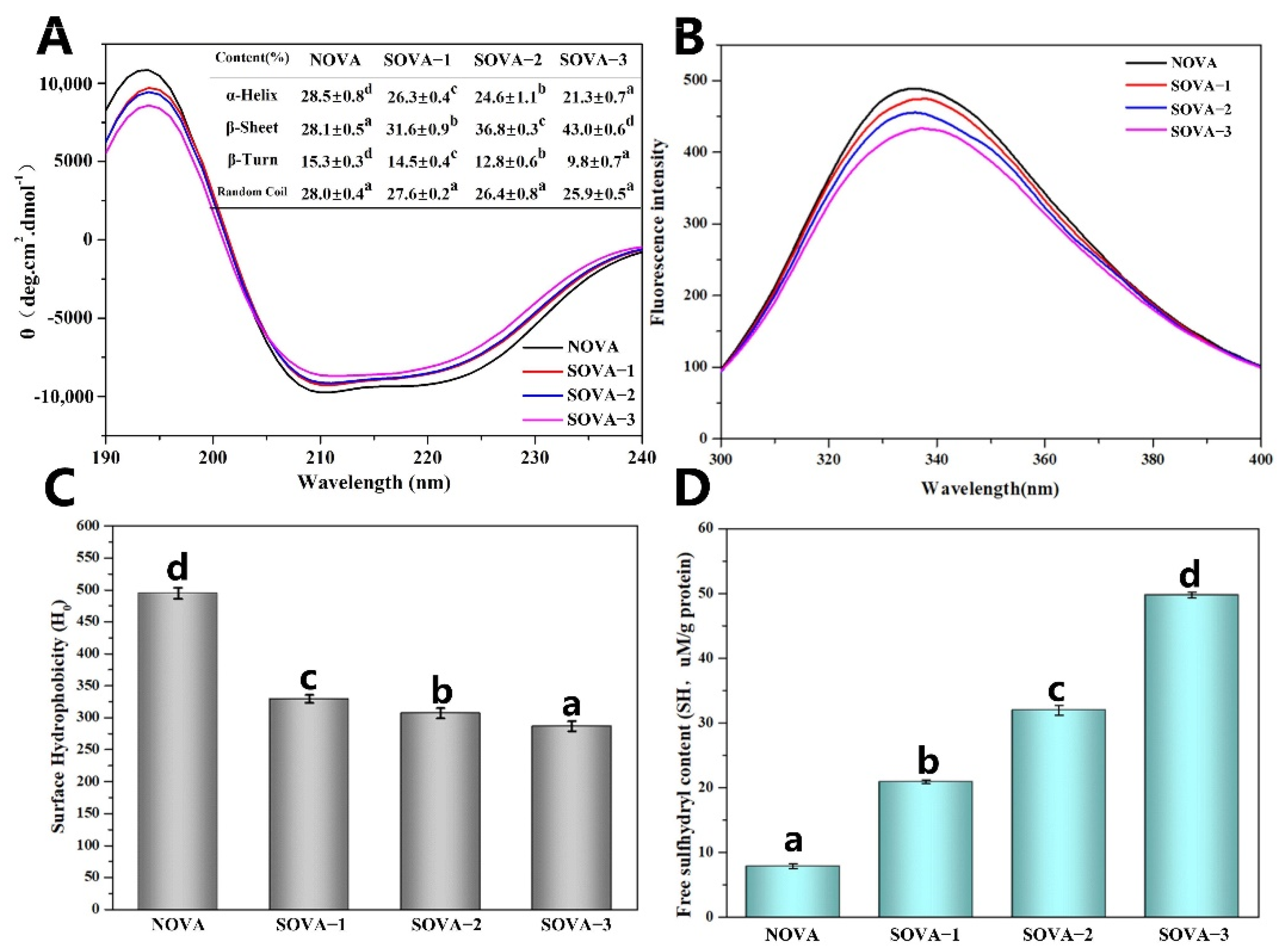
2.7. Circular Dichroism (CD) Analysis
2.8. Intrinsic Fluorescence Emission Spectrum
2.9. Free sulfhydryl Groups (SH) Determination
2.10. Surface Hydrophobicity (H0) Determination
2.11. Differential Scanning Calorimetry (DSC)
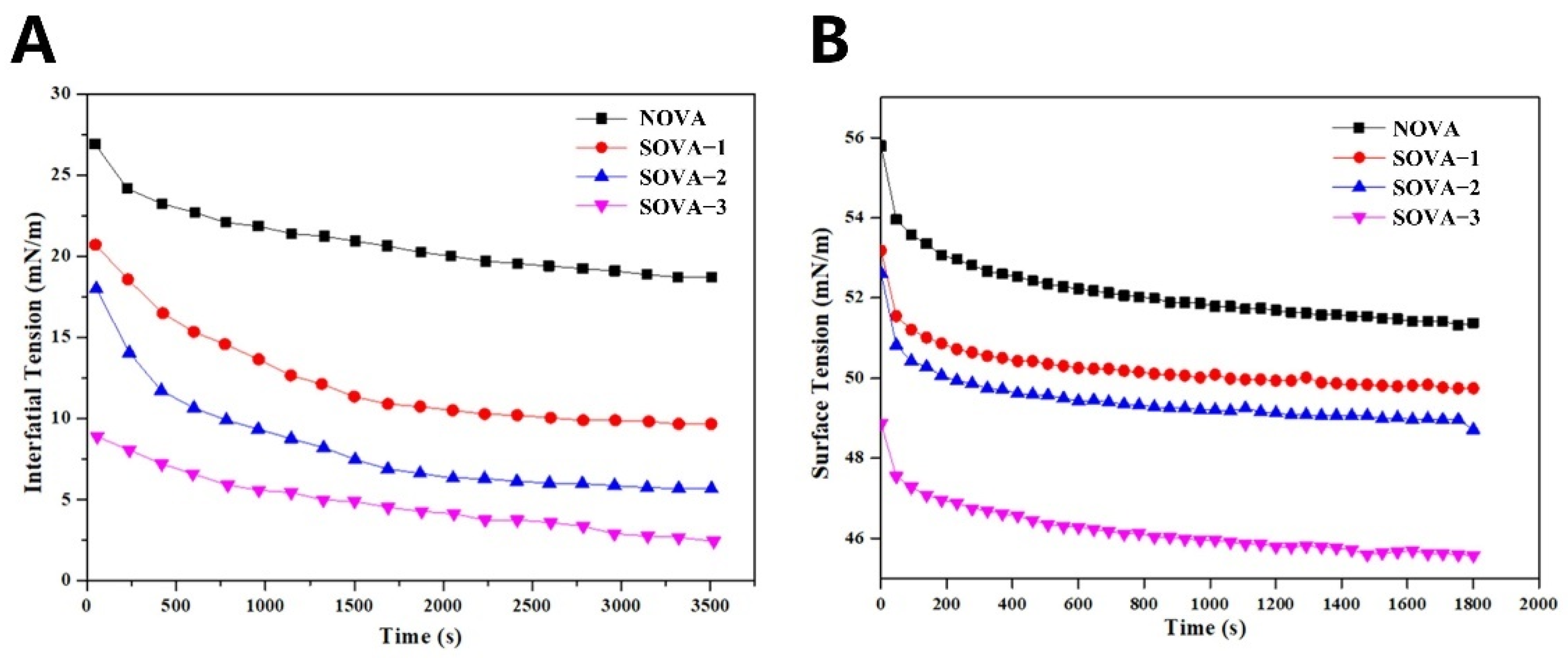
2.12. Interfacial Tension Measurements
2.13. Solubility
2.14. Emulsifying Properties
2.15. Foaming Properties
2.16. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
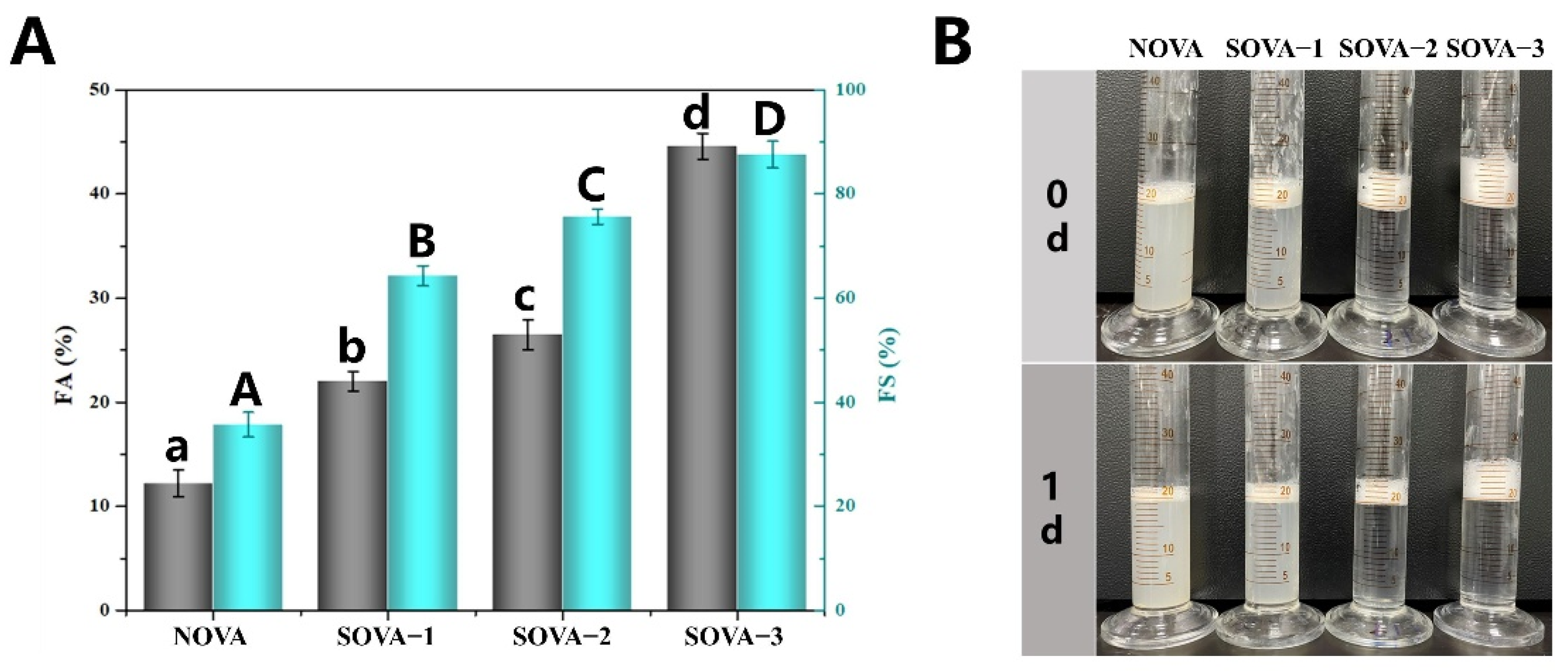
3.1.1. Degree of Succinylation
3.1.2. FTIR
3.1.3. SDS–PAGE
3.1.4. DSC
3.2. Structural Properties
3.2.1. CD spectroscopy
3.2.2. Intrinsic Fluorescence
3.2.3. Surface Hydrophobicity (H0)
3.2.4. Free Sulfhydryl Groups
3.3. Interface Properties
3.3.1. Oil–Water Interfacial Tension
3.3.2. Air–Water Interfacial Tension
3.4. Functional Properties
3.4.1. Protein Solubility
3.4.2. Emulsifying Properties
3.4.3. Foaming Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gharbi, N.; Labbafi, M. Effect of processing on aggregation mechanism of egg white proteins. Food Chem. 2018, 252, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.; Hoffmann-Sommergruber, K.; Have, T.T.; Foss, N.; Briza, P.; Oberhuber, C.; Radauer, C.; Alessandri, S.; Knulst, A.C.; Fernandez-Rivas, M.; et al. The panel of egg allergens, Gal d 1–Gal d 5: Their improved purification and characterization. Mol. Nutr. Food Res. 2008, 52, S176–S185. [Google Scholar] [CrossRef]
- Delahaije, R.J.; Lech, F.J.; Wierenga, P.A. Investigating the effect of temperature on the formation and stabilization of ovalbumin foams. Food Hydrocoll. 2019, 91, 263–274. [Google Scholar] [CrossRef]
- Karbasi, M.; Madadlou, A. Interface-related attributes of the Maillard reaction-born glycoproteins. Crit. Rev. Food Sci. Nutr. 2018, 58, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Pourmohammadi, K. Chemical modifications and their effects on gluten protein: An extensive review. Food Chem. 2021, 343, 128398. [Google Scholar] [CrossRef]
- Shilpashree, B.; Arora, S.; Chawla, P.; Vakkalagadda, R.; Sharma, A. Succinylation of sodium caseinate and its effect on physicochemical and functional properties of protein. LWT Food Sci. Technol. 2015, 64, 1270–1277. [Google Scholar] [CrossRef]
- Smith, J.; Hong-Shum, L. Food Additives Data Book; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chem. 2009, 114, 127–131. [Google Scholar] [CrossRef]
- Charoensuk, D.; Brannan, R.G.; Chanasattru, W.; Chaiyasit, W. Physicochemical and emulsifying properties of mung bean protein isolate as influenced by succinylation. Int. J. Food Prop. 2018, 21, 1633–1645. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, D.; Xia, Q.; Cao, J.; Zhou, C.; He, J.; Sun, Y.; Xu, S. Impact of pH-dependent succinylation on the structural features and emulsifying properties of chicken liver protein. Food Chem. 2021, 358, 129868. [Google Scholar] [CrossRef]
- Hu, G.; Batool, Z.; Cai, Z.; Liu, Y.; Ma, M.; Sheng, L.; Jin, Y. Production of self-assembling acylated ovalbumin nanogels as stable delivery vehicles for curcumin. Food Chem. 2021, 355, 129635. [Google Scholar] [CrossRef]
- Zhao, C.-B.; Zhang, H.; Xu, X.-Y.; Cao, Y.; Zheng, M.-Z.; Liu, J.-S.; Wu, F. Effect of acetylation and succinylation on physicochemical properties and structural characteristics of oat protein isolate. Process Biochem. 2017, 57, 117–123. [Google Scholar] [CrossRef]
- Geng, F.; Huang, Y.; Huang, Q.; He, D.; Li, S.; Ma, M. Effect of hydroxyl radical-induced oxidation on the structure and heat-induced gel properties of ovalbumin. J. Food Process. Preserv. 2018, 42, e13626. [Google Scholar] [CrossRef]
- Lajnaf, R.; Picart-Palmade, L.; Attia, H.; Marchesseau, S.; Ayadi, M. Foaming and air-water interfacial properties of camel milk proteins compared to bovine milk proteins. Food Hydrocoll. 2022, 126, 107470. [Google Scholar] [CrossRef]
- Ellman, G.L. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 1958, 74, 443–450. [Google Scholar] [CrossRef]
- Sheng, L.; Ye, S.; Han, K.; Zhu, G.; Ma, M.; Cai, Z. Consequences of phosphorylation on the structural and foaming properties of ovalbumin under wet-heating conditions. Food Hydrocoll. 2019, 91, 166–173. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.; Zhang, C.; Wan, J.; Shah, B.R.; Pei, Y.; Zhou, B.; Li, J.; Li, B. High intensity ultrasound modified ovalbumin: Structure, interface and gelation properties. Ultrason. Sonochemistry 2016, 31, 302–309. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Sharma, V.; Chawla, P.; Vakkalagadda, R. Changes in physicochemical and functional properties of whey protein concentrate upon succinylation. Int. J. Dairy Technol. 2017, 70, 137–145. [Google Scholar] [CrossRef]
- Classics, L.O.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, T.; Xing, T.; Xu, X.-L.; Zhou, G. Rheological and physical properties of O/W protein emulsions stabilized by isoelectric solubilization/precipitation isolated protein: The underlying effects of varying protein concentrations. Food Hydrocoll. 2019, 95, 580–589. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, C.-Y.; Yuen, A.S.-N.; Phillips, D.L. Study of Succinylated Food Proteins by Raman Spectroscopy. J. Agric. Food Chem. 2004, 52, 1815–1823. [Google Scholar] [CrossRef]
- Liu, G.; Hu, M.; Du, X.; Qi, B.; Lu, K.; Zhou, S.; Xie, F.; Li, Y. Study on the interaction between succinylated soy protein isolate and chitosan and its utilization in the development of oil-in-water bilayer emulsions. Food Hydrocoll. 2022, 124, 107309. [Google Scholar] [CrossRef]
- Guan, J.J.; Qiu, A.Y.; Liu, X.Y.; Hua, Y.F.; Ma, Y.H. Microwave Improvement of Soy Protein Isolate–Saccharide Graft Reactions. Food Chemistry. 2006, 97, 577–585. [Google Scholar] [CrossRef]
- Bunaciu, A.; Fleschin, S.; Aboul-Enein, H. Infrared Microspectroscopy Applications—Review. Curr. Anal. Chem. 2014, 10, 132–139. [Google Scholar] [CrossRef]
- Spada, J.C.; Marczak, L.D.; Tessaro, I.C.; Cardozo, N.S. Interactions between soy protein from water-soluble soy extract and polysaccharides in solutions with polydextrose. Carbohydr. Polym. 2015, 134, 119–127. [Google Scholar] [CrossRef]
- Cuadri, A.; Bengoechea, C.; Romero, A.; Guerrero, A. A natural-based polymeric hydrogel based on functionalized soy protein. Eur. Polym. J. 2016, 85, 164–174. [Google Scholar] [CrossRef]
- Ren, L.; Liu, J.; Zhang, X.; Zhao, S.; Lv, Y.; Guo, H. Emulsion, gelation, physicochemical properties and microstructure of phosphorylated and succinylated egg yolk. LWT 2020, 131, 109675. [Google Scholar] [CrossRef]
- Rao, S.; Xu, G.; Lu, X.; Zhang, R.; Gao, L.; Wang, Q.; Yang, Z.; Jiao, X. Characterization of ovalbumin-carvacrol inclusion complexes as delivery systems with antibacterial application. Food Hydrocoll. 2020, 105, 105753. [Google Scholar] [CrossRef]
- Feng, J.; Wu, S.; Wang, H.; Liu, S. Improved Oxidative Stability of Fish Oil Emulsion by Grafted Ovalbumin-Catechin Conjugates. Food Chemistry. 2018, 241, 60–69. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- He, R.; Dai, C.; Li, Y.; Wang, Z.; Li, Q.; Zhang, C.; Ju, X.; Yuan, J. Effects of Succinylation on the Physicochemical Properties and Structural Characteristics of Edible Rapeseed Protein Isolate Films. J. Am. Oil Chem. Soc. 2019, 96, 1103–1113. [Google Scholar] [CrossRef]
- Feng, M.; Pan, L.; Yang, X.; Sun, J.; Xu, X.; Zhou, G. Thermal gelling properties and mechanism of porcine myofibrillar protein containing flaxseed gum at different NaCl concentrations. LWT 2018, 87, 361–367. [Google Scholar] [CrossRef]
- Dufour, E.; Hoa, G.H.B.; Haertlé, T. High-pressure effects on β-lactoglobulin interactions with ligands studied by fluorescence. Biochim. et Biophys. Acta (BBA)—Protein Struct. Mol. Enzym. 1994, 1206, 166–172. [Google Scholar] [CrossRef]
- Spotti, M.J.; Martinez, M.J.; Pilosof, A.M.; Candioti, M.; Rubiolo, A.C.; Carrara, C.R. Influence of Maillard conjugation on structural characteristics and rheological properties of whey protein/dextran systems. Food Hydrocoll. 2014, 39, 223–230. [Google Scholar] [CrossRef]
- Feng, J.; Wu, S.; Wang, H.; Liu, S. Gliadin nanoparticles stabilized by a combination of thermally denatured ovalbumin with gemini dodecyl O-glucoside: The modulating effect of cosurfactant. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 516, 94–105. [Google Scholar] [CrossRef]
- Yang, M.; Cui, N.; Fang, Y.; Shi, Y.; Yang, J.; Wang, J. Influence of succinylation on the conformation of yak casein micelles. Food Chem. 2015, 179, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Lv, L.; Chi, Y. Improvement of functional properties of ovalbumin phosphorylated by dry-heating in the presence of pyrophosphate. Eur. Food Res. Technol. 2012, 235, 981–987. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, Y.; Chen, J.; Zou, J.; Wang, Q.; Ma, M. Influence of high-intensity ultrasound on foaming and structural properties of egg white. Food Res. Int. 2018, 108, 604–610. [Google Scholar] [CrossRef]
- Sebii, H.; Karra, S.; Bchir, B.; Nhouchi, Z.; Ghribi, A.M.; Karoui, R.; Blecker, C.; Besbes, S. Effect of succinylation on the secondary structures, surface, and thermal properties of date palm pollen protein concentrate. J. Food Sci. Technol. 2021, 58, 632–640. [Google Scholar] [CrossRef]
- Yin, S.-W.; Tang, C.-H.; Wen, Q.-B.; Yang, X.-Q.; Yuan, D.-B. The relationships between physicochemical properties and conformational features of succinylated and acetylated kidney bean (Phaseolusvulgaris L.) protein isolates. Food Res. Int. 2010, 43, 730–738. [Google Scholar] [CrossRef]
- Lawal, O.; Adebowale, K.; Adebowale, Y. Functional properties of native and chemically modified protein concentrates from bambarra groundnut. Food Res. Int. 2007, 40, 1003–1011. [Google Scholar] [CrossRef]
- Xia, N.; Lu, X.; Zheng, Z.; Mu, D.; Zhong, X.; Luo, S.; Zhao, Y. Study on preparation of acylated soy protein and stability of emulsion. J. Sci. Food Agric. 2021, 101, 4959–4968. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Umesh, K.V.; Singhal, R.S. Hydrophobically modified pea proteins: Synthesis, characterization and evaluation as emulsifiers in eggless cake. J. Food Eng. 2019, 255, 15–23. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Zhang, J.; Wang, Q.; Ma, M.; Ma, L.; Li, S. Succinylation Modified Ovalbumin: Structural, Interfacial, and Functional Properties. Foods 2022, 11, 2724. https://doi.org/10.3390/foods11182724
Hu G, Zhang J, Wang Q, Ma M, Ma L, Li S. Succinylation Modified Ovalbumin: Structural, Interfacial, and Functional Properties. Foods. 2022; 11(18):2724. https://doi.org/10.3390/foods11182724
Chicago/Turabian StyleHu, Gan, Jiemin Zhang, Qi Wang, Meihu Ma, Lulu Ma, and Shugang Li. 2022. "Succinylation Modified Ovalbumin: Structural, Interfacial, and Functional Properties" Foods 11, no. 18: 2724. https://doi.org/10.3390/foods11182724
APA StyleHu, G., Zhang, J., Wang, Q., Ma, M., Ma, L., & Li, S. (2022). Succinylation Modified Ovalbumin: Structural, Interfacial, and Functional Properties. Foods, 11(18), 2724. https://doi.org/10.3390/foods11182724






